Copyright
©The Author(s) 2005.
World J Gastroenterol. Jul 14, 2005; 11(26): 4018-4023
Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4018
Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4018
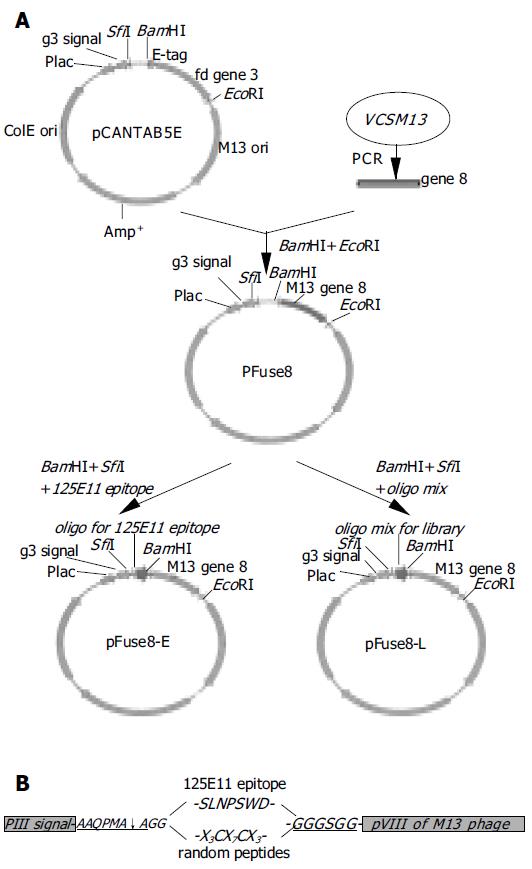
Figure 1 Diagram of library construction.
A: a schematic representation of the steps in the construction of the phagemid encoding the pVIII fusion protein. pCANTAB5E, a pIII displaying vector; pFuse8, the vector harboring gene 8 coding sequence in replace of gene 3; pFuse8-E, the coding sequence for a specific epitope recognized by the 125E11 mAb is inserted into pFuse8; pFuse8-L, the coding sequences for random peptides are inserted into pFuse8; B: Inserted sequences in pFuse8-E and pFuse8-L after translation. SLNPSWD is the specific epitope recognized by the 125E11 mAb. In pFuse8-L, the epitope is replaced with X3CX7CX3.
Figure 2 Surface display of the epitope recognized by 125E11.
The SLNPSWD epitope displayed on the surface of the phage particle was detected by phage ELISA. The circles indicate the phages that bind 125E11 coated wells. Triangles represent phages captured by an irrelevant mAb (anti-HBs). The synthesis of pVIII fusion proteins with or without IPTG induction is denoted as white or black, respectively.
Figure 3 Diversity of the constructed library.
In the region coding for the random peptides, four kinds of nucleotides are equally distributed at the first two loci of the triplet codons, while T or G varies at the last. Oligonucleotides for cysteine confined random peptides (5’-GCAGCAGGC-(NNS)3-TGT(NNS)7-TGC(NNS) 3-GGTGGTGGA-3’, N = A, T, C or G; S = A or C) are shown.
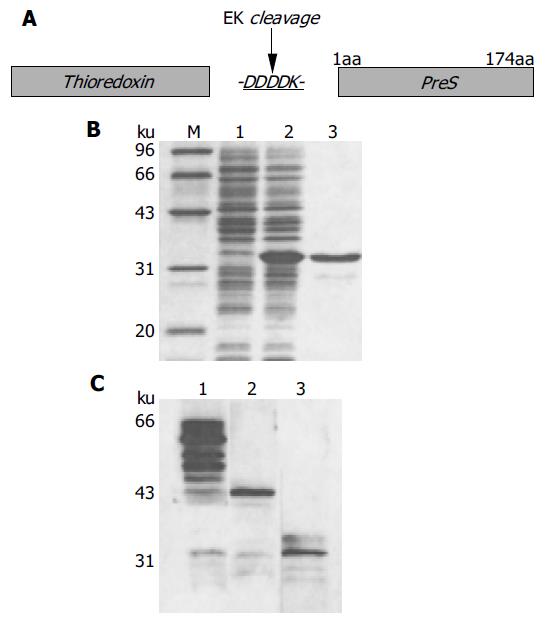
Figure 4 Synthesis of thio-PreS.
A: a schematic representation of the thio-PreS fusion protein, DDDDK is a cleavage site of rEK; B: SDS-PAGE analysis. Lane 1, the E.coli lysate without IPTG induction; lane 2, the soluble lysate after IPTG induction, thio-PreS of 33 ku is indicated with a triangle; lane 3, the purified thio-PreS; C: Western blot with the anti-thio mAb. Lane 1, thio-PreS coupled to the ThiobondTM beads; lane 2, thio-PreS coupled beads treated in the absence of rEK; lane 3, thio-PreS coupled beads treated in the presence of rEK.
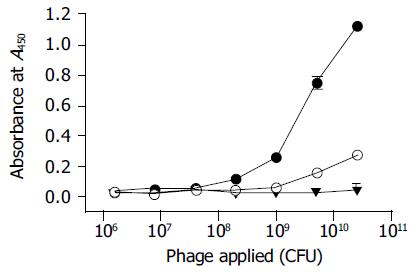
Figure 5 Phage ELISA of the enriched phages after the final round of screening.
Black dots, the enriched phages bind to thio-PreS; Empty dots, the enriched phages bind to thioredoxin as a control; Black triangles, phages in the original library do not bind to thio-PreS.
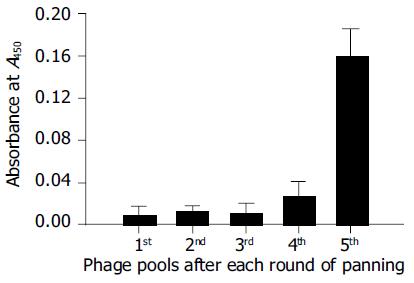
Figure 6 Virus capture assay.
The phage pool (1011 CFU) after each round of screening shows an increasing binding capacity to HBV virions in the culture medium of HepG2.2.15.
Figure 7 Amino acid sequences of PreS specific oligopeptides.
The amino acid sequences of the peptides encoded by the phagemids derived from five of the PreS-binding phages were deduced from DNA sequencing analysis. The C in bold indicates the invariable residues of cysteines.
- Citation: Deng Q, Zhuang M, Kong YY, Xie YH, Wang Y. Screening for PreS specific binding ligands with a phage displayed peptides library. World J Gastroenterol 2005; 11(26): 4018-4023
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4018