Published online Oct 6, 2018. doi: 10.12998/wjcc.v6.i11.459
Peer-review started: May 13, 2018
First decision: June 5, 2018
Revised: June 16, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: October 6, 2018
Pancreatic pseudocyst (PPC), a common sequela of acute or chronic pancreatitis, was defined by the revised Atlanta classification as “a collection.” Endoscopic ultrasound (EUS)-guided drainage is often considered a standard first-line therapy for patients with symptomatic PPC. This effective approach exhibits 90%-100% technical success and 85%-98% clinical success. Bleeding is a deadly adverse event associated with EUS-guided drainage procedures, and the bleeding rate ranges from 3% to 14%. Hemostasis involves conservative treatment, endoscopy, interventional radiology-guided embolization and surgery. However, few studies have reported on EUS-guided drainage with massive, multiple hemorrhages related to severe pancreatogenic portal hypertension (PPH). Thus, the aim of this case report was to present a case using a balloon dilator to achieve successful hemostasis for PPH-related massive bleeding in EUS-guided drainage of PPC. To our knowledge, this method has not been previously reported.
Core tip: There has been considerable research in recent years dedicated to the development of endoscopic ultrasound-guided drainage, which is viewed as the first-line therapy for the management of pancreatic pseudocyst due to the minimized invasiveness, lower mortality, better physical and mental condition of patient compared with surgical and percutaneous approaches. Although the procedure is safe and effective, bleeding is one of the deadly adverse events. This is the first report using a balloon dilator to control pancreatogenic portal hypertension-related bleeding in endoscopic ultrasound-guided drainage for pseudocyst.
- Citation: Wang BH, Xie LT, Zhao QY, Ying HJ, Jiang TA. Balloon dilator controls massive bleeding during endoscopic ultrasound-guided drainage for pancreatic pseudocyst: A case report and review of literature. World J Clin Cases 2018; 6(11): 459-465
- URL: https://www.wjgnet.com/2307-8960/full/v6/i11/459.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i11.459
Acute pancreatitis is the most common cause of hospitalization associated with gastrointestinal disease in the United States[1]. It is estimated that 5%-20% of pancreatitis episodes are complicated by the development of pancreatic pseudocyst (PPC)[2,3]. PPC, a common sequela of acute or chronic pancreatitis, was defined by the revised Atlanta classification as an encapsulated collection of fluid with a well-defined wall with minimal or no necrosis[4]. The majority of PPC cases was asymptomatic and may resolve spontaneously. However, PPCs can become symptomatic when they are infected or increase in size, which warrants intervention[5].
Previous retrospective and prospective studies have demonstrated that endoscopic ultrasound (EUS)-guided drainage for symptomatic PPC is increasingly used as a primary therapy with 90%-100% technical and 85%-98% clinical success rates[5,6]. However, hemorrhage is one of the deadly adverse events, and bleeding rates range from 3% to 14%[7,8]. Bleeding presents a challenging problem for physicians dealing with PPC. Management of this condition requires the cooperation of surgeons, endoscopists and radiologists. Historically, hemorrhages have been treated with conservative therapy, endoscopy, interventional radiology-guided embolization or surgery[9].
Herein, we present a meaningful case using a small balloon dilator to control PPH-related massive bleeding in EUS-guided drainage of PPC. To our knowledge, this method has not been previously reported.
A 55 years old woman presented with abdominal pain and distension resulting from PPC. She reported no nausea or vomiting. The patient had a complicated medical history (Supplementary file 1). She developed acute pancreatitis due to a gallstone and chronic cholecystitis 2.5 years ago. Then, the patient progressed from acute to chronic pancreatitis and PPC. Therefore, some treatment approaches, such as endoscopic nasopancreatic drainage (ENPD) and ultrasound-guided percutaneous drainage, were attempted. However, the PPC continually recurred. She visited our hospital again due to PPC growth and abdominal pain symptoms.
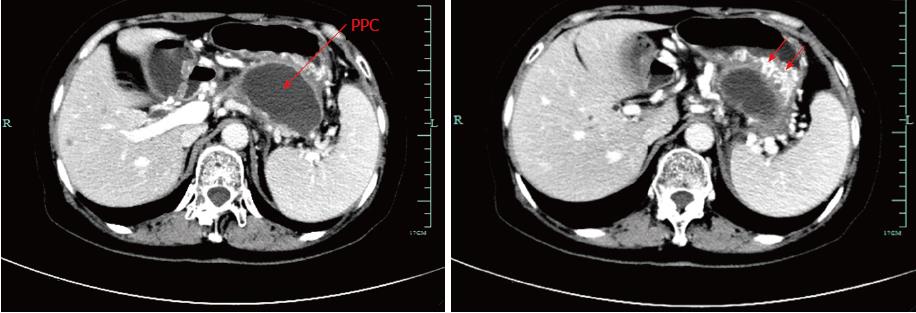
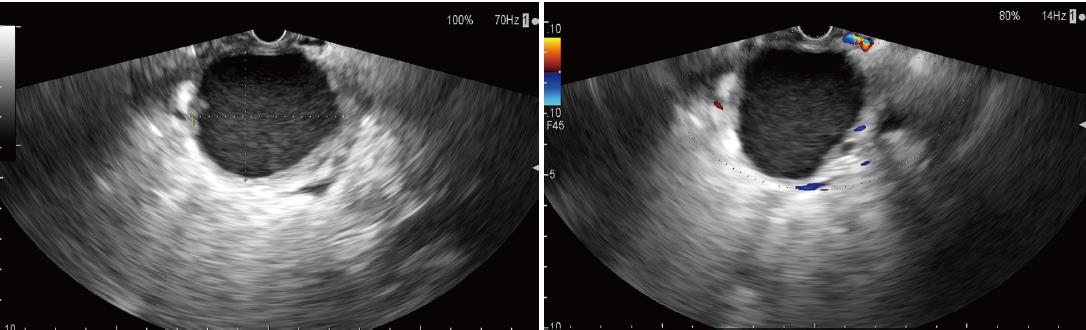
An abdominal contrast-enhanced computed tomography (CECT) scan (Figure 1) revealed a 7.4 cm × 6.2 cm pseudocyst in the tail of pancreas. Notably, most of the splenic vein compression, splenomegaly, perisplenic and gastric varices suggested severe varices due to pancreatogenic portal hypertension (PPH). In addition, pre-procedural magnetic resonance cholangiopancreatography (MRCP) revealed a homogeneous pseudocyst mass in the tail of the pancreas. Contact was observed between the pseudocyst and the pancreatic duct, and a high-intensity fluid tract was detected. Cross-sectional imaging and patient medical history helped to confirm the pseudocyst. Particularly, EUS (Figure 2) revealed that the cyst wall had a thickness of approximately 10 mm and good adhesion (within 10 mm) between the cyst wall and posterior gastric wall. After sufficient pre-procedural preparation, EUS-guided trans-gastric drainage was performed.
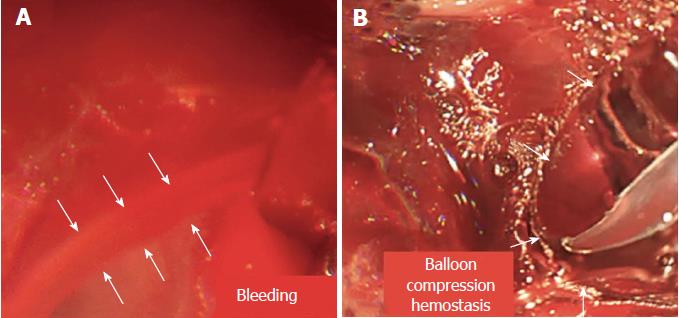
The patient underwent endoscopy performed by experienced interventional endoscopists using a linear array echoendoscope (Olympus Ltd, Tokyo, Japan) (Supplementary file 2). All procedures were performed while the patient was under general anesthesia. EUS imaging was used to determine the cyst puncture site and confirm the lack of intervening vessels at the puncture site. A 19-gauge needle was employed to perform the primary PPC puncture and access the cavity, which helped to create a fistula between the PPC and gastric lumen. Aspiration of PPC contents was then conducted to confirm the location, and the aspirate was microbiologically assessed. A 0.035 in guidewire was inserted through the needle and then coiled into the cyst cavity. The needle was withdrawn, while the guidewire remained in the cyst. Next, a 10F cystotome was utilized to dilate the fistula. Unfortunately, after we removed the cystotome, an acute, massive hemorrhage surrounding the fistula was noted under EUS, and the blood flow was similar to a stream (Figure 3A, video 1). Hemoglobin decreased by 2 g, and a blood transfusion was performed immediately. We transfused 1.5 units of fresh red cells, but the transfusion did not improve the situation.
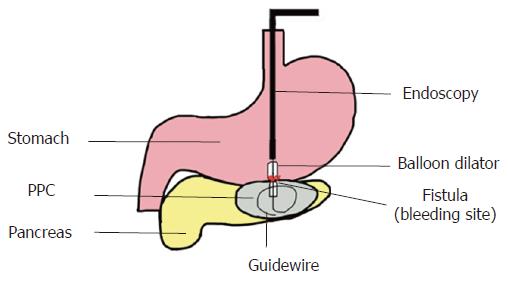
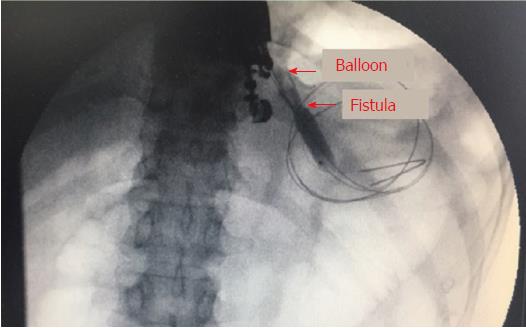
Ultimately, we used a 10-mm balloon dilator (Boston Scientific, Natick, Mass) guided by digital subtraction angiography (DSA) to compress the bleeding area (Figure 4, schematic diagram). DSA was used to ensure that the balloon was placed in the correct location (Figure 5). We used a pressure pump to inject 1:1 contrast agent into the balloon to expand it to 1 cm. Suddenly, the “blood stream” was controlled (Figure 3B). After half an hour, we loosened the balloon, and bleeding was observed in EUS images. Thus, we continued compression for two hours. Surprisingly, the serious hemorrhage had completely stopped when we loosened the balloon again. Furthermore, the fistula was simultaneously dilated. One small balloon, as a dilator and an effective tool to achieve hemostasis, made a significant impact. Finally, a 1.0 cm double-pigtail plastic stent (DPPS) was successfully deployed. Considering the substantial bleeding, we decided to deploy only one stent to avoid excessive damage.
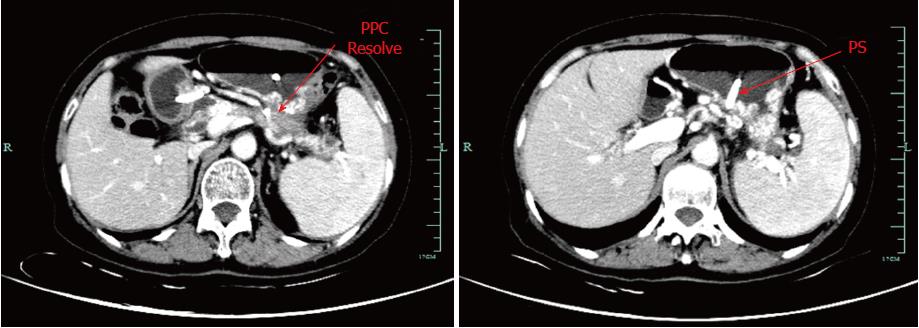
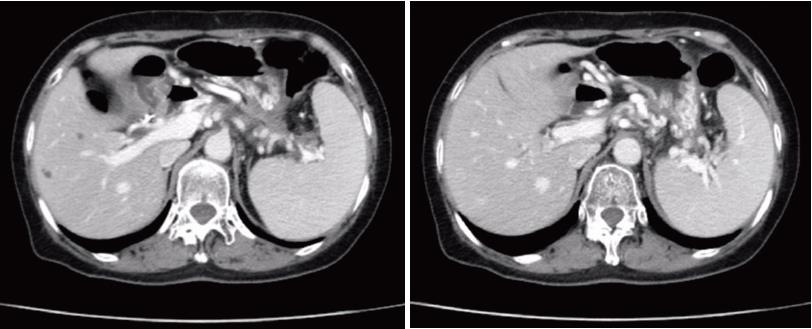
The patient underwent CECT after two months to evaluate resolution of the fluid collection. CECT revealed that the PPC completely resolved without any remaining fluid component, and the severe PPH was alleviated (Figure 6). Ultimately, the stent was safely removed. Post-procedural CT indicated that the PPC lacked a stent and that PPH was significantly alleviated (Figure 7). The patient reported no bleeding and no recurrence at the six month follow-up.
PPC typically develops as a result of sequelae of acute or chronic pancreatitis, pancreatic trauma, malignancy, and surgery[10]. Ultrasound, CT, MRI (including MRCP) and EUS are routine diagnostic approaches for pancreatic fluid collections. A CT scan of PPC is the most commonly used modality for diagnosis, and EUS is used for observation and as a therapeutic procedure[11,12]. Rapid progression in the improvement of diagnostic methods enables examination with high sensitivity and specificity. The differentiation of PPC can be challenging and depends on high-quality imaging and thorough knowledge of medical history and disease pathophysiology (Supplementary file 3).
Currently, the management options for symptomatic PPC mainly include surgical, percutaneous and endoscopic drainage[13]. In a previous randomized trial comparing endoscopic and surgical drainage for a pseudocyst, Varadarajulu et al[14] revealed that these procedures had equal efficacy for PPC drainage. However, endoscopic treatment was associated with lower mortality, better physical and mental health of patients, shorter hospital stays, and lower cost. A recent systematic review and meta-analysis[15] concluded that endoscopic drainage rather than percutaneous drainage should be the preferred therapeutic method for PFC. In recent years, the use of EUS-guided drainage has gradually increased. Moreover, this procedure has been recognized as the first-line approach for managing symptomatic PPC[16].
In our case, the patient chose EUS-guided drainage for three reasons. First, the patient tried other treatments, including conservative approaches as well as endoscopic nasopancreatic and percutaneous methods. However, the PPC continuously recurred. Second, although another possible treatment for this patient was pseudocyst-jejunum anastomosis due to the connection between the pseudocyst and the pancreatic duct. Although surgery is the traditional therapy for PPC, it is associated with more complications, such as pancreatic fistula and intestinal fistula, longer length of recovery and hospital stays, and increased cost[17]. In contrast, EUS-guided drainage is a minimally invasive treatment for pseudocysts with fewer adverse events and shorter length of recovery[11]. Third, the patient experienced serious PPH. Surgery causes substantial trauma that could potentially injure the variceal vessels and increase the risk of bleeding. In consideration of all these factors, we selected EUS-guided drainage for this patient, and it was effective.
However, some treatment methods related to EUS-guided drainage are associated with bleeding. A recent retrospective study assessed 103 pancreatic fluid collection patients treated by EUS-guided drainage. In total, five patients experienced bleeding (5%), and one patient died from splenic artery pseudoaneurysms[9]. In addition, stent erosion of the gastric wall was noted in one patient who was treated by cauterization for durable hemostasis under esophagogastroduodenoscopy (EGD). In another patient, collateral vessel bleeding was managed conservatively. Moreover, one patient experienced intracavity variceal bleeding and was treated by intracavity tamponade under endoscopy. In another multicenter study by Siddiqui[5], EUS-guided drainage of PPC was employed, and 4% of patients experienced bleeding. Two patients experienced severe bleeding due to inadvertent puncture of an artery, which was successfully treated with interventional radiology-guided coil embolization. Recently, Puri et al[18] performed EUS-guided cyst puncture and drainage on 40 patients with PPC. The success rate was 100%, and only one patient underwent surgery due to bleeding. The authors also believed that EUS-guided drainage of PPC was a safe, successful method.
In the present case, the patient experienced acute, massive bleeding surrounding the puncture site, and surgery seemed to be an appropriate option. However, considering the serious situation with PPH, surgery could possibly damage the variceal vessels, while EUS with color Doppler ultrasound can identify the surrounding vessels and avoid intervening vessels at the puncture site[19]. Furthermore, because the bleeding was so severe, we needed a rapid, efficient, less damaging form of hemostasis to control it, and balloon compression quickly controlled the bleeding. In addition, the outcome of EUS appeared poor when the bleeding occurred like a “blood stream,” and there were multiple, indefinite bleeding points. Thus, endoscopic cauterization or tamponade treatment and interventional embolization may have been impossible to perform. Under these conditions, balloon compression represented optimal hemostasis with the advantages of convenience, quickness, inexpensive cost, and minimal invasiveness. From this case, we learned the following. Although EUS-guided drainage for PPC is safe and effective, there are some adverse events, and interventional endoscopists need to prepare in advance to address different problems. In addition, management of bleeding requires integrated and multidisciplinary cooperation between surgeons, endoscopists, and radiologists.
In conclusion, balloon compression may represent a successful treatment for a fistula surrounding massive bleeding during EUS-guided drainage for PPC and provides a novel form of hemostasis. Although this method was effective in our patient, additional successful cases are needed to confirm the validity of this new hemostasis method in future studies.
A 55-year-old woman was referred to our hospital with abdominal pain and distension resulting from a history of pancreatic pseudocyst.
Pancreatic pseudocyst.
Walled-off necrosis and pancreatic cystic tumors.
No specific laboratory testing contributed to the diagnosis of the pancreatic pseudocyst.
Abdominal contrast-enhanced computed tomography, magnetic resonance cholangiopancreatography and endoscopic ultrasound examinations showed a pseudocyst in the tail of pancreas.
Pancreatic pseudocyst.
We performed endoscopic ultrasound-guided drainage with massive bleeding and used a balloon dilator to compress the bleeding sites.
To our knowledge, using balloon compression to achieve effective hemostasis in EUS-guided drainage for pancreatogenic portal hypertension-related bleeding has not been previously reported.
Endoscopic ultrasound-guided drainage with stenting is recognized as the standard first-line approach for a symptomatic pancreatic pseudocyst.
Balloon compression is a novel and effective form of hemostasis for endoscopic ultrasound-guided drainage with bleeding. Although this method was successful in this case, additional cases are needed to confirm our findings.
CARE Checklist statement: The guidelines of the CARE Checklist (2013) have been adopted.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Andrianello S, Kin T, Luo HS, Sun X S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179-1187.e1-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1355] [Cited by in F6Publishing: 1391] [Article Influence: 115.9] [Reference Citation Analysis (1)] |
| 2. | Cui ML, Kim KH, Kim HG, Han J, Kim H, Cho KB, Jung MK, Cho CM, Kim TN. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci. 2014;59:1055-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Poornachandra KS, Bhasin DK, Nagi B, Sinha SK, Rana SS, Shafiq N, Greer K, Gupta R, Kang M, Malhotra S. Clinical, biochemical, and radiologic parameters at admission predicting formation of a pseudocyst in acute pancreatitis. J Clin Gastroenterol. 2011;45:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4134] [Cited by in F6Publishing: 3699] [Article Influence: 336.3] [Reference Citation Analysis (38)] |
| 5. | Sharaiha RZ, DeFilippis EM, Kedia P, Gaidhane M, Boumitri C, Lim HW, Han E, Singh H, Ghumman SS, Kowalski T. Metal versus plastic for pancreatic pseudocyst drainage: clinical outcomes and success. Gastrointest Endosc. 2015;82:822-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Aburajab M, Smith Z, Khan A, Dua K. Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest Endosc. 2018;87:1248-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Lakhtakia S, Basha J, Talukdar R, Gupta R, Nabi Z, Ramchandani M, Kumar BVN, Pal P, Kalpala R, Reddy PM. Endoscopic “step-up approach” using a dedicated biflanged metal stent reduces the need for direct necrosectomy in walled-off necrosis (with videos). Gastrointest Endosc. 2017;85:1243-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Bang JY, Hasan M, Navaneethan U, Hawes R, Varadarajulu S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. 2017;66:2054-2056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 9. | Lang GD, Fritz C, Bhat T, Das KK, Murad FM, Early DS, Edmundowicz SA, Kushnir VM, Mullady DK. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. 2018;87:150-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Akshintala VS, Saxena P, Zaheer A, Rana U, Hutfless SM, Lennon AM, Canto MI, Kalloo AN, Khashab MA, Singh VK. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc. 2014;79:921-928; quiz 983.e2, 983.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Zerem E, Hauser G, Loga-Zec S, Kunosić S, Jovanović P, Crnkić D. Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol. 2015;21:6850-6860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Dhaka N, Samanta J, Kochhar S, Kalra N, Appasani S, Manrai M, Kochhar R. Pancreatic fluid collections: What is the ideal imaging technique? World J Gastroenterol. 2015;21:13403-13410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, Kahaleh M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22:2256-2270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 109] [Cited by in F6Publishing: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 300] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 15. | Khan MA, Hammad T, Khan Z, Lee W, Gaidhane M, Tyberg A, Kahaleh M. Endoscopic versus percutaneous management for symptomatic pancreatic fluid collections: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E474-E483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Isby L, Kahaleh M, Karia K, Yoo J. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: clinical outcomes and success. Gastrointest Endosc. 2017;85:758-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 17. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 506] [Cited by in F6Publishing: 457] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 18. | Puri R, Mishra SR, Thandassery RB, Sud R, Eloubeidi MA. Outcome and complications of endoscopic ultrasound guided pancreatic pseudocyst drainage using combined endoprosthesis and naso-cystic drain. J Gastroenterol Hepatol. 2012;27:722-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Vazquez-Sequeiros E, Baron TH, Pérez-Miranda M, Sánchez-Yagüe A, Gornals J, Gonzalez-Huix F, de la Serna C, Gonzalez Martin JA, Gimeno-Garcia AZ, Marra-Lopez C. Evaluation of the short- and long-term effectiveness and safety of fully covered self-expandable metal stents for drainage of pancreatic fluid collections: results of a Spanish nationwide registry. Gastrointest Endosc. 2016;84:450-457.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |