Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 14, 2007; 13(14): 2083-2088
Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2083
Published online Apr 14, 2007. doi: 10.3748/wjg.v13.i14.2083
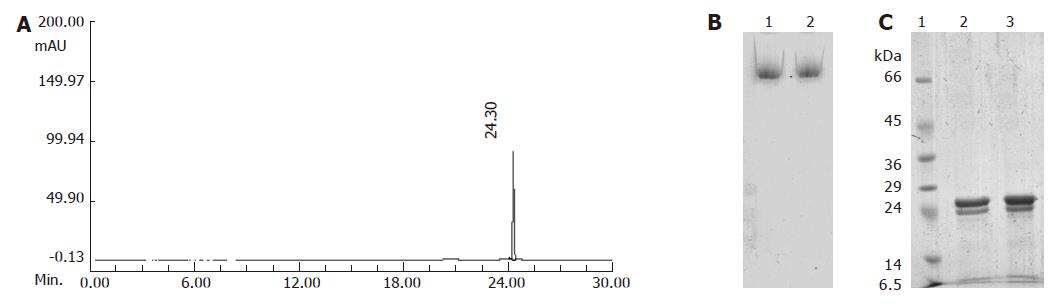
Figure 1 Characterization of purified pea ferritin: A: Capillary zone electrophoretogram; B: 6% PAGE; C: 12.
5% SDS-PAGE of pooled fractions obtained after gel filtration chromatography on Sepharose-6B column. B1 and 2: Purified ferritin (10 μg). C1: Molecular weight markers; C 2 and 3: 10 μg of purified ferritin.
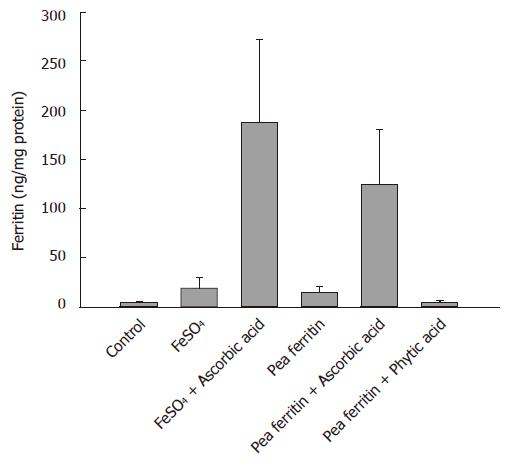
Figure 2 Pea ferritin iron bioavailabilityin the presence and absence of ascorbic acid and phytic acid.
Bars represent mean and SD of ferritin formed in caco 2 cells. Control (saline), FeSO4 (78 μg iron) and pea ferritin (78 μg iron in 0.24 mg protein) in the absence or presence of ascorbic acid (1:5 ratio) and pea ferritin in presence of phytic acid (1:1 ratio). Bar that do not share common alphabets differ significantly (P < 0.004).
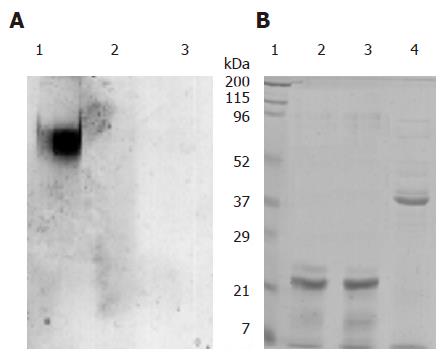
Figure 3 Digestive stability of pea ferritin: Purified pea ferritin (1 mg/mL) was incubated at pH 7.
2 and at pH 2 in the presence and absence of 1.6 mg/mL of pepsin for 2 h at 37°C and analyzed by 6% PAGE (A) A1. pH 7.2; A2: pH 2; A3: pH 2 + pepsin, and 12.5% SDS-PAGE (B) B1: Molecular weight markers; B2: pH 7.2; B3: pH 2; B4: pH 2 + pepsin.
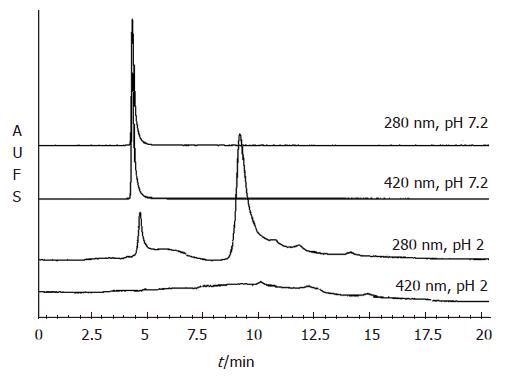
Figure 4 Pea ferritin dissociates under gastric conditions and releases bound iron.
The elution profile of purified pea ferritin (200 μg) either at pH 7.2 or pH 2 fractionated on TSK 2000 column. The protein was monitored at 280 nm and protein bound iron at 420 nm.
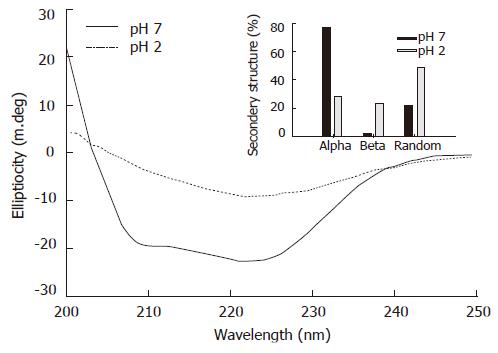
Figure 5 Pea ferritin looses its secondary structure at gastric pH.
Purified pea ferritin (1 mg/mL) was incubated either at pH 7.2 (10 mmol/L phosphate buffer saline) or at pH 2 (saline HCl) for 20 min and the far UV CD spectra recorded in the range of 200-250 nm. Inset, the percentage of secondary structure calculated from the CD data using K2D program.
- Citation: Bejjani S, Pullakhandam R, Punjal R, Nair KM. Gastric digestion of pea ferritin and modulation of its iron bioavailability by ascorbic and phytic acids in caco-2 cells. World J Gastroenterol 2007; 13(14): 2083-2088
- URL: https://www.wjgnet.com/1007-9327/full/v13/i14/2083.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i14.2083