Published online Apr 30, 2019. doi: 10.13105/wjma.v7.i4.170
Peer-review started: March 4, 2019
First decision: April 11, 2019
Revised: April 20, 2019
Accepted: April 23, 2019
Article in press: April 23, 2019
Published online: April 30, 2019
Anthracyclines and taxanes are more active group of chemotherapy regimen. Randomized controlled trials (RCTs) reported variable evidences regarding efficacy of taxanes over anthracyclines for tumor response and survival outcomes. The present study compares the relative efficacy of taxanes over anthracyclines using pathological complete response (pCR), clinical responses, breast-conserving surgeries and survival outcomes in female breast cancer patients by systematic review and meta-analysis of available RCTs.
To assess the effectiveness of taxanes over anthracyclines in neoadjuvant setting in terms of tumor response and survival outcomes.
All RCTs assessing efficacy of taxanes over anthracyclines in neoadjuvant setting for management of breast cancer searched through PubMed and Cochrane register of controlled trials on 28 April 2017 and published in English language were considered. Following PRISMA guideline, retrieved records were screened and data were extracted by two independent reviewers. Meta-analysis was performed using fixed effect or random effect method depending on heterogeneity assessed using I2 statistic. Subgroup meta-analyses on the basis of taxane alone or taxane along with anthracycline in comparison to anthracycline alone were also performed for each considered outcomes.
A total of 16 RCTs involving 6752 breast cancer patients were found eligible. Taxanes based chemotherapy significantly improved pCR (n = 7, RR = 1.48, 95%CI: 1.04-2.12), disease free survival [n = 6, RR = 0.89 (0.80-0.99)] and loco-regional recurrence free survival [n = 4, RR = 0.74 (0.59-0.94)]. Interestingly in subgroup analysis, addition of taxane to anthracyclines showed better effectiveness regarding these survivals over anthracyclines than taxane alone over anthracycline.
Addition of taxanes to anthracyclines based chemotherapy significantly improves pCR, disease free survival and loco-regional recurrence free survival but with no significant impact on breast conservation rates.
Core tip: There is contradictory reporting through randomized controlled trials regarding relative efficacy of taxanes over anthracyclines which are used in neo-adjuvant setting for treatment of breast cancer patients. As a first systematic review and Meta analysis on the topic, present study is to assess the relative efficacy of taxanes (docetaxel and paclitaxel) alone or their addition to anthracyclines over anthracyclines alone in terms of pathological complete response, clinical response, breast conserving surgery, survival outcomes and toxicity.
- Citation: Pathak M, Dwivedi SN, Deo S, Thakur B, Sreenivas V, Rath GK. Effectiveness of taxanes over anthracyclines in neoadjuvant setting: A systematic-review and meta-analysis. World J Meta-Anal 2019; 7(4): 170-183
- URL: https://www.wjgnet.com/2308-3840/full/v7/i4/170.htm
- DOI: https://dx.doi.org/10.13105/wjma.v7.i4.170
Neoadjuvant chemotherapy, given prior to loco-regional treatment (surgery/ radiotherapy) is standard of care for locally advanced breast cancer but now became popular for early breast cancer as well[1]. The response to chemotherapy depends on the used regimen. Anthracyclines and taxanes are more active group of chemotherapy regimen used for breast cancer[2]. These regimens are usually administered with other chemotherapy drugs like cyclophosphamide, flurourocil. Anthracyclines based drugs include doxorubicin, epirubicin and mitoxantrone. On the other hand, widely used taxanes, originally identified from plant of genus Taxus, are docetaxel and paclitaxel[3]. Neoadjuvant chemotherapy increases the chance of breast conserving surgery (BCS) but there is no consensus regarding role of chemotherapy drugs in further increasing BCS rate[4,5]. Further, pathological complete response (pCR) to neoadjuvant chemotherapy predict long term survival outcomes as the breast cancer patients achieving pCR have better survival than the patients who do not[6]. The reported results from randomized controlled trials (RCTs) were contradictory as some favored taxanes based chemotherapy over anthracyclines based chemotherapy[7,8] regarding pCR, while some showed the other way[9,10]. The efficacy of taxanes over anthracyclines has been examined and found to be associated with increased overall survival in adjuvant setting[11]. Two reviews have discussed about the relative effectiveness of taxanes but could not synthesize the results for response because of very few RCTs at that point of time[12,13]. Further, these reviews could not comment on the effect of taxanes on long term outcomes. To the best of our knowledge, the relative efficacy of taxanes over anthracyclines has not been synthesized in neoadjuvant setting. Accordingly, present study aims to assess the effectiveness of taxanes based Neoadjuvant chemotherapy in comparison to anthracyclines based neoadjuvant chemotherapy on the basis of pCR, clinical responses, breast conserving surgeries and survival outcomes in female breast cancer patients by systematic review and meta-analysis of RCTs.
The present systematic review is designed as per the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)[14-16]. This study has been registered with International prospective register of systematic reviews and the registration Number is CRD42016027236.
All RCTs assessing efficacy of taxanes based Neoadjuvant Chemotherapy (NACT) in comparison to anthracyclines based NACT in the management of breast cancer, published in English language were considered. There was no restriction regarding the regimens used in the chemotherapy. The Population, Intervention, Comparator, Outcome and Time considered in the present systematic review is given below: (1) Population: Non-metastatic Female Breast Cancer Patients; (2) Intervention: Taxanes (Docetaxel or Paclitaxel); (3) Comparator: Anthracyclines; (4) Outcomes: PCR, overall response (OR) and BCS; (5) Design:RCTs; and (6) Time: Assessed on and up to 28 April 2017.
pCR: pCR was reported under three definitions as follows: (1) pCR1: pCR was defined as complete response of primary as well as axilla; (2) pCR2: pCR was defined as complete response of primary regardless of axilla; and (3) pCR3: pCR was defined as complete response of primary allowing for ductal carcinoma in situ (DCIS).
Considering variability in the definitions, results were synthesized separately under these three definitions.
OR: OR was defined as complete disappearance of clinically palpable tumor or more than 50% reduction in tumor volume.
BCS: BCS rate was defined as rate of breast conserving surgery, i.e., removal of lump only or removal of partial breast including tumor as well as some normal tissues.
Long term outcomes, i.e., overall survival, disease free survival, loco-regional recurrence free survival and metastasis free survival were also considered as secondary outcomes.
Details of search strategies development as well as electronic search strategies for PubMed and Cochrane register of controlled trials along with methodologies for study selection are available in the published protocol[17]. Data collection process, data extraction tool and method for risk of bias assessment are also available under published protocol. There was no deviation from the published protocol[17].
Effect sizes under consideration were “risk ratio” for all response outcomes and BCS. However, long term outcomes including, overall survival, disease free survival, loco-regional recurrence and distant metastasis, it was “hazards ratio”.
Statistical heterogeneity was examined by I2 statistics[18]. Publication bias assessment was performed using Eggers test and visualized using funnel plot[19]. In case of very low extent of heterogeneity (i.e., I2 = 0-25%), fixed effect method of synthesizing the effect size was used. However, for moderate to large extent of heterogeneity random effect method of meta-analysis was used. All analyses were performed using Stata 14 (StataCorp, Texas, United States) and RevMan 5.3.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
To derive additional inferences, Subgroup analyses were performed for the RCTs comparing taxanes versus anthracycline; and, addition of taxanes to anthracyclines versus anthracyclines under neoadjuvant setting.
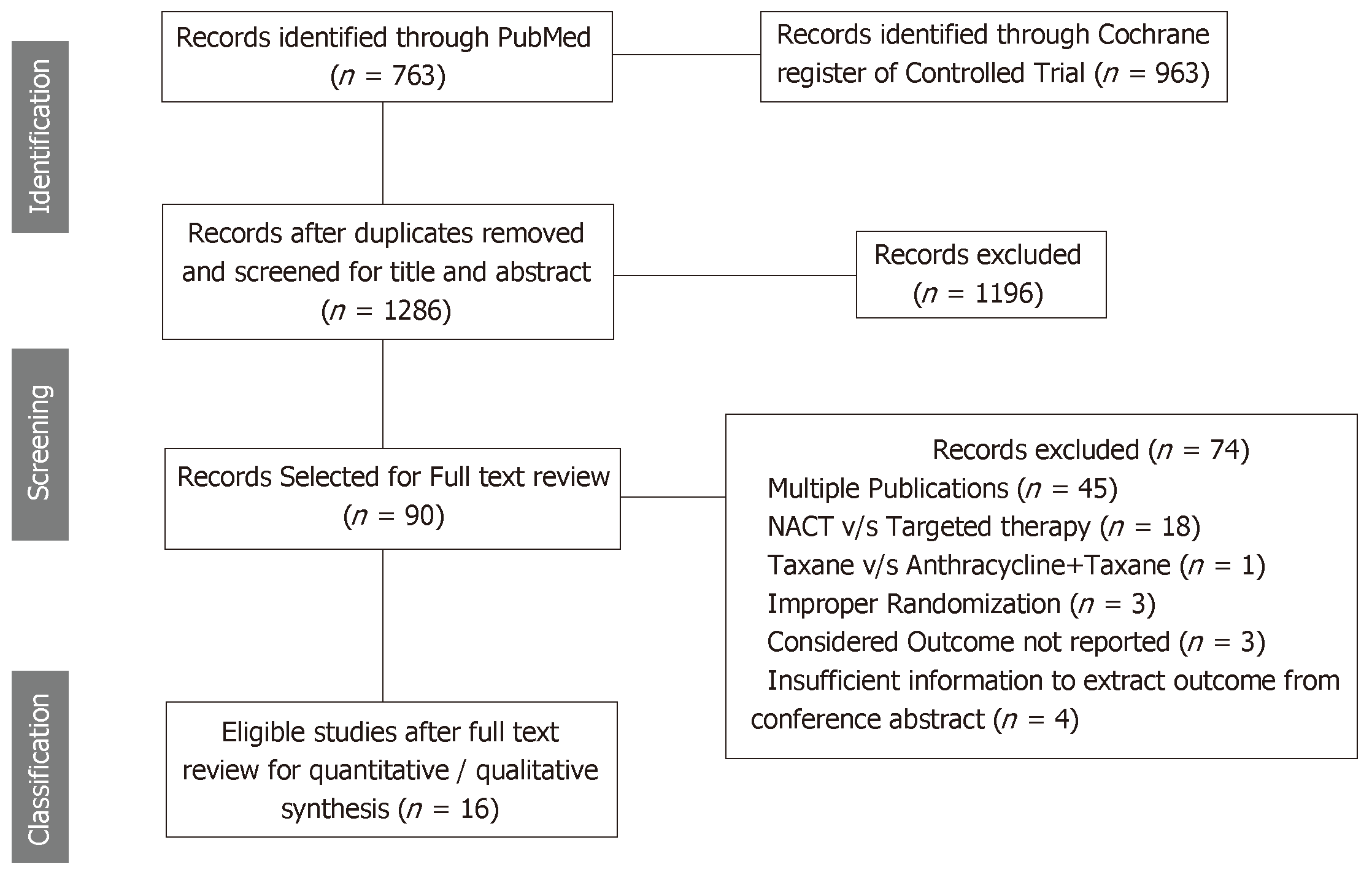
A total of 16 RCTs comparing effectiveness of taxanes versus anthracyclines involving 6752 breast cancer patients and measuring atleast one of the considered outcomes were found eligible out of 1286 searched records. These details are presented using PRISMA flow chart (Figure 1).
RCTs assessing the effectiveness of taxanes were sub-divided in two groups, i.e., RCTs comparing taxanes alone to anthracyclines alone[20-23] (n = 5); and RCTs comparing taxanes and anthracyclines together to anthracyclines alone[4-6,9,24-30] (n = 11). Out of these 16 RCTs, 10 RCTs assessed the effectiveness of docetaxel (3 RCT assessed the effectiveness of docetaxel vs doxorubicin along with other chemotherapy drugs however 7 RCTs assessed effectiveness of addition of docetaxel to anthracyclines based chemotherapy). However, six RCTs assessed effectiveness of paclitaxel, more precisely two RCT assessed paclitaxel and four RCT assessed addition of paclitaxel to anthracyclines based chemotherapy. The details of Population, intervention and outcome are presented in Table 1.
| Study | Accrual | Population | Regimen Comparison | Outcomes |
| ABDREEN[9], 2002 | 104 | Locally advanced breast cancer patients in which only responders of previous four cycles of CVAP | Anthracycline Arm: CVAP chemotherapy, comprised of cyclophosphamide (C) 1000 mg/m2, doxorubicin (A) 50 mg/m2, vincristine (V) 1.5 mg/m2 (I.V.), and prednisone 40 mg/d p.o. for 5 d; Taxane Arm: 4 cycles of Docetaxel (D) 100 mg/m2 was given as an I.V. infusion over 1 h and repeated at 21-d intervals. In addition, these patients received prednisone 100 mg for 5 d, beginning 24 h prior to docetaxel administration. | pCR1, pCR2, OR, cCR, OS |
| ACCOG[24], 2010 | 363 | Patients with primary tumour>3 cm, inflammatory or locally advanced non-metastatic breast cancer patients | Anthracycline Arm: Six cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) both administered every 3 wk (6xAC); Taxane Arm: Six cycles of doxorubicin (50 mg/m2) and docetaxel (75 mg/m2) administered as a 1-h I.V., with both drugs being given every 3 wk (6xAD). | pCR1, pCR2, pCR3, OR, cCR, BCS; Toxicity, OS, DFS, LRR, DM |
| Amsterdam trial[25], 2005 | 57 | Invasive breast cancer greater than 3 cm and/or at least one tumor-positive auxiliary lymph node | Anthracycline Arm: Six cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 administered every 3 ws (6xAC); Taxane Arm: Six cycles of doxorubicin 50 mg/m2 and docetaxel 75 mg/m2 (6xAD) 3 wk. | pCR2, |
| EORCT BIG-01[26], 2011 | 1856 | Invasive breast cancer <71 years with large operable/inflammatory breast cancer patients suitable for neoadjuvant chemotherapy | Anthracycline Arm: Six cycles of iv FEC (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) or tailored FEC (F600, E75, C900) starting on day 1 and then every 21 d with GCF (6xFEC); Taxane Arm: Three cycles of docetaxel 100 mg/m2 iv, followed by 3 cycles of epirubicin 90 mg/m2 and docetaxel 75 mg/m2 on day 1 every 21 d, without GCF. | pCR2, cCR, BCS; Toxicity |
| NSABP FB-9[8], 2015 | 50 | HER2 negative breast cancer patients with palpable mass of ≥ 2cm in breast or axilla or inflammatory breast cancer patients | Anthracycline Arm: 4 cycles of Eribuline 1.4 mg/m2 on days 1 and 8 of a 21-d cycle followed by A60 C600, every 21 d for 4 cycles; Taxane Arm: Weekly Paclitaxel 80 mg/m2 for 12 doses followed by standard A60C600 every 21 d for 4 cycles. | pCR1, OR, cCR, BCS, Toxicity |
| Madrid trial[20], 2011 | 211 | Female breast cancer patients aged 18-78 years of clinical stage IIB, IIIA or IIIB and with palpable breast cancer not amenable to BCS | Anthracycline Arm: Four cycles of doxorubicin (75 mg/m2 body surface area); Taxane Arm: Four cycles docetaxel 100 mg/m2 with G-CSF support every 3 wk. | pCR1 |
| Saura et al[4], 2013 | 295 | Breast cancer patients of stage T2-3N0-3M0 | Pretreatment: patients received four cycles of doxorubicin (60mg/m2 iv) and cyclophosphamide (600 mg/m2 iv) every 3 wk; Anthracycline Arm: Ixabepilone (40 mg/m2, 3-h infusion) every 3 wk for 4 cycles; Taxane Arm: paclitaxel (80 mg/m2, 1-h infusion) weekly for 12 wk. | pCR1, pCR3, OR, cCR, BCS, Toxicity |
| NCC Korea[21], 2008 | 209 | Previously untreated stage II/III breast cancer patients with auxiliary lymph node involvement of age ≥ 18 years, ECOG performance status ≤ 1 | Anthracycline Arm: doxorubicin 60 mg/m2 IV on day 1 plus cyclophosphamide 600 mg/m2 IV on day 1 every 3 wk for four cycles; Taxane Arm: docetaxel 75 mg/m2 1-h infusion on day 1 plus capecitabine 1000 mg/m2 orally twice daily on days 1-14 every 3 wk for four cycles. | pCR3, OR, cCR, Toxicity, OS, DFS |
| Norwagian trial[22], 2012 | 223 | Primary stage III breast cancer patients | Anthracycline Arm: 4x Epirubicin 90 mg/m2 administered at 3 wk interval; Taxane Arm: four cycles of paclitaxel 200 mg/m2 administered at 3 wk intervals. | OR, cCR, BCS, OS |
| Learn et al[27], 2005 | 144 | Invasive breast carcinoma with clinical staging T1c-T3, N0M0 or T1-3, N1M0 | Anthracycline Arm: 4 cycles of doxorubicin and cyclophosphamide (A60 C600) every 21 as well as tamoxifen 20 mg per day for 5 yr as NACT; Taxane Arm: 4 cycles of A60 C600 every 21 d further 4 cycles of docetaxel at 100 mg/m2 every 21 d as NACT; Arm 3 (Docetaxel as ACT): 4x AC as ACT (not part of the current study). | pCR1; OR |
| Diéras et al[5], 2004 | 240 | Breast cancer patients of stage T2-3N0-1M0, who were not assessable for breast conserving surgery | Anthracycline Arm: 4 cycles of A60 C600 i.v. every 21 d; Taxane Arm: doxorubicin 60 mg/m2 as (IV) bolus during 5 to 15 min immediately followed by paclitaxel 200 mg/m2 as a 3-h infusion every 21 d for 4 cycles. | pCR3, OR, cCR, cPR, BCS; Toxicity, OS, DFS, LRR, DM |
| Tabchy et al[28], 2010 | 273 | Breast cancer patients with clinical stage I to III | Anthracycline Arm: six courses of 5-fluorouracil (500 mg/m2), doxorubicin50/epirubicine100, and cyclophosphamide (500 mg/m2) all on day 1 repeated in 21-d cycles; Taxane Arm: 12 courses of weekly paclitaxel (80 mg/m2/wk) followed four cycles of anthracycline chemotherapy all on day 1 repeated in 21-d cycles. | pCR1; BCS |
| NSABP-27[6], 2006 | 2411 | Primary operable breast cancer patients with palpable tumor of stage T1c-3, N0-1 M0. | Arm1- 4 cycles of Doxorubicin 60 mg/m2 Cyclophosphamide 600 mg/m2 every 3 wk; Arm 2-Doxorubicin 60 mg/m2 Cyclophosphamide 600 mg/m2 every 3 wk × 4 followed by Docetaxel 100 mg/m2 every 3 wk × 4 followed by surgery; Arm3 (ACT arm)- Doxorubicin 60 mg/m2 Cyclophosphamide 600 mg/m2 every 3 wk × 4 followed by surgey--> Docetaxel 100 mg/m2 every 3 wk × 4 | pCR2, pCR3, OR, cCR, BCS; Toxicity, OPS, DFS, LRR, DM |
| Buzdar et al[23], 1999 | 174 | Invasive, but non-inflammatory, breast cancer with stage II to IIIA disease | Anthracycline Arm: 4 × FAC (fluorouracil 500, cyclophosphamide 500 mg/m2, doxorubicin 50 mg/m2) every 3 wk interval; Taxane Arm: Paclitaxel 250 mg/m2 as a 24-h continuous infusion at 3-wk intervals for four cycles. | pCR3, OR, cCR, BCS; Toxicity, DFS |
| Cortés-Flores et al[30], 2008 | 41 | Stage IIB and IIIA, locally advanced breast cancer patients | Anthracycline Arm: 5-fluorouracil epirubicine cyclophosphamide; Taxane Arm: docetaxel and epirubicine. | pCR2 |
| Sivasanker et al[29],2017 | 101 | Locally advanced breast cancer patients’ candidates for NACT | Anthracycline Arm: Cyclophosphamide 500 mg/m2, Doxorubicin 50 mg/m2 and 5-FU 500/m2 as IV infusion repeated every 21 d; Taxane Group: Paclitaxel 175 mg/m2 as a 3 h IV infusion, Doxorubicin 50 mg/m2 as IV infusion. | pCR1, pCR2, OR, cCR, BCS |
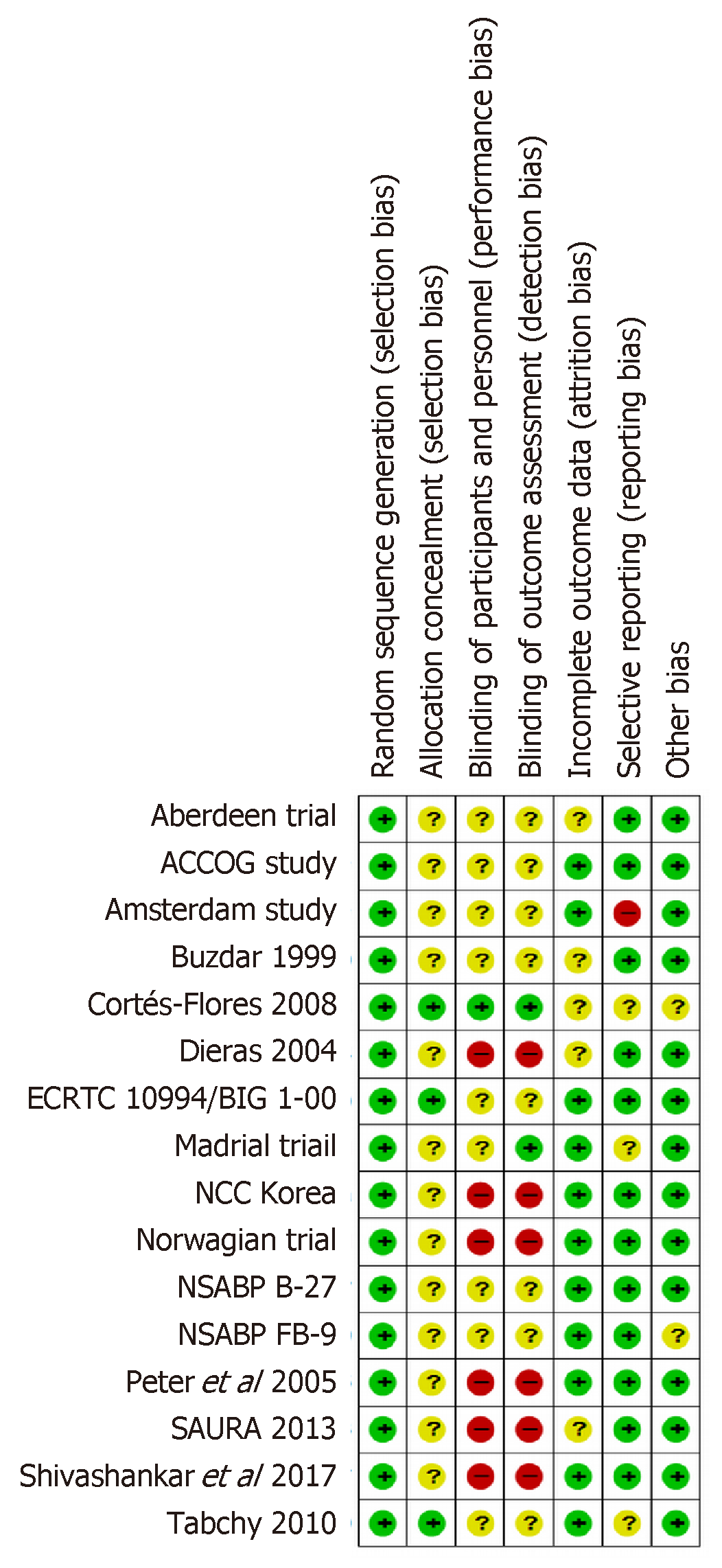
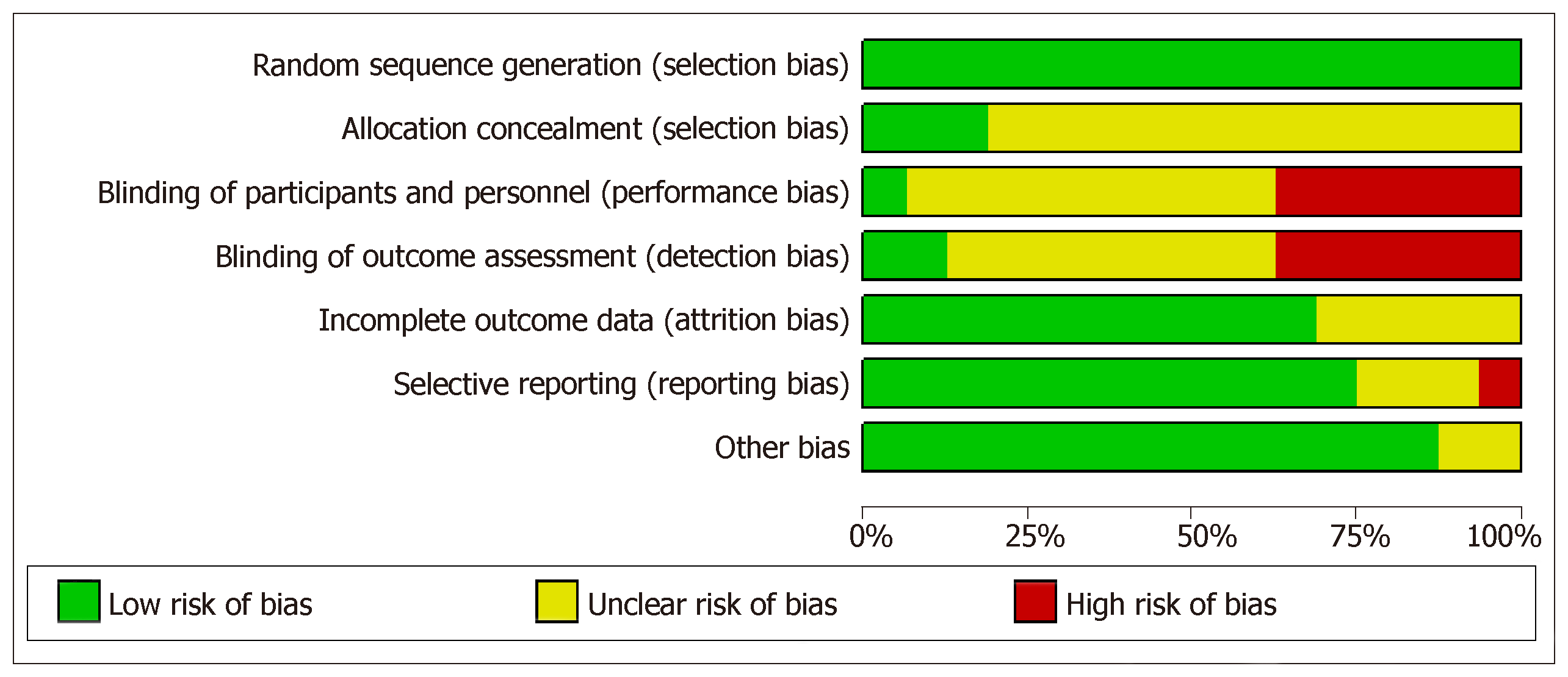
Risk of bias was assessed for each individual study using Cochrane bias assessment tool Figure 2. However, overall summary of risk of bias for all considered studies is presented in Figure 3. In summary, there were around 30% of the studies, which did not perform blinding of patients and/or outcome assessment. In addition, a large proportion of the trials did not report sufficient details to judge blinding. However, it is worthwhile to mention here that objective measurement of pCR and BCS was not affected by non-blinding of outcome assessment. But clinical responses might get affected by non-blinding of outcome assessment because of obvious subjectivity.
There was no publication bias for any of the outcomes except OR while assessing the effectiveness of taxane based chemotherapy tested using Egger’s test (Table 2) and visualized using Funnel Plots.
| Outcome | Sub-Group | Number of studies | Events taxane | Events anthracycline | Egger’s test P-value | I2 Statistic | Risk Ratio(95%CI) | Grade |
| pCR (BA) | Taxane v/s Anthracycline | 2 | 21/125 | 24/128 | - | 38.3 | 0.74 (0.23-2.39) | High |
| Taxane + Anthracyline v/s Anthracycline | 6 | 106/562 | 103/627 | 0.110 | 41.9 | 1.23 (0.86-1.76) | High1 | |
| Overall | 8 | 127/687 | 127/755 | 0.573 | 34.4 | 1.14 (0.84-1.55) | High1 | |
| pCR (B) | Taxane v/s Anthracycline | 1 | 16/47 | 8/50 | - | - | 2.13 (1.01-4.50) | Moderate2 |
| Taxane + Anthracyline v/s Anthracycline | 6 | 443/1951 | 329/1959 | 0.475 | 72.6 | 1.48 (1.04-2.12) | Moderate1, 3 | |
| Overall | 7 | 459/1998 | 337/2009 | 0.331 | 69.6 | 1.54 (1.11-2.15) | Moderate1, 3 | |
| pCR (DCIS) | Taxane v/s Anthracycline | 2 | 29/189 | 24/186 | - | 85.4 | 1.06 (0.25-4.47) | Moderate1, 3 |
| Taxane + Anthracyline v/s Anthracycline | 4 | 761/1679 | 183/683 | 0.339 | 71.4 | 1.23 (0.86-1.75) | Moderate1, 3 | |
| Overall | 6 | 790/1868 | 207/869 | 0.277 | 71.7 | 1.20 (0.84 -1.70) | Moderate1, 3 | |
| Overall response | Taxane v/s Anthracycline | 4 | 249/356 | 221/349 | 0.956 | 66.2 | 1.12 (0.94-1.33) | Low3, 4 |
| Taxane + Anthracyline v/s Anthracycline | 7 | 1098/1348 | 1024/1345 | 0.045 | 71.4 | 1.14 (1.02-1.27) | Low3, 4 | |
| Overall | 11 | 1347/1704 | 1245/1694 | 0.031 | 69.1 | 1.13 (1.04-1.24) | Low3, 4 | |
| Complete clinical response | Taxane v/s Anthracycline | 4 | 62/356 | 45/349 | 0.908 | 00.0 | 1.40 (1.01-1.93) | Moderate4 |
| Taxane + Anthracyline v/s Anthracycline | 7 | 548/2231 | 511/2176 | 0.273 | 60.1 | 1.13 (0.88-1.43) | Low3, 4 | |
| Overall | 11 | 610/2587 | 556/2525 | 0.106 | 49.5 | 1.18 (0.97-1.44) | Moderate4 | |
| Breast conserving surgery | Taxane v/s Anthracycline | 1 | 40/86 | 30/85 | - | - | 1.32 (0.91-1.90) | Moderate2 |
| Taxane + Anthracyline v/s Anthracycline | 8 | 1040/2206 | 1007/2199 | 0.633 | 00.0 | 1.03 (0.97-1.09) | High1 | |
| Overall | 9 | 1080/2292 | 1037/2284 | 0.406 | 01.1 | 1.04 (0.98-1.10) | High1 | |
| Overall survival | Taxane v/s Anthracycline | 3 | 18/255 | 31/251 | 0.002 | 74.9 | 0.41 (0.13-1.31) | Low1, 35 |
| Taxane + Anthracyline v/s Anthracycline | 4 | 424/2026 | 456/1960 | 0.899 | 0.0 | 0.91 (0.79-1.05) | High1 | |
| Overall | 7 | 442/2281 | 487/2211 | 0.059 | 37.4 | 0.86 (0.70-1.05) | High1 | |
| Disease free survival | Taxane v/s Anthracycline | 3 | 71/285 | 84/289 | 0.144 | 0.00 | 0.92 (0.63-1.36) | High1 |
| Taxane + Anthracyline v/s Anthracycline | 4 | 722/2026 | 772/1958 | 0.685 | 0.00 | 0.89 (0.80-0.99) | High1 | |
| Overall | 7 | 793/2311 | 856/2247 | 0.791 | 0.00 | 0.89 (0.80-0.99) | High1 | |
| Loco-regional recurrence | Overall | 4 | 120/2026 | 161/1960 | 0.808 | 0.00 | 0.74 (0.59-0.94) | High |
| Distant metastasis | Overall | 4 | 426/2026 | 441/1960 | 0.264 | 0.00 | 0.94 (0.82-1.07) | High |
Pathological complete response: As mentioned earlier, effect sizes were synthesized separately under three definitions of pCR. Considering pCR to breast as well axilla reported under eight RCTs randomizing 1442 patients, 127 (16.8%) in anthracycline arm and 127 (18.5%) in taxane arm achieved pCR. But this increase in pCR with taxane (especially with addition of taxane to anthracycline) was not statistically significant (n = 8, I2 = 34.4%, RR = 1.14, 95%CI: 0.84-1.55). Further, subgroup analyses also revealed the similar results for anthracycline versus taxane (n = 2, I2 = 38.3%, RR=0.74, 95%CI: 0.23-2.39) as well as anthracycline versus taxane along with anthracycline (n = 6, I2 = 41.9%, RR = 1.23, 95%CI: 0.86-1.76). Although some of the RCTs on which evidence is based were non-blinded but objective measurement of pCR would not change drawn evidences which were graded as high (Table 2).
PCR of breast regardless of axilla was reported by seven RCTs randomizing 4007 patients due to inclusion of two large RCTs[26,31] which contribute around 50% in the pooled effect estimates. Out of these seven RCTs, six RCTs assessed the effectiveness of addition of taxanes. pCR by this definition was observed to be 16.8% with anthracycline group and 23.0% in taxane group which was found to be statistically significant because of significant results under two big RCTs involving 390 patients (I2 = 72.6%, RR = 1.48, 95%CI: 1.04-2.12). Similarly, pCR of breast with DCIS was found to be significantly higher in taxanes group (I2 = 69.6%, RR = 1.54, 95%CI: 1.11-2.15). Apart from inclusion of some non-blinded trials for the objectively measured outcomes, evidences were downgraded one label to moderate due to high heterogeneity. The third definition of pCR, i.e., allowing for DCIS revealed beneficial effect of addition of taxanes to anthracycline based chemotherapy.
Clinical response: OR measured clinically was higher with taxane based che-motherapy (79.0%) in comparison to anthracycline based chemotherapy (73.5%) (I2 =69.1%, RR = 1.13, 95%CI: 1.04-1.24). Similarly, addition of taxane based chemotherapy also improved clinical complete response in comparison to anthracyclines alone (I2 = 49.5%, RR = 1.18, 95%CI: 0.97-1.44). Subjective measurement of these outcomes was based on few non-blinded trials which downgraded the evidence one level. Further, due to high heterogeneity and presence of publication bias, evidence for OR further downgraded to Low but that for complete clinical response remains at moderate level.
Breast conserving surgery: Taxane based chemotherapy could not further improve BCS, weather it was given with or without anthracycline in comparison to anthracyclines (I2 = 1%, RR = 1.04, 95%CI: 0.98-1.10) with high grade of evidence.
Survival and recurrences: Long term outcomes like overall survival outcomes, disease free survival and recurrences were reported by very few RCTs. A total of seven RCTs (Three compared taxane v/s anthracycline and four RCTs compared anthracycline + taxane v/s anthracycline) reported overall survival (Table 2). Overall survival was relatively better with taxane based NACT in comparison to anthracyclines but due to few RCTs, it could not reach at significance level [n = 7, RR = 0.86 (0.70-1.05)]. However, taxanes based NACT especially addition of taxanes to anthracycline based NACT significantly improved disease-free survival [n = 6, RR = 0.89 (0.80-0.99)]. Recurrences, i.e., loco-regional recurrence and distant metastasis were reported by only four RCTs comparing addition of taxanes to anthracycline based NACT in comparison to anthracycline alone. Taxanes played a significant role in combating loco-regional recurrence [RR=0.74 (0.59-0.94)] but not distant metastasis [RR=0.94 (0.82-1.07)].
Toxicities: Meta-analysis of toxicities involve both group of RCTs comparing taxane alone vs anthracycline alone as well as taxane and anthracycline combination vs anthracycline alone. In comparison to anthracycline based chemotherapy, taxane based chemotherapy was found to lower down the risk for nausea (n=7, RR = 0.33, 95%CI: 0.24-0.44) and vomiting (n = 4, RR = 0.23, 95%CI: 0.16-0.33) (Table 3). In contrary, it was found to raise the chance of febrile Neutropenia [n = 4, RR = 2.67 (2.33-3.07)] and infection [n = 4, RR = 2.53 (2.00-3.19)] significantly. However, due to lack of sufficient sample size/event rate, stable results could not be obtained for allergic reaction, hand foot syndrome, sensory neuropathy, gastrointestinal problems and dermatological problems as the confidence interval of the related risk ratio was observed to be very wide while comparing taxanes and anthracyclines.
| Toxicity | Number of studies | RR (95%CI) |
| Hematological toxicity | ||
| Neutropenia | 7 | 1.00 (0.78-1.29) |
| Febrile neutropenia | 4 | 2.67 (2.33-3.07) |
| Leucopenia | 4 | 0.72 (0.36-1.45) |
| Anemia | 3 | 0.75 (0.12-4.53) |
| Thrombocytopenia | 4 | 0.07 (0.03-0.19) |
| Thrombosis | 2 | 1.07 (0.59-1.96) |
| Cardiac and nervous system toxicity | ||
| Neuropathy | 2 | 1.01 (0.35-2.93) |
| Sensory neuropathy | 3 | 18.26 (5.87-56.80) |
| Cardiac left ventricular function | 1 | 0.33 (0.01-8.14) |
| Cardiovascular toxicity | 1 | 2.74 (0.88-8.57) |
| Dermatological toxicities | ||
| Hand foot syndrome | 2 | 27.43 (3.75-200.84) |
| Rash | 1 | 8.96 (0.48-166.20) |
| Dermatological toxicity | 2 | 3.71 (1.18-11.67) |
| Alopecia | 1 | 0.78 (0.73-0.83) |
| Diarrhea | 5 | 1.90 (0.97-3.73) |
| Gastro | 1 | 4.23 (1.43-12.53) |
| constipation | 1 | 3.49 (0.73-16.73) |
| Oral toxicities | ||
| Stomatitis | 6 | 1.89 (1.23-2.91) |
| Musculoskeletal pain | 1 | 1.01 (0.06-15.95) |
| General toxicity | ||
| Nausea | 7 | 0.33 (0.24-0.44) |
| Fatigue | 5 | 1.29 (0.96-1.73) |
| Infection | 4 | 2.53 (2.00-3.19) |
| Other | 6 | 1.12 (0.61-2.06) |
| Vomiting | 4 | 0.23 (0.16-0.33) |
| Allergic reaction | 3 | 21.25 (2.74-164.67) |
| Myalgia | 3 | 1.99 (0.36-11.01) |
| Serious adverse event | 1 | 0.65 (0.29-1.45) |
| edema | 1 | 6.97 (0.36-134.74) |
| Fever | 1 | 15.85 (0.96-260.89) |
| Hypotension | 1 | 6.97 (0.36-134.74) |
| Pulmonary | 1 | 3.54 (0.92-13.65) |
| Arthelgia | 3 | 0.02 (0.01-0.04) |
| Bone pain | 1 | 0.07 (0.00-1.17) |
A total of 16 RCTs assessing efficacy of taxanes based NACT (taxanes alone or addition of taxanes) in comparison to anthracyclines based NACT in the treatment of breast cancer, reporting at least one of the considered outcomes were included for this systematic review and meta analyses. Out of these trials, most of the big RCTs compared addition of taxanes to anthracyclines over anthracyclines (having 86% of the total randomized patients among all 16 RCTs). Meta-analysis sample size varied for outcome-wise synthesis. It was highest with 11 RCTs reporting clinical responses, i.e., OR and clinical complete response. Further, pCR was reported by six to eight RCTs with varying definitions and BCS rates were reported by 9 RCTs. Survival outcomes (overall survival and disease free survival) were reported by only seven RCTs and recurrences (loco-regional recurrences and distant metastasis) were reported by only four RCTs assessing the effectiveness of addition of taxanes over anthracyclines alone. Pattern of reported toxicities were also varied among RCTs. Most of the trials (n = 7) reported Neutropenia. All of the included RCTs used proper method for randomization but enough information to assess concealment was not reported by many RCTs. Further, six out of 16 RCTs were open label RCTs and may have an obvious impact on subjectively measured outcomes like OR and clinically complete response. Most of the RCTs have reported results based on intention to treat analysis. Overall, quality of included RCTs can be treated as adequate for objectively measured outcomes. Further, quantity of RCTs was sufficient to assess the relative effectiveness of addition of taxanes to anthracyclines over anthracyclines alone but not for subgroup assessing efficacy of taxanes alone versus anthracyclines alone.
The effectiveness of neoadjuvant chemotherapy depends on the used regimens. Response to neoadjuvant chemotherapy predict the prognosis and recurrence in breast cancer patients regardless of the type of surgery performed[32]. Patients having pCR have prolonged disease-free survival and overall survival. NSABP B-18 and B-27 trials revealed significantly better disease-free survival and overall survival among patients achieving pCR in comparison to the patients who could not[6]. It revealed that pCR is valid surrogate point for long term outcomes. This may be the reason; most of the trials comparing two neoadjuvant chemotherapy regimens reported tumor response instead of survival outcomes. Anthracyclines and taxanes are most active groups of chemotherapy regimens. Taxanes are standard of care for metastatic breast cancer patients because they are significantly more effective than anthracyclines based regimens[33]. Systematic review to assess the role of taxanes was performed a long back in 2004 and 2005 by Nowak et al[12] and Trudeau et al[13]. But most of the RCTs assessing effectiveness of taxanes over anthracyclines were reported after publication of these two reviews. Further, these two reviews included abstracts of ongoing trials which were not complete at that point of time. Since trials were not mature to report survival outcomes, these two studies could not comment on efficacy of taxanes for long term outcomes like survival and recurrences[13]. Further, Due to availability of very few trials, these two reviews could not perform meta-analysis for response outcomes and limit their finding with qualitative synthesis of these trials. As a matter of fact, present study could be able to quantitatively synthesize the results of tumor responses as well as for long term outcomes like overall survival, disease free survival, loco-regional recurrence and distant metastasis.
Addition of taxanes to anthracyclines based chemotherapy was found to be associated with higher pCR, better disease-free survival and decreased loco-regional recurrence. It was also found beneficial for clinical responses like OR and clinical complete response. But evidences on clinical responses have limited use due to downgrading of their quality because of involvement of some non-blinded trials. Also, clinical response to NACT also guides for further systemic therapy[34,35]. NACT increases the chance of BCS[36] but taxanes could not further improve the conservation surgery rate over anthracyclines alone. Evidences for the subgroup assessing effectiveness of addition of taxanes to anthracyclines over anthracyclines were rated as of high grade and stable due to availability of large RCTs with adequate number. On the other hand, synthesized results for the subgroup comparing taxanes alone to anthracyclines alone involved few RCTs that too of small sample size. Further, the conclusive results for toxicities cannot be summarized because these were reported by very few RCTs and reporting of toxicities also involved variation in their definitions. In summary, addition of taxanes improves pCR, disease free survival and loco-regional recurrence free survival but has no major impact on BCT rates.
There are mainly two active group of chemotherapy regimen namely Anthracyclines and taxanes. Randomized controlled trials (RCTs) have reported variable evidences regarding efficacy of taxanes over anthracyclines especially for tumor response and survival outcomes. Hence, as required, the present study synthesizes the relative efficacy of taxanes over anthracyclines using pathological complete response, clinical responses, breast-conserving surgeries and survival outcomes in female breast cancer patients by systematic review and meta-analysis of available RCTs.
There is contradictory reporting regarding relative efficacy of taxanes over anthracycline. To resolve this, for the first time, present meta-analysis is to assess the relative efficacy of taxanes (Docetaxel and Paclitaxel) alone or their addition to anthracyclines over anthracyclines alone in terms of pCR, clinical response, breast conserving surgery, survival outcomes and toxicity in neo-adjuvant setting. As and when there is further addition in regimes, such appraisals from time to time are unavoidable.
Keeping in view of contradictory reporting, this study aimed to assess the relative effectiveness of taxanes over anthracyclines in neo-adjuvant setting in terms of tumor response and survival outcomes through systematic review and Meta analysis. This was expected to provide important clues regarding appropriate clinical practice.
The RCTs have reported contradictory findings on relative effectiveness of taxanes over anthracyclines in neo-adjuvant setting regarding treatment of breast cancer patients. In spite of this, no earlier attempt is made to synthesize relative effectiveness of taxanes over anthracyclines. For the first time, using a focused systematic review and Meta analysis of existing RCTs, this study attempted to synthesize relative effectiveness of taxanes over anthracyclines in terms of pCR, clinical response, BCS, survival outcomes and toxicity in neo-adjuvant setting. For this, all related RCTs were searched through PubMed and Cochrane register of controlled trials on 28 April 2017 and published in English language. Using PRISMA guidelines, retrieved records were screened and data were extracted by two independent reviewers. Depending on heterogeneity assessed through I2 statistic, Meta-analysis was performed using either fixed effect or random effect method. Subgroup meta-analyses were also performed for each considered outcomes on the basis of taxanes alone or taxanes along with anthracyclines in comparison to anthracyclines alone.
Through a search through PubMed and Cochrane register of controlled trials on 28 April 2017, for this study, a total of 16 RCTs were found eligible in view of reporting at least one of the considered outcomes. The analytical results revealed that taxanes based chemotherapy significantly improved pCR, disease free survival and loco-regional recurrence free survival. Further, subgroup analysis showed that addition of taxanes to anthracyclines has better effectiveness regarding these survivals over anthracyclines alone than taxanes alone over anthracyclines alone.
This study hypothesized that effectiveness of neo-adjuvant chemotherapy may rely on used regimens. Keeping in view of varying reporting under related RCTs, as an appraisal, to assess relative effectiveness of taxanes in comparison to anthracyclines was planned. For this, it was carried out as a first systematic review and Meta analysis of the related RCTS. As obvious, as a first-time observation, the synthesized results suggest that taxanes based chemotherapy may significantly improve pCR, disease free survival and loco-regional recurrence free survival. Further, as additional clues, subgroup analysis showed that addition of Taxanes to anthracyclines emerged to be more effective regarding these survivals over anthracyclines alone than taxanes alone over anthracyclines alone.
In presence of contradictory findings under RCTs, a systematic review and Meta analysis of available RCTs may provide important clues towards clinical practice. Completeness of data is crucial for such studies. To achieve this, as true in case of other study designs, an appropriate protocol needs to be written for carrying out such studies. Although such studies are being carried out on other study designs as well, to ensure high level of evidence, such studies on RCTs need to be preferred.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng THS-Editor:Dou Y L-Editor:A E-Editor: Wu YXJ
| 1. | Costa R, Hansen NM, Gradishar WJ. Locally Advanced Breast Cancer, In: The Breast: Comprehensive Management of Benign and Malignant Diseases. Elsevier Inc. 2017;e6:819-830. [DOI] [Cited in This Article: ] |
| 2. | Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med. 2015;13:195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Estévez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res. 2004;10:3249-3261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Saura C, Tseng LM, Chan S, Chacko RT, Campone M, Manikhas A, Nag SM, Leichman CG, Dasappa L, Fasching PA, Hurtado de Mendoza F, Symmans WF, Liu D, Mukhopadhyay P, Horak C, Xing G, Pusztai L. Neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early stage breast cancer and evaluation of βIII-tubulin expression as a predictive marker. Oncologist. 2013;18:787-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Diéras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, Guastalla JP, Pujade-Lauraine E, Kerbrat P, Maillart P, Pénault-Llorca F, Buyse M, Pouillart P. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004;22:4958-4965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1211] [Cited by in F6Publishing: 1241] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 7. | Romero A, García-Sáenz JA, Fuentes-Ferrer M, López Garcia-Asenjo JA, Furió V, Román JM, Moreno A, de la Hoya M, Díaz-Rubio E, Martín M, Caldés T. Correlation between response to neoadjuvant chemotherapy and survival in locally advanced breast cancer patients. Ann Oncol. 2013;24:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Abraham J, Robidoux A, Tan AR, Limentani S, Sturtz K, Shalaby I, Alcorn H, Buyse ME, Wolmark N, Jacobs SA. Phase II randomized clinical trial evaluating neoadjuvant chemotherapy regimens with weekly paclitaxel or eribulin followed by doxorubicin and cyclophosphamide in women with locally advanced HER2-negative breast cancer: NSABP Foundation Study FB-9. Breast Cancer Res Treat. 2015;152:399-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, Smith I, Walker LG, Eremin O; Aberdeen Breast Group. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer. 2002;3 Suppl 2:S69-S74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Mansi JL. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an anglo-celtic cooperative oncology group study. J Clin Oncol. 2005;23:2988-2995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH, Valero V, Shen Y, Ueno NT. Effectiveness of an Adjuvant Chemotherapy Regimen for Early-Stage Breast Cancer: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2015;1:1311-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Nowak AK, Wilcken NR, Stockler MR, Hamilton A, Ghersi D. Systematic review of taxane-containing versus non-taxane-containing regimens for adjuvant and neoadjuvant treatment of early breast cancer. Lancet Oncol. 2004;5:372-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Trudeau M, Sinclair SE, Clemons M; Breast Cancer Disease Site Group. Neoadjuvant taxanes in the treatment of non-metastatic breast cancer: a systematic review. Cancer Treat Rev. 2005;31:283-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18463] [Cited by in F6Publishing: 16685] [Article Influence: 1112.3] [Reference Citation Analysis (0)] |
| 15. | Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, Gøtzsche PC, Lasserson T, Tovey D; PRISMA for Abstracts Group. PRISMA for Abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:e1001419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 445] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 16. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9970] [Cited by in F6Publishing: 10289] [Article Influence: 685.9] [Reference Citation Analysis (0)] |
| 17. | Pathak M, Dwivedi SN, Deo SVS, Thakur B, Sreenivas V, Rath GK. Neoadjuvant chemotherapy regimens in treatment of breast cancer: a systematic review and network meta-analysis protocol. Syst Rev. 2018;7:89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Pathak M, Dwivedi SN, Deo S, Vishnubhatla S, Thakur B. Which is the Preferred Measure of Heterogeneity in Meta-Analysis and Why? A Revisit. Biostat Biom Open Acc J. 2017;1:55555. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34245] [Cited by in F6Publishing: 36234] [Article Influence: 1342.0] [Reference Citation Analysis (1)] |
| 20. | Martin M, Romero A, Cheang MC, López García-Asenjo JA, García-Saenz JA, Oliva B, Román JM, He X, Casado A, de la Torre J, Furio V, Puente J, Caldés T, Vidart JA, Lopez-Tarruella S, Diaz-Rubio E, Perou CM. Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat. 2011;128:127-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, Chung KW, Kang HS, Kim EA, Kim SW, Shin KH, Kim SK. A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat. 2008;109:481-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Cao MD, Giskeødegård GF, Bathen TF, Sitter B, Bofin A, Lønning PE, Lundgren S, Gribbestad IS. Prognostic value of metabolic response in breast cancer patients receiving neoadjuvant chemotherapy. BMC Cancer. 2012;12:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Buzdar AU, Singletary SE, Theriault RL, Booser DJ, Valero V, Ibrahim N, Smith TL, Asmar L, Frye D, Manuel N, Kau SW, McNeese M, Strom E, Hunt K, Ames F, Hortobagyi GN. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J Clin Oncol. 1999;17:3412-3417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Mansi JL, Yellowlees A, Lipscombe J, Earl HM, Cameron DA, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Evans TR. Five-year outcome for women randomised in a phase III trial comparing doxorubicin and cyclophosphamide with doxorubicin and docetaxel as primary medical therapy in early breast cancer: an Anglo-Celtic Cooperative Oncology Group study. Breast Cancer Res Treat. 2010;122:787-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Hannemann J, Oosterkamp HM, Bosch CA, Velds A, Wessels LF, Loo C, Rutgers EJ, Rodenhuis S, van de Vijver MJ. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2005;23:3331-3342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Bonnefoi H, Piccart M, Bogaerts J, Mauriac L, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J, Blot E, Zaman K, Cufer T, Lortholary A, Lidbrink E, André S, Litière S, Lago LD, Becette V, Cameron DA, Bergh J, Iggo R; EORTC 10994/BIG 1-00 Study Investigators. TP53 status for prediction of sensitivity to taxane versus non-taxane neoadjuvant chemotherapy in breast cancer (EORTC 10994/BIG 1-00): a randomised phase 3 trial. Lancet Oncol. 2011;12:527-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 27. | Learn PA, Yeh IT, McNutt M, Chisholm GB, Pollock BH, Rousseau DL, Sharkey FE, Cruz AB, Kahlenberg MS. HER-2/neu expression as a predictor of response to neoadjuvant docetaxel in patients with operable breast carcinoma. Cancer. 2005;103:2252-2260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Tabchy A, Valero V, Vidaurre T, Lluch A, Gomez H, Martin M, Qi Y, Barajas-Figueroa LJ, Souchon E, Coutant C, Doimi FD, Ibrahim NK, Gong Y, Hortobagyi GN, Hess KR, Symmans WF, Pusztai L. Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy response predictor in a multicenter randomized trial in breast cancer. Clin Cancer Res. 2010;16:5351-5361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Sivasanker M, Sistla SC, Manwar SA, Vivekanandam S. Clinical and pathologic response following taxane based neoadjuvant chemotherapy in locally advanced breast cancer patients in a tertiary care centre in India. Indian J Cancer. 2016;53:220-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Cortés-Flores AO, Morgan-Villela G, Castro-Cervantes JM, Vázquez-Camacho G, Fuentes-Orozco C, González-Ojeda A. [Neoadjuvant treatment for locally advanced breast cancer. Comparison of two schemes based on docetaxel-epirubicin vs. 5-fluorouracil-epirubicin-cyclophosphamide]. Cir Cir. 2008;76:23-28. [PubMed] [Cited in This Article: ] |
| 31. | Bear HD, Anderson S, Smith RE, Geyer CE, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, Paik S, Soran A, Wickerham DL, Wolmark N. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019-2027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 703] [Cited by in F6Publishing: 681] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 32. | Hanrahan EO, Hennessy BT, Valero V. Neoadjuvant systemic therapy for breast cancer: an overview and review of recent clinical trials. Expert Opin Pharmacother. 2005;6:1477-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Ghersi D, Wilcken N, Simes J, Donoghue E. Taxane containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2005;CD003366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:1747-1749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Kümmel S, Paepke S, Schneeweiss A, Untch M, Zahm DM, Mehta K, Loibl S. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623-3630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 250] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Pathak M, Deo SVS, Dwivedi SN, Vishnubhatla S, Thakur B, Julka PK, Rath GK. Role of Neoadjuvant Chemotherapy in breast cancer patients: Systematic Review and Meta-analysis. Indian J Med Pediatr Oncol. 2019;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |