Copyright
©2005 Baishideng Publishing Group Inc.
World J Gastroenterol. Dec 28, 2005; 11(48): 7569-7578
Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7569
Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7569
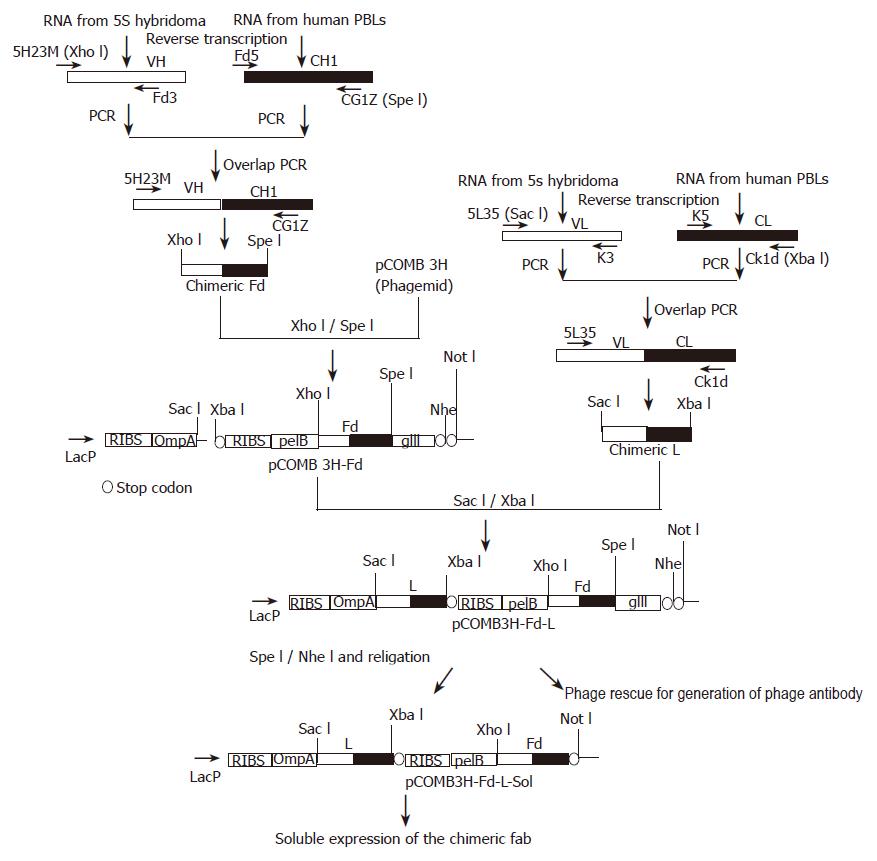
Figure 1 Schematic representation of the strategy for cloning and expression of the anti-HBs chimeric Fab.
The VH and VL fragments of 5S hybridoma were fused with human CH1 and CL by overlap PCR. The resulting chimeric fragments were cloned in the bi-cistronic phagemid vector pCOMB3H. Both the fragments are under the control of a single LacZ promoter. ompA and pelB are two leader sequences, provided for directing the light chain and Fd fragment to the bacterial periplasm. Two ribosome-binding sites are present in the vector for stable expression of both the fragments. The Fd fragment was cloned upstream to the phage gIII sequence, for surface display of the chimeric Fab. This gIII fragment was removed by digestion with Spe I/Nhe I and after self-ligation of the vector, the Fab fragment was expressed in soluble form.
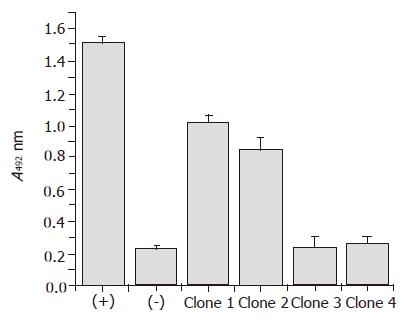
Figure 2 Phage ELISA to identify antigen binding clones.
After three rounds of biopanning, selected phage was rescued and antigen-binding clones were detected by phage ELISA. In this experiment 5S hybridoma supernatant and helper phage M13 KO7 were used as positive (+) and negative (–) control respectively. Clone 1 was used for soluble expression of the chimeric Fab.
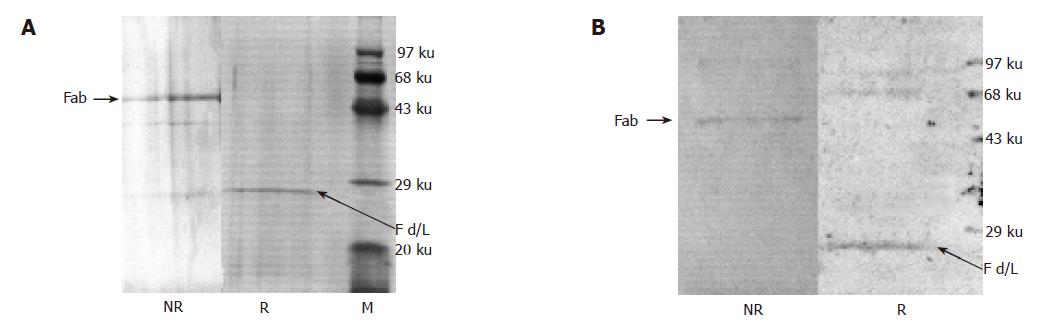
Figure 3 SDS-PAGE (A) and Western Blot (B) analysis of the chimeric Fab.
The purified chimeric Fab was resolved in 12% SDS-PAGE under reducing (R) and non-reducing conditions (NR). In non-reducing conditions the Fab is a heterodimer of molecular weight ~50 ku. In reducing conditions the heterodimer dissociates into chimeric light chain (~25 ku) and Fd (~25 ku). Both the gels were stained by silver staining. M is the lane for the protein marker. Similar observations were made in Western Blots (B) of the chimeric Fab in reducing (R) and non-reducing (NR) conditions. Electroblotted antibody fragments were detected by Rabbit anti-Human IgG-HRP conjugate (1:1 000 dilution; Dako).
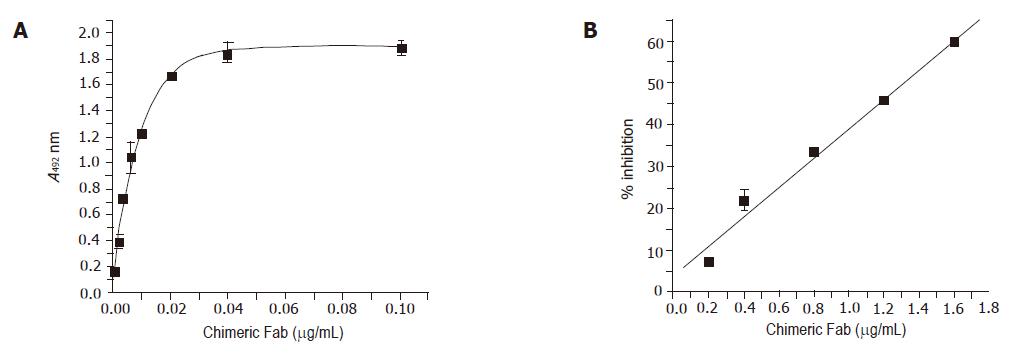
Figure 4 Binding properties of the anti-HBs chimeric Fab.
1 Solid phase ELISA was performed with different dilutions of the soluble chimeric Fab. The result of the competitive ELISA between the chimeric Fab and the mouse monoclonal 5S is shown in Figure (B). Different amounts of the chimeric Fab were allowed to compete with 1:80 dilution of the 5S-hybridoma culture supernatant for binding to HBsAg. Bound mouse monoclonal was detected with anti-mouse HRP and the % inhibition of binding was calculated.
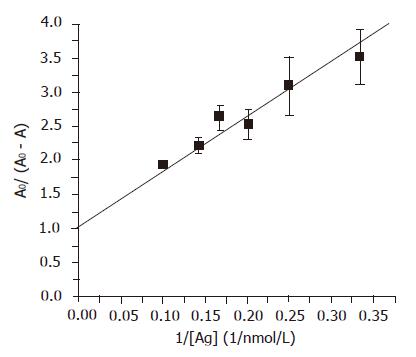
Figure 5 Dissociation constant (KD) of the chimeric Fab.
The KD was determined using an ELISA based method. Different amounts of HBsAg were incubated with concentrated periplasmic extract of the clone expressing the anti-HBs chimeric Fab for 16 h and unbound antibody was detected by ELISA using rabbit anti-human antibody-HRP (Dako). The data was fitted to the equation AO/(AO–A) = KD*1/[Ag]+1, where Ao = absorbance when the antibody was incubated without any antigen, A = absorbance corresponding to free antibody after incubation with the antigen. Dissociation constant of the chimeric Fab was determined from the slope of the straight line (KD = 8.166±0.14 nmol/L).
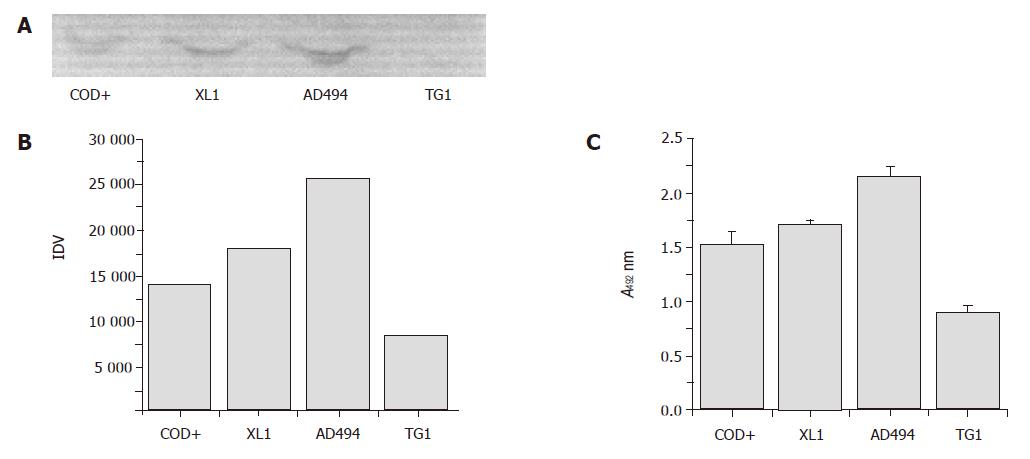
Figure 6 Expression profile of the chimeric Fab in different strains of E.
coli. The chimeric Fab was expressed in soluble form in different E. coli strains in identical conditions. Levels of expression of the chimeric Fab were checked by Western blot in non-reducing conditions (A). Intensities of bands were measured densitometrically (B). Levels of expression were also checked by ELISA (C). Equal amount of the periplasmic extracts were allowed to bind to HBsAg coated on ELISA plate and bound chimeric Fab was detected. (XL1 = XL1-Blue, AD494 = AD494, COD+ = BL21 Codon Plus, TG1 = TG1, IDV = integrated density value).
- Citation: Bose B, Khanna N, Acharya SK, Sinha S. High affinity mouse-human chimeric Fab against Hepatitis B surface antigen. World J Gastroenterol 2005; 11(48): 7569-7578
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7569