Published online Dec 26, 2016. doi: 10.13105/wjma.v4.i6.118
Peer-review started: July 18, 2016
First decision: September 7, 2016
Revised: September 13, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 26, 2016
To evaluate the incidence of lymph node metastasis (LNM) and its risk factors in patients with Siewert type I and type II pT1 adenocarcinomas.
We enrolled 85 patients [69 men, 16 women; median age (range), 67 (38-84) years] who had undergone esophagectomy or proximal gastrectomy for Siewert type I and type II pT1 adenocarcinomas. Predictive risk factors of LNM included age, sex, location of the tumor center, confirmed Barrett’s esophageal adenocarcinoma, tumor size, macroscopic tumor type, pathology, invasion depth, presence of ulceration, and lymphovascular invasion. Multivariate logistic regression analysis was used to identify factors predicting LNM. We also evaluated the frequencies of LNM for Siewert type I and type II pT1 adenocarcinomas in meta-data analysis.
LNMs were found in 11 out of 85 patients (12.9%, 95%CI: 5.8-20.0). Only 1 of the 15 patients (6.6%, 95%CI: 0.0-19.2) who had a final diagnosis of pT1a adenocarcinoma had a positive LNM, whereas 10 of the 70 patients (14.2%, 95%CI: 6.0-22.4) with a final diagnosis of pT1b adenocarcinoma had positive LNM. Furthermore, only one of the 30 patients (3.3%, 95%CI: 0.0-9.7) with a tumor invasion depth within 500 μm from muscularis mucosae had positive LNM. Poor differentiation and lymphovascular invasion were independently associated with a risk of LNM. In meta-data analysis, 12 of the 355 patients (3.3%, 95%CI: 1.5-5.2) who had a final diagnosis of pT1a adenocarcinoma had a positive LNM, whereas 91 of the 438 patients (20.7%, 95%CI: 16.9-24.5) with a final diagnosis of pT1b adenocarcinoma had positive LNM.
We consider endoscopic submucosal dissection (ESD) is suitable for patients with Siewert type I and type II T1a adenocarcinomas. For patients with T1b adenocarcinoma, especially invasion depth is within 500 μm from muscularis mucosae with no other risk factor for LNM, diagnostic ESD could be a treatment option according to the overall status of patients and the presence of comorbidities.
Core tip: We evaluated meta-analysis of the incidence of lymph node metastasis (LNM) in patients with Siewert type I and type II pT1 adenocarcinomas. Of previous 5 reports and our study, 12 of the 355 patients (3.38%, 95%CI: 1.5-5.2) in pT1a adenocarcinoma had LNM, whereas 91 of the 438 patients (20.7%, 95%CI: 16.9-24.5) in pT1b adenocarcinoma had LNM. We consider endoscopic submucosal dissection (ESD) to be a reasonable for patients that have well differentiated, limited to the mucosa, and within 30 mm in diameter with no lymphovascular invasion. For patients with T1b adenocarcinoma, especially invasion depth within 500 μm from muscularis mucosae with no other risk factor for LNM, diagnostic ESD could be a treatment option.
- Citation: Osumi H, Fujisaki J, Omae M, Shimizu T, Yoshio T, Ishiyama A, Hirasawa T, Tsuchida T, Yamamoto Y, Kawachi H, Yamamoto N, Igarashi M. Meta-analysis of lymph node metastasis in Siewert type I and II T1 adenocarcinomas. World J Meta-Anal 2016; 4(6): 118-123
- URL: https://www.wjgnet.com/2308-3840/full/v4/i6/118.htm
- DOI: https://dx.doi.org/10.13105/wjma.v4.i6.118
Barrett’s esophagus is most often diagnosed in people who have long term gastroesophageal reflux disease (GERD), which is a chronic regurgitation of acid from the stomach into the lower esophagus. It is associated with an increased risk of developing esophageal adenocarcinoma. The frequency of Barrett’s esophageal adenocarcinoma (BEA) from Barrett’s esophagus is about 0.5% per year[1]. However, the frequency of BEA is thought to be increasing because of the Westernization of dietary habits, obesity, and increased frequency of GERD associated with a decreasing frequency of Helicobacter pylori (H. pylori) infection in Japan.
Endoscopic submucosal dissection (ESD) for esophageal and gastric cancer is limited by the possible incidence of regional lymph node metastasis (LNM). There is robust data about the frequencies of LNM of squamous cell carcinoma or esophageal adenocarcinoma over the full length of esophagus. In contrast, there is a few data about the frequency of LNM for Siewert type I and type II pathological T1 (pT1) adenocarcinomas. Especially, there is only one report about the frequency of LNM for Siewert type II pT1 adenocarcinomas from 2005 to 2015 in the PubMed database[2]. Siewert type I was defined as adenocarcinoma of the distal esophagus, which usually arises from an area with Barrett’s esophagus and may infiltrate the esophagogastric junction (EGJ) from above[3]. On the other hand, Siewert type II was defined true carcinoma of the cardia arising immediately at the EGJ3. In this range, there are two types of adenocarcinomas: BEA from short or long segment Barret’s esophagus develops from inflammation caused by exposure of the esophagus to gastric acid and bile; and gastric adenocarcinoma develops from mucosal atrophy and intestinal metaplasia, mainly caused by H. pylori infection[4].
If the frequency of LNM and the risk factors driving this process in this range can be determined, then patient treatment can be stratified: ESD can be offered to patients with tumors that have a low frequency of LNM; and surgical resection can be offered to patients with tumors that have a high frequency of LNM. The aim of this study was to evaluate the frequency of LNM for Siewert type I and II pT1 adenocarcinomas and its risk factors of LNM.
There were 85 patients who received esophagectomy or proximal gastrectomy or additional surgery after ESD in Siewert type I and type II pT1 adenocarcinomas between January 2006 and December 2014 in our hospital. Our selection criteria were: (1) the center of the tumor was within 2 cm of the EGJ at the gastric side or within 5 cm of the EGJ at the oral side; (2) invasion depth was intramucosal or submucosal and was not reached the muscularis propria; and (3) patients had received primary surgery or additional surgery after ESD. Pathological evaluation was performed by two experienced pathologists (Kawachi H and Yamamoto N).
Differentiated pathology included papillary adenocarcinoma and tubular adenocarcinoma. Undifferentiated pathology included poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma. For the condition to be considered Barrett’s esophagus, one of the following criteria must have been met: We could identify these pathologic findings in anal side of the tumor; esophageal glands, squamous island, and double layer of muscularis mucosae. Or we could find palisade vessels around the tumor endoscopically. Invasion depth was divided into T1a (Tumor confined to the mucosa) and T1b (Tumor confined to the submucosa) groups. T1b lesions were subclassified as: SM1 (tumor invasion is within 500 μm of the muscularis mucosae) or SM2 (tumor invasion is 500 μm or more deep into the muscularis mucosae). Assessment of the depth of tumor infiltration into the SM layer was based on the Japanese Classification of Gastric Carcinoma[5].
Meta-data analysis of the frequencies of LNM for Siewert type I and II pT1 adenocarcinomas
We searched for articles which were mentioned about the frequency of LNM for Siewert type I and II pT1 adenocarcinomas in the PubMed database from 2005 to 2015 using following terms: “T1,”“esophagogastric junction adenocarcinoma”, “esophageal adenocarcinoma”, “lymph node metastasis”, “early”, “superficial”. Terms were combined with “and/or” and asterisks. The main reasons of initial exclusion were as follows; squamous cell carcinoma was also included, esophageal adenocarcinoma of over the full length of esophagus, non-English literature, case reports, reviews and double publications.
This study was performed in accordance with the Declaration of Helsinki and approved by our Institutional Review Board (Registry number: 2015-1143).
Predictive risk factors included age, sex, location of tumor center (Siewert type I or II), presence of confirmed BEA (yes or no), tumor size (< 30 mm or ≥ 30 mm), macroscopic tumor type (elevated or depressed), pathology (undifferentiated or differentiated), depth of invasion (mucosal or SM, ≥ 500 μm or < 500 μm), presence of ulceration (yes or no), and presence of lymphovascular invasion (yes or no). All P values were the result of two-sided tests, and a P value of < 0.05 was considered statistically significant. Prognostic factors with a P value of < 0.2 in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), a graphical user interface for R (The R Foundation for Statistical Computing).
Patient characteristics are shown in Table 1. This cohort included 85 patients (81.1% men and 18.9% women). The median age of patients at the time of surgery was 67 years (38-84). In total, 22 patients had pT1a tumors (25.9%) and 63 patients had pT1b tumors (74.1%). Median tumor size was 26 mm (± 14.6 mm). 72 patients (84.7%) had differentiated type tumor pathology and 13 patients (15.3%) had undifferentiated type tumor pathology. A total of 50 patients (58.8%) had lymphovascular invasion and 43 patients (50.5%) had underlying Barrett’s esophagus.
| Characteristic | Data |
| n | 85 |
| Median age (range), yr | 67 (38-84) |
| Male sex, n (%) | 69 (81.1) |
| Depth, n (%) | |
| T1a | 22 (25.9) |
| T1b | 63 (74.1) |
| Differentiation, n (%) | |
| Differentiated | 72 (84.7) |
| Undifferentiated | 13 (15.3) |
| Median size (SD), mm | 26 (± 14.6) |
| Lymphovascular invasion, n (%) | 50 (58.8) |
| Underlying Barrett's esophagus, n (%) | 43 (50.5) |
| Lymph node metastasis, n (%) | 11 (12.9) |
Overall, 11 out of 85 patients (12.9%, 95%CI: 5.8-20) had LNM. Table 2 shows the rate of LNM for each depth of invasion. There was a higher incidence of LNM in patients with pT1b compared with pT1a disease; however, this was not significant [14.2% (10/70) vs 6.6% (1/15), OR = 2.3, 95%CI: 0.28-108.3, P = 0.67]. Furthermore, for the actual depth of invasion, the frequencies of LNM were: < 500 μm, 3.3% (1/30, 95%CI: 0-9.7); < 1000 μm, 4.3% (2/46 95%CI: 0-10.2) (Table 3).
| Ref. | n | Siewert classification | TNM classification | SM subdivision | |||
| T1a, n (%) | T1b, n (%) | SM1, n (%) | SM2, n (%) | SM3, n (%) | |||
| Westerterp et al[6] | 120 | I, II | 1/54 (1.8) | 18/66 (27.2) | 0/25 (0) | 6/23 (20) | 12/18 (56) |
| Barbour et al[7] | 85 | I, II | 0/35 (0) | 9/50 (18) | - | - | - |
| Lees et al[8] | 126 | I, II | 1/75 (1.3) | 11/51 (21.6) | 4/19 (21) | 1/9 (11.1) | 6/23 (26.1) |
| Griffin et al[9] | 119 | I, II | 0/54 (0) | 8/65 (12.3) | - | - | - |
| Lee et al[10] | 258 | I, II | 9/122 (7.3) | 35/136 (25.7) | - | - | - |
| Present study | 85 | I, II | 1/15 (6.6) | 10/70 (14.2) | 0/7 (0) | 4/43 (9.3) | 6/20 (30) |
| Total | 793 | I, II | 12/355 | 91/438 | |||
| (3.4%, 95%CI: 1.5-5.2) | (20.7%, 95%CI: 16.9-24.5) | ||||||
| Invasion depth (μm) | Lymphatic invasion frequency | Venous invasion frequency | Frequency of lymph node metastasis |
| SM < 500, n (%, 95%CI) | 7/30 (23.3, 8.1-38.4) | 2/30 (6.6, 0-15.5) | 1/30 (3.3, 0-9.7) |
| SM < 1000, n (%, 95%CI) | 11/46 (23.9, 11.5-36.2) | 7/46 (15.2, 4.5-25.5) | 2/46 (4.3, 0-10.2) |
In the univariate analysis, poor differentiation (OR 6.6, 95%CI: 1.29-33.7, P = 0.01), and lymphovascular invasion (OR = 5.1, 95%CI: 1.04-25.1, P = 0.02) were risk factors for LNM; tumor size > 30 mm showed a tendency to be a risk factor (OR = 3.1, 95%CI: 0.72-14.8, P = 0.08). Multivariate logistic regression analysis identified poor tumor differentiation (OR = 6.08, 95%CI: 1.4-26.4, P = 0.01) and lymphovascular invasion (OR = 4.66, 95%CI: 1.09-19.9, P = 0.03) as independent predictors of a positive lymph node status (Table 4).
| Statistical test | OR | Lower 95%CI | Upper 95%CI | P value |
| Univariate analysis | ||||
| Age (< 70 or ≥ 70 yr) | 0.32 | 0.03 | 1.75 | 0.19 |
| Sex (male or female) | 1.04 | 0.18 | 11 | 1 |
| Location of tumor center (Siewert type I or II) | 2.1 | 0.31 | 10.8 | 0.37 |
| Depth of invasion (M or SM) | 2.3 | 0.28 | 108.3 | 0.67 |
| Depth of invasion (≥ 500 μm or < 500 μm) | 4.89 | 0.58 | 40.8 | 0.14 |
| Differentiation (undifferentiated or differentiated) | 6.6 | 1.29 | 33.7 | 0.01 |
| Tumor size (< 30 mm or ≥ 30 mm) | 3.1 | 0.72 | 14.8 | 0.08 |
| Macroscopic tumor type (elevated or depressed) | 1.43 | 0.31 | 9.1 | 0.74 |
| Ulceration (yes or no) | 1.91 | 0.44 | 8.7 | 0.33 |
| Barrett’s esophageal adenocarcinoma (yes or no) | 0.79 | 0.17 | 3.42 | 0.75 |
| Lymphovascular invasion (yes or no) | 5.1 | 1.04 | 25.1 | 0.02 |
| Multivariate analysis | ||||
| Differentiation (undifferentiated or differentiated) | 6.08 | 1.4 | 26.4 | 0.01 |
| Lymphovascular invasion (yes or no) | 4.66 | 1.09 | 19.9 | 0.03 |
Meta-data analysis of the frequencies of LNM for Siewert types I and II pT1 adenocarcinomas
In total, we could find only 5 articles except for our study that were mentioned about the frequency of LNM for Siewert type I and II pT1 adenocarcinomas in the PubMed database from 2005 to 2015. The overall frequency of LNM was 3.38% (12/355, 95%CI: 1.5-5.2) for pT1a tumors and 20.7% (91/438, 95%CI: 16.9-24.5) for pT1b tumors. Furthermore, the frequencies of LNM were 9.1% (4/44, 95%CI: 0.5-17.5) for SM1, 22.5% (7/31, 95%CI: 7.8-37.2) for SM2, and 43.9% (18/41, 95%CI: 27-59) for SM3 (Table 2).
Our date showed that the frequency of LNM was 14.2% (10/70, 95%CI: 6-22.4) for pT1b and 6.6% (1/15, 95%CI: 0-19.2) for pT1a disease. The frequencies of LNM were 3.3% (1/30, 95%CI: 0-9.7) and 4.3% (2/46, 95%CI: 0-10.2) for invasion depths of < 500 µm and < 1000 μm, respectively. Logistic regression multivariate analysis identified poor differentiation and lymphovascular invasion as independent risk factors of LNM. The overall frequency of LNM was 3.38% (12/355, 95%CI: 1.5-5.2) for pT1a tumors and 20.7% (91/438, 95%CI: 16.9-24.5) for pT1b tumors in meta-analysis.
As I mentioned before, fewer data of LNM are available for Siewert type I and type II pT1 adenocarcinomas. Especially, we could find only one report which mentioned the frequency of LNM for Siewert typeIIpT1 adenocarcinoma using pubmed data base from 2005 to 2015[2]. The study included 453 patients: The incidence of LNM was 9.5% (16/173, 95%CI: 4.9-13.5) for pT1a tumors and 22.9% (61/280, 95%CI: 16.6-28.1) for pT1b tumors. Infiltration of the submucosa, tumor size of over 10 mm, and poor tumor differentiation were independently associated with a risk of LNM. On the other hand, when the search was restricted to patients with Siewert type I and II pT1 adenocarcinomas (as in the present study), there were five reports that reviewed the frequency of LNM[6-10]. Table 2 and 3 shows summary data from those studies. There was an increase in the rate of LNM with increasing SM category. In a study of the risk factors for LNM, Lees et al[10] described the features of LNM of a pT1a adenocarcinoma with lymphovascular invasion: a tumor size of 22 mm and poor differentiation. Barbour et al[7] recommended that patients with lymphovascular invasion or poorly differentiated adenocarcinomas should undergo adjuvant chemotherapy after surgery.
Thus far we described published data on each site of adenocarcinomas and then evaluated the frequency of LNM for each invasion depth category for both BEA and gastric adenocarcinoma. Dunbar and Spechler reported the frequency of LNM in Barrett’s esophagus patients with high grade dysplasia (HGD) and pT1a adenocarcinoma in a systematic review[11]. In a total of 70 relevant reports, there were 1874 Barrett’s esophagus patients who had undergone esophagectomy for HGD or pT1a adenocarcinoma. LNM were found in 26 patients (1.4%, 95%CI: 0.9-1.9). There were no metastases in the 524 patients with a final pathology diagnosis of HGD; in contrast, 26 (1.9%, 95%CI: 1.2-2.7) of the 1350 patients with a final diagnosis of pT1a adenocarcinoma had LNM. Gotoda et al[12] reported the frequency of LNM of pT1a gastric cancer. Of the 3016 pT1a cancers; only 65 (2.2%, 95%CI: 1.6-2.6) patients were associated with regional LNM. Depressed or ulcerated lesions of over 30 mm diameter, undifferentiated histology and invasion into lymph nodes or venules were associated with an increased risk of LNM. Therefore, the risk of unexpected LNM in both intramucosal BEA and gastric adenocarcinoma patients is in the range of 1%-2%.
On the other hand, Gockel et al[13] reported the risk of LNM in pT1b esophageal adenocarcinoma patients in a systematic review. The pooled outcomes for 7645 patients with esophageal adenocarcinoma involving tumor infiltration to the submucosal level were analyzed. Esophageal adenocarcinoma patients with SM1 lesions had the lowest incidence of LNM, and there was an increasing rate of LNM with increasing depth of SM invasion: 6% (4/65, 95%CI: 0.3-11.9) for SM1, 23% (10/44, 95%CI: 10.3-35.1) for SM2, and 58% (33/57, 95%CI: 45-70.7) for SM3. In gastric pT1b adenocarcinoma, Gotoda et al[12] also reported that 2249 tumors had penetrated the SM and 402 tumors invading the SM (17.9%, 95%CI: 16.2-19.4) were associated with LNM. There was a significant correlation of both tumor size over 30 mm and lymphovascular involvement with an increased risk of LNM. In addition, cancers that penetrated deep into the SM were the most likely to be associated with regional LNM.
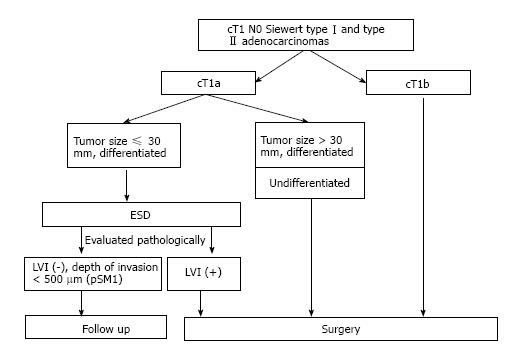
Based on these results, we currently consider ESD to be a reasonable treatment for Siewert types I and II T1a adenocarcinomas that is well differentiated, limited to the mucosa, and within 30 mm in diameter with no lymphovascular invasion (Figure 1). In this study, although only one patient with LNM had pT1a adenocarcinoma, this patient had other risk factors for LNM (tumor size was 82 mm. Pathology was mixed type of tubular adenocarcinoma and signet cell adenocarcinoma. Vascular invasion was positive). On the other hand, the frequency of LNM was high in previous report on pT1b tumors, therefore we think T1b tumors are not appropriate for ESD. Indeed, However, the frequency of LNM was relatively low for tumors of within 500 µm from muscularis mucosae in this study (3.3%; 1/30, 95%CI: 0-9.7). Gotoda et al[12] reported that 145 patients with a tumor size of under 30 mm, differentiated histology, no lymphovascular invasion, and submucosal penetration of under 500 µm were entirely free of nodal metastasis (95%CI: 0-2.5%). Furthermore, although the 5-year survival rate for pT1b gastric cancer patients (except for death caused other disease) was 96.7%[14], and esophagectomy has a mortality rate that is 2%-11% higher than that of gastrectomy[3,15,16]. Therefore, diagnostic ESD could be a treatment option for patients with T1b tumors, especially those within 500 µm from muscularis mucosae without other risk factors of LNM, according to the patient’s overall status and the presence of comorbidities (Figure 1). Even so, it is difficult to diagnose invasion depth correctly before ESD in this range. More patients undergoing surgery should be persuaded to accept ESD.
Barrett’s esophagus is most often diagnosed in people who have long term gastroesophageal reflux disease (GERD), which is a chronic regurgitation of acid from the stomach into the lower esophagus. It is associated with an increased risk of developing esophageal adenocarcinoma. The frequency of Barrett’s esophageal adenocarcinoma (BEA) from Barrett’s esophagus is about 0.5% per year. However, the frequency of BEA is thought to be increasing because of the Westernization of dietary habits, obesity, and increased frequency of GERD associated with a decreasing frequency of Helicobacter pylori (H. pylori) infection in Japan.
If the frequency of lymph node metastasis (LNM) and the risk factors driving this process in this range can be determined, then patient treatment can be stratified: ESD can be offered to patients with tumors that have a low frequency of LNM; and surgical resection can be offered to patients with tumors that have a high frequency of LNM.
These date showed that the frequency of LNM was 14.2% (10/70, 95%CI: 6-22.4) for pT1b and 6.6% (1/15, 95%CI: 0-19.2) for pT1a disease. The frequencies of LNM were 3.3% (1/30, 95%CI: 0-9.7) and 4.3% (2/46, 95%CI: 0-10.2) for invasion depths of < 500 μm and < 1000 μm, respectively. Logistic regression multivariate analysis identified poor differentiation and lymphovascular invasion as independent risk factors of LNM. The overall frequency of LNM was 3.38% (12/355, 95%CI: 1.5-5.2) for pT1a tumors and 20.7% (91/438, 95%CI: 16.9-24.5) for pT1b tumors in meta-analysis.
The authors evaluated the frequencies of LNM for Siewert type I and type II pT1 adenocarcinomas in meta-data analysis.
This paper has shown accurate incidence of lymph nodes metastasis of esophageal adenocarcinomas. Their study provides us important information related to treatment of esophageal adenocarcinomas.
Manuscript source: Unsolicited manuscript
Specialty type: Evidence-Based Medicine
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Matsuda Y S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 2. | Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg. 2015;99:1879-1885; discussion 1886. [PubMed] [Cited in This Article: ] |
| 3. | Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 515] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 4. | Horii T, Koike T, Abe Y, Kikuchi R, Unakami H, Iijima K, Imatani A, Ohara S, Shimosegawa T. Two distinct types of cancer of different origin may be mixed in gastroesophageal junction adenocarcinomas in Japan: evidence from direct evaluation of gastric acid secretion. Scand J Gastroenterol. 2011;46:710-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 6. | Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Barbour AP, Jones M, Brown I, Gotley DC, Martin I, Thomas J, Clouston A, Smithers BM. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol. 2010;17:2494-2502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Griffin SM, Burt AD, Jennings NA. Lymph node metastasis in early esophageal adenocarcinoma. Ann Surg. 2011;254:731-776; discussion 731-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Lee L, Ronellenfitsch U, Hofstetter WL, Darling G, Gaiser T, Lippert C, Gilbert S, Seely AJ, Mulder DS, Ferri LE. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg. 2013;217:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2012;107:850-862; quiz 863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1308] [Cited by in F6Publishing: 1270] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 13. | Gockel I, Sgourakis G, Lyros O, Polotzek U, Schimanski CC, Lang H, Hoppo T, Jobe BA. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Sasako M, Kinoshita T, Maruyama K. The prognosis of early gastric cancer. Stomach Intest. 1983;28:139-146. [Cited in This Article: ] |
| 15. | Yamashita H, Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg. 2011;254:274-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |