Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5708
Peer-review started: December 11, 2021
First decision: February 8, 2022
Revised: March 8, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: June 16, 2022
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma (BCLu-DLBCL/cHL), also referred to as gray zone lymphoma (GZL), is known to share features with cHL and DLBCL. However, GZL is often difficult to diagnose. There is no consensus regarding the optimal therapeutic regimen. Most reported cases of GZL have been in Caucasian and Hispanic individuals, and its incidence is lower in African-American and Asian populations, including the Japanese population.
A 69-year-old female presented at our hospital with a growing mass on the right side of her neck. An elastic, soft mass measuring 9 cm × 6 cm was palpable in the right cervical region. Laboratory analyses showed pancytopenia, increased serum lactate dehydrogenase levels, and markedly increased levels of soluble interleukin-2 receptor. Enhanced computed tomography (CT) and fluorodeoxyglucose positron emission tomography (PET)/CT revealed multiple lesions throughout her body. She was diagnosed with GZL based on the characteristic pathological findings, the immunophenotype [CD20+, PAX5+, OCT2+/BOB1 (focal+), CD30+, CD15-], and the strong positive expression of neoplastic programmed cell death protein ligand 1 (PD-L1) in her lymphoma cells. The lymphoma was stage IV according to the Lugano classification and high-risk according to the International Prognostic Index for aggressive non-Hodgkin lymphoma. The patient received cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab (R-CHOP) chemotherapy because the tumor cells were CD20+. She has remained in complete remission for 3 years.
GZL was diagnosed based on histopathology and immunophenotyping with ancillary PD-L1 positivity. R-CHOP chemotherapy was an effective treatment.
Core Tip: The incidence of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma (BCLu-DLBCL/cHL), also known as gray zone lymphoma (GZL), is low in Asian populations. A patient presented with right-sided cervical lymph node enlargement. The patient was diagnosed based on the characteristic pathological findings, the immunophenotype [CD20+, PAX5+, OCT2+/BOB1 (focal+), CD30+, CD15-], and the strong positive expression of neoplastic programmed cell death protein ligand 1 in her lymphoma cells. There is no consensus regarding the optimal therapeutic regimen for GZL. The addition of rituximab to the chemotherapy regimen should be considered if the tumor cells are CD20+. The patient was successfully treated with the cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab regimen and has remained in complete remission for 3 years.
- Citation: Hojo N, Nagasaki M, Mihara Y. Gray zone lymphoma effectively treated with cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab chemotherapy: A case report. World J Clin Cases 2022; 10(17): 5708-5716
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5708.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5708
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma (BCLu-DLBCL/cHL), also referred to as GZL, was first recognized in the World Health Organization (WHO) classification of lymphoid neoplasms in 2008[1,2]. The recognition of the disease as a distinct pathological and clinical entity has increased, although its diagnosis remains complex[3]. The diagnostic category was formally included in the WHO classification, which defined the histological and immunophenotypic criteria. In general, GZL tends to have a more aggressive clinical course and is associated with poorer outcomes than either cHL or primary mediastinal large B-cell lymphoma (PMBL). The application of rituximab chemotherapy appears to be beneficial for improving GZL patient outcomes [4,5].
Here, we report the case of a patient with GZL, which is rare in Asian populations[6-9], who was successfully treated with cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab (R-CHOP) chemotherapy.
A 69-year-old female presented at the surgical outpatient department of our hospital with a growing mass on the right side of her neck.
The patient was admitted to the hospital for further examination and treatment. The patient had no B symptoms, such as fever, night sweats, or weight loss.
The patient had a past medical history of subarachnoid hemorrhage resulting in the impairment of higher cognitive functions at 61 years of age.
There was no personal history of tobacco or alcohol consumption or any other family medical history.
Anemia was suspected based on the color of the palpebral conjunctiva. An elastic, soft mass measuring 9 cm × 6 cm was palpable in the right cervical region. Other superficial lymph nodes and the liver and spleen were not palpable. An increased bleeding tendency was not observed.
Laboratory analyses showed pancytopenia (white blood cells, 1500/μL; red blood cells, 371 × 104/μL; hemoglobin, 10.7 g/dL; mean corpuscular volume, 87.3 fL; and platelets, 5.0 × 104/μL), increased serum lactate dehydrogenase levels (451 U/L; reference value 124–222 U/L), and markedly increased levels of soluble interleukin-2 receptor (6220 U/mL; reference value 145–519 U/mL).
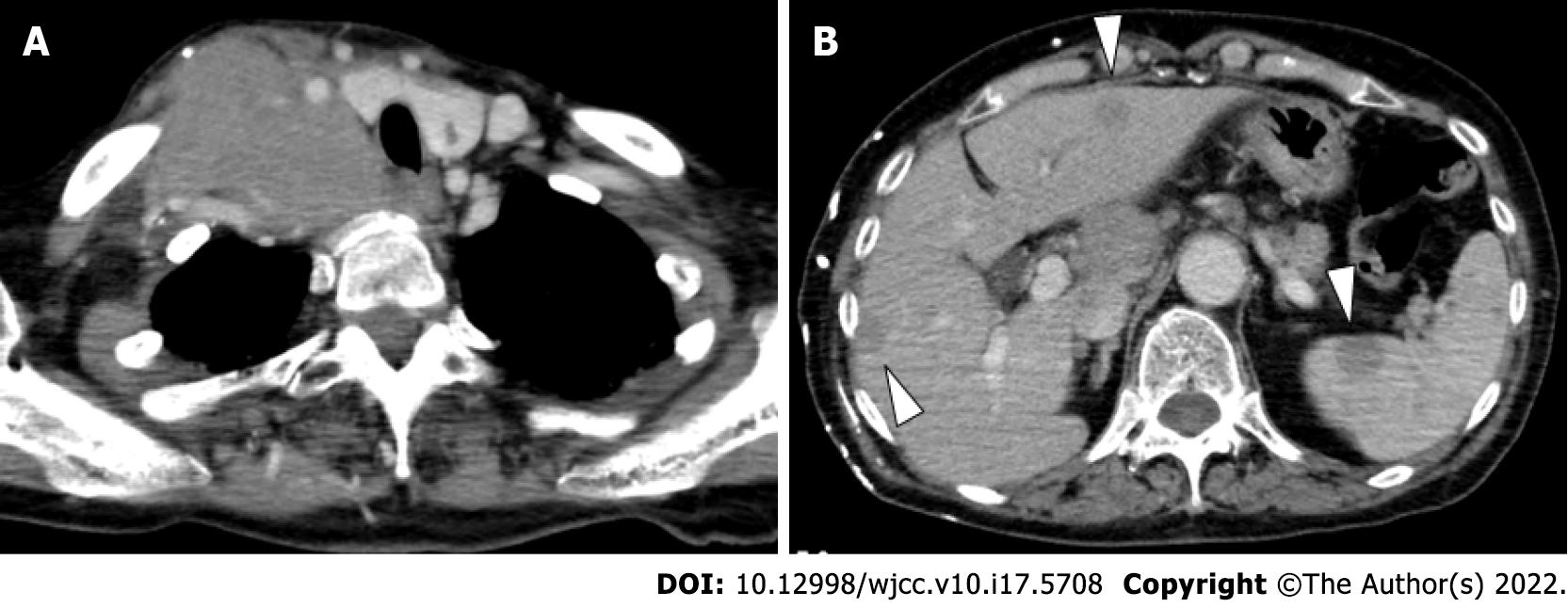
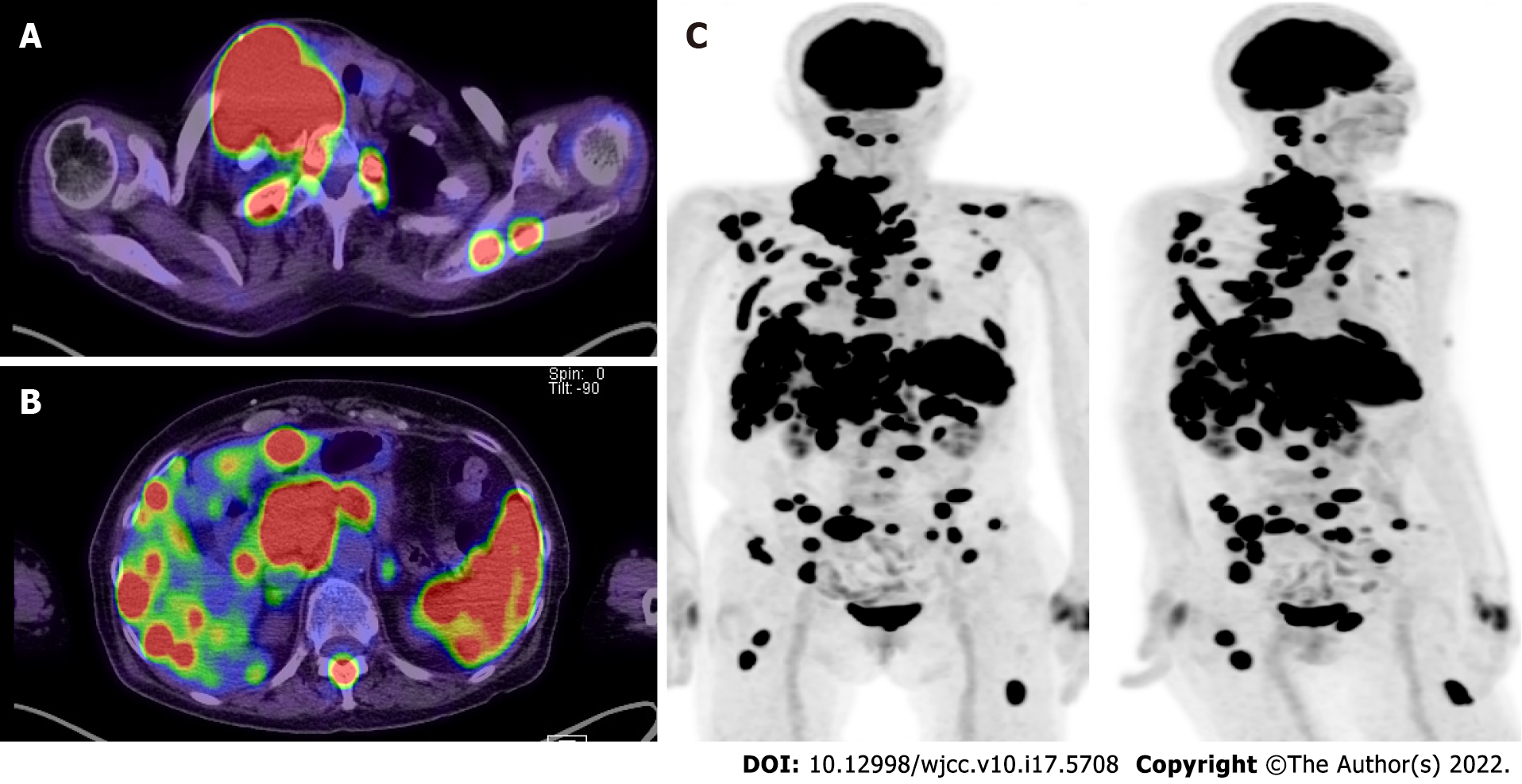
Enhanced CT (Figure 1) and fluorodeoxyglucose PET /CT (Figure 2) revealed multiple lesions throughout the patient’s body, including the right neck.
A bone marrow biopsy was obtained from the iliac crest. Fine-needle biopsies were obtained from the right cervical mass (supraclavicular lymph node). Pathological examinations were performed using immunohistochemical (IHC) studies and outsourced flow cytometry (FCM). IHC staining and Epstein–Barr virus (EBV)-encoded small RNA in situ hybridization (EBER-ISH) were performed using standard methods with an automated immunostainer (Ventana Benchmark Ultra, Tucson, AZ, United States). Antibodies against the following markers were used: CD20 (L26, Ventana), CD3 (GV6, Ventana), CD30 (BerH2, Ventana), CD15 (MMA, Ventana), PAX5 (DAKO-Pax5, Dako, Agilent Technologies, Inc., Santa Clara, CA, USA), CD45 (PD7/26,2B11, Nichirei Biosciences, Tokyo, Japan), OCT2 (Oct-207, Leica Biosystems, Wetzlar, Germany), BOB1 (TG14, Leica), CD79a (JCB117, Nichirei), MUM1 (MUM1p, Dako), and ALK (ALK1, Dako). Appropriate positive controls were used for IHC staining. The immunostaining of programmed cell death protein ligand 1 (PD-L1) (clone SP142, Ventana OptiView, Roche) was outsourced.
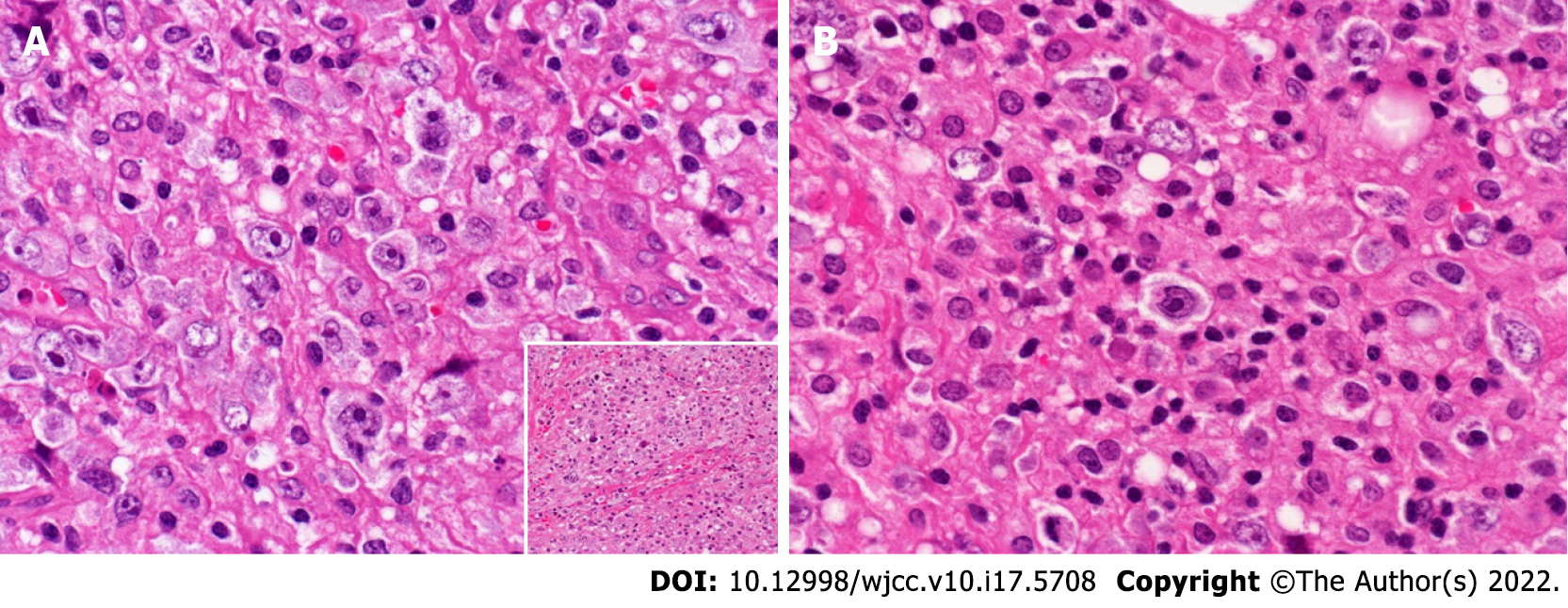
Histopathological analysis of the iliac crest biopsy revealed hemophagocytosis without bone marrow infiltration. Supraclavicular lymph nodes were infiltrated by large, atypical, and pleomorphic cells (Figure 3). These cells showed sheet-like proliferation and were scattered among the inflammatory cells [small lymphocytes (CD3+ T-cells) and histiocytes]. These large, atypical cells, including Hodgkin and Reed-Sternberg cells or lacunar-like cells, were immunohistochemically positive for CD20, CD79a, CD30, CD45, OCT2, BOB1 (focally), PAX5, and MUM1 but negative for CD15 and ALK. EBV was not detected by EBER-ISH (some of the results are shown in Figure 4). CD20 and PAX5 expression levels were strong, and CD30 expression levels were variable (weak to strong). Nearly 50% of the lymphoma cells were MUM1-positive. As shown in Figure 5, PD-L1 expression (clone SP142) was found in > 75% of the tumor cells (neoplastic PD-L1). Clonal expression of surface and cytoplasmic light chains (sIg and cyIg, respectively) was not detected by FCM (sIg, kappa 12.5%, lambda 12.9%; cyIg, kappa 13.2%, lambda 11.9%). On the other hand, immunoglobulin heavy chain gene rearrangement was detected (data not shown).
The patient was diagnosed with stage IV GZL according to the Lugano classification, the International Prognostic Index 4 (high-risk lymphoma) for aggressive non-Hodgkin lymphoma, and the International Prognostic Score 4 for Hodgkin lymphoma.
The patient was initially treated with a reduced dose (60% of the scheduled dose) of CHOP chemotherapy to prevent tumor lysis syndrome, and it was initially unclear whether the tumor cells were CD20-positive. Subsequently, five cycles of R-CHOP chemotherapy were administered at standard doses.
The patient achieved complete remission and has remained in complete remission for 3 years since the last chemotherapy session.
GZL is known to share features with cHL and DLBCL; however, it is often difficult to diagnose[10]. The differential diagnosis of the present case included DLBCL [common DLBCL with CD30 expression and anaplastic variant DLBCL (avDLBCL)], T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL), PMBL, and cHL.
Regarding the clinical manifestations, the patient had an elastic, soft mass in the right cervical region. Enhanced CT and fluorodeoxyglucose PET/CT revealed abnormal lymph node enlargement in the right neck, right clavicular region, and anterior mediastinum. Multiple lesions in the lymph nodes throughout the body, including the liver, spleen, and multiple bones, were also found. DLBCL involves nodal or extranodal lesions in any location, including the liver, spleen, and bone marrow[11]. THRLBCL mainly involves the lymph nodes, but bone marrow, liver, and spleen involvement is frequently found[12]. CHL also involves the liver, spleen, and bone marrow[13]. PMBL usually lacks bone marrow lesions[14]. Therefore, PMBL was excluded from the differential diagnosis. The most common presentation of GZL is a large anterior mediastinal mass, with or without the involvement of supraclavicular lymph nodes. The tumor may spread directly to the lung, and spread to the liver, spleen, and bone marrow can also be observed[5].
Pathologically, the distribution of large, atypical cells, including centroblastic cells and lacunar-like cells, varied from sheet-like to scattered (with predominantly sheet-like proliferation). Except for the moderate expression of MUM1, the immunophenotype of the tumor cells [positive for CD30 (variable expression) and CD20 (diffuse, strong expression), and negative for CD15] was compatible with the diagnosis of consensus-confirmed GZL but not with cHL[10]. The Lymphoma Study Association has proposed a classification system for GZL with four subcategories based on the morphological and phenotypical spectrum[15]. According to this classification system, the patient’s tumor was categorized into the transitional group of cHL-like GZL and large B-cell lymphoma-like GZL. The FCM analysis of the same pathologic specimen did not detect clonal sIg and cyIg expression, although CD20+ B-cells represented a minor population in the gated fraction. Kappa or lambda Ig expression in large tumor cells cannot be estimated by IHC due to background staining. Strong nuclear expression of OCT2 was present in > 50% of the cells; on the other hand, most of the cells were weak to faintly positive for BOB1 (Figure 4E and F), with strong expression observed in only 7.5% (Figure 4E and F, inserted). As previously reported, positive evaluation of OCT2 and BOB1 was based on at least 30% of large tumor cells[16-18], and in one study, strong staining intensity was also a condition[17]. Thus, we interpreted the focal positive BOB1 expression as abnormal in the present case. The focal expression of BOB1 and lack of detectable clonal expression of sIg and cyIg in the FCM analysis were possibly related to abnormal regulation of immunoglobulin expression, similar to cHL but not ordinal DLBCL. The findings were not compatible with the diagnosis of THRLBCL due to the presence of a sheet-like growth area and the positive expression of CD30. Because the primary site of the tumor was not considered to be the mediastinum, PMBL was not considered. Because large tumor cells did not display a sinusoidal growth pattern, the diagnosis of avDLBCL was incompatible.
Recently, neoplastic PD-L1 has been found to be a useful marker of GZL[17-20], enabling researchers to differentiate GZL from nodal avDLBCL[18]. We detected abundant PD-L1-positive (clone SP142) large lymphoma cells (75%) without an EBV association (EBER-ISH negative). Tanaka et al[20] reported PD-L1 IHC expression (30% cut off) in 77% of GZL cases (10/13). Sakakibara et al[21] reviewed PD-L1 expression (clone SP142) and found it to be useful for the diagnosis of GZL (Nodal DLBCL, EBV-negative 0/275 (0%); Nodal GZL, EBV-negative 3/3 (100%); Nodal avDLBCL, EBV-negative 0/11 (0%). Considering these findings, we diagnosed this patient with nodal GZL.
GZL can be divided into mediastinal GZL (MGZL) and nonmediastinal GZL (NMGZL) depending on the presence or absence of mediastinal lesions, and there are several clinical differences between these two subtypes. It has been reported that patients with MGZL more commonly have bulky disease than patients with NMGZL. Patients with NMGZL are typically significantly older, have a higher likelihood of bone marrow involvement, more often have extranodal disease sites, and present much more commonly with advanced-stage disease[5]. The patient in this case was elderly, had bone marrow involvement, and was diagnosed with advanced-stage disease; therefore, the patient’s clinical manifestations were similar to those of NMGZL.
Most reported cases of GZL have been in Caucasian and Hispanic individuals, and its incidence is lower in African-American and Asian populations, including the Japanese population[6,7]. Therefore, reporting cases of rare diseases is important, especially when these diseases occur in regions where they are usually not prevalent. This practice aids in investigating the factors underlying the epidemiology of the disease.
According to the National Comprehensive Cancer Network guidelines, aggressive large B-cell lymphoma treatment regimens are preferred for GZL, though there is no consensus regarding the optimal regimen. If the tumor cells are CD20+, the addition of rituximab to the chemotherapy regimen should be considered[22]. R-CHOP or dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) regimens are recommended[23-25].
Recently, brentuximab vedotin, an anti-CD30 antibody, has been used to treat Hodgkin lymphoma and CD30-positive lymphoma[9,26,27]. Pembrolizumab, an anti-PD-L1 monoclonal antibody, is also used for patients with refractory GZL[28]. If the response to the abovementioned chemotherapy regimens is poor, tandem high-dose chemotherapy supported by autologous stem cell transplantations and consolidative radiotherapy can be considered[29]. In this case, the tumor cells were CD20+, and the patient was initially treated with one cycle of CHOP followed by five cycles of R-CHOP chemotherapy. She has remained in complete remission for 3 years. These regimens can also be considered for treating recurrent disease.
GZL was diagnosed based on histopathology and immunophenotyping with ancillary PD-L1 positivity. R-CHOP chemotherapy was an effective treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chuang SS, Taiwan; Pan H, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri S, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer, 2008: 267-268. [Cited in This Article: ] |
| 2. | Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-5032. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Magyari F, Barna S, Miltényi Z, Rajnai H, Csomor J, Udvardy M, Illés Á, Váróczy L. Histopathological difficulties in an adolescent lymphoma patient. Pathol Oncol Res. 2015;21:213-217. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri S, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: International Agency for Research on Cancer, 2008: 342-344. [Cited in This Article: ] |
| 5. | Evens AM, Kanakry JA, Sehn LH, Kritharis A, Feldman T, Kroll A, Gascoyne RD, Abramson JS, Petrich AM, Hernandez-Ilizaliturri FJ, Al-Mansour Z, Adeimy C, Hemminger J, Bartlett NL, Mato A, Caimi PF, Advani RH, Klein AK, Nabhan C, Smith SM, Fabregas JC, Lossos IS, Press OW, Fenske TS, Friedberg JW, Vose JM, Blum KA. Gray zone lymphoma with features intermediate between classical Hodgkin lymphoma and diffuse large B-cell lymphoma: characteristics, outcomes, and prognostication among a large multicenter cohort. Am J Hematol. 2015;90:778-783. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Iwaki N, Sato Y, Kurokawa T, Maeda Y, Ohno K, Takeuchi M, Takata K, Orita Y, Nakao S, Yoshino T. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma without mediastinal disease: mimicking nodular sclerosis classical Hodgkin lymphoma. Med Mol Morphol. 2013;46:172-176. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Shi F, Zhou Q, Gao Y, Cui XQ, Chang H. Primary splenic B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: A case report. Oncol Lett. 2016;12:1925-1928. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Ebisawa K, Koya J, Shinozaki-Ushiku A, Nakamura F, Kurokawa M. Paraneoplastic limbic encephalitis in B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and classical Hodgkin lymphoma. Ann Hematol. 2016;95:1011-1012. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Mallipudi RM, Alquran L, Shenoy VA, Leslie LA, Conti JA. A Rare Case of Grey Zone Lymphoma Successfully Treated with Brentuximab Vedotin and R-CHP Chemotherapy. Case Rep Oncol Med. 2019;2019:4121234. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Pilichowska M, Pittaluga S, Ferry JA, Hemminger J, Chang H, Kanakry JA, Sehn LH, Feldman T, Abramson JS, Kritharis A, Hernandez-Ilizaliturri FJ, Lossos IS, Press OW, Fenske TS, Friedberg JW, Vose JM, Blum KA, Jagadeesh D, Woda B, Gupta GK, Gascoyne RD, Jaffe ES, Evens AM. Clinicopathologic consensus study of gray zone lymphoma with features intermediate between DLBCL and classical HL. Blood Adv. 2017;1:2600-2609. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, Connors JM, Gascoyne RD. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2011;29:1452-1457. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Shimabukuro-Vornhagen A, Haverkamp H, Engert A, Balleisen L, Majunke P, Heil G, Eich HT, Stein H, Diehl V, Josting A. Lymphocyte-rich classical Hodgkin's lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin's Study Group trials. J Clin Oncol. 2005;23:5739-5745. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Barth TF, Leithäuser F, Joos S, Bentz M, Möller P. Mediastinal (thymic) large B-cell lymphoma: where do we stand? Lancet Oncol. 2002;3:229-234. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Sarkozy C, Copie-Bergman C, Damotte D, Ben-Neriah S, Burroni B, Cornillon J, Lemal R, Golfier C, Fabiani B, Chassagne-Clément C, Parrens M, Herbaux C, Xerri L, Bossard C, Laurent C, Cheminant M, Cartron G, Cabecadas J, Molina T, Salles G, Steidl C, Ghesquières H, Mottok A, Traverse-Glehen A. Gray-zone Lymphoma Between cHL and Large B-Cell Lymphoma: A Histopathologic Series From the LYSA. Am J Surg Pathol. 2019;43:341-351. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Gualco G, Natkunam Y, Bacchi CE. The spectrum of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma: a description of 10 cases. Mod Pathol. 2012;25:661-674. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | O'Malley DP, Fedoriw Y, Weiss LM. Distinguishing Classical Hodgkin Lymphoma, Gray Zone Lymphoma, and Large B-cell Lymphoma: A Proposed Scoring System. Appl Immunohistochem Mol Morphol. 2016;24:535-540. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Megahed NA, Kohno K, Sakakibara A, Eladl AE, Elsayed AA, Wu CC, Suzuki Y, Takahara T, Kato S, Nakamura S, Satou A, Asano N. Anaplastic variant of diffuse large B-cell lymphoma: Reappraisal as a nodal disease with sinusoidal involvement. Pathol Int. 2019;69:697-705. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Kohno K, Suzuki Y, Harada T, Sakai A, Takeuchi Y, Inagaki Y, Megahed NA, Takahara T, Satou A, Sakakibara A, Nakamura S. Nodal diffuse large B-cell lymphoma with neoplastic PD-L1 positivity, but without EBV association: Three cases highlighting an aspect of gray zone lymphoma. Pathol Int. 2020;70:695-697. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Tanaka Y, Maeshima AM, Nomoto J, Makita S, Fukuhara S, Munakata W, Maruyama D, Tobinai K, Kobayashi Y. Expression pattern of PD-L1 and PD-L2 in classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and gray zone lymphoma. Eur J Haematol. 2018;100:511-517. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Sakakibara A, Kohno K, Ishikawa E, Suzuki Y, Tsuyuki Y, Shimada S, Shimada K, Satou A, Takahara T, Ohashi A, Takahashi E, Kato S, Nakamura S, Asano N. Diagnostic utility of programmed cell death ligand 1 (clone SP142) immunohistochemistry for malignant lymphoma and lymphoproliferative disorders: A brief review. J Clin Exp Hematop. 2021;61:182-191. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | NCCN Guidelines for Patients. Diffuse large b-cell lymphoma, grey zone lymphoma (intermediate between DLBCL and classical HL). Version 2. Plymouth Meeting, PA: NCCN, 2018. [Cited in This Article: ] |
| 23. | Kritharis A, Pilichowska M, Evens AM. How I manage patients with grey zone lymphoma. Br J Haematol. 2016;174:345-350. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Chihara D, Westin JR, Miranda RN, Cheah CY, Oki Y, Turturro F, Romaguera JE, Neelapu SS, Nastoupil LJ, Fayad LE, Rodriguez MA, Fowler NH, Orlowski RZ, Wang M, Hagemeister FB, Medeiros LJ, Fanale MA. Dose adjusted-EPOCH-R and mediastinal disease may improve outcomes for patients with gray-zone lymphoma. Br J Haematol. 2017;179:503-506. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Simon Z, Virga B, Pinczés L, Méhes G, Miltényi Z, Barna S, Szabó R, Illés Á. Transition Between Diffuse Large B-Cell Lymphoma and Classical Hodgkin Lymphoma- Our Histopathological and Clinical Experience With Patients With Intermediate Lymphoma. Pathol Oncol Res. 2021;27:625529. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Terao T, Yuda J, Yamauchi N, Guo YM, Shimada K, Sugano M, Ishii G, Minami Y. Brentuximab vedotin maintenance after autologous stem cell transplantation for refractory gray zone lymphoma with long-term remission. Mol Clin Oncol. 2021;14:125. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Svoboda J, Bair SM, Landsburg DJ, Dwivedy Nasta S, Nagle SJ, Barta SK, Khan N, Filicko-O'Hara J, Gaballa S, Strelec L, Chong E, Mitnick S, Waite TS, King C, Ballard H, Youngman M, Gerson J, Plastaras JP, Maity A, Bogusz AM, Hung SS, Nakamura H, Nejati R, Steidl C, Lim M, Ruella M, Schuster SJ. Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas. Haematologica. 2021;106:1705-1713. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Rosales YMZ, Mesquita JL, Garcia YDO, Paz FRF, Campos NCB, de Vasconcelos Leitão JP, Filho FDR, Filho RVAO, Lemes RPG, Duarte FB. Use of checkpoint inhibitors in gray zone lymphoma. Hematol Oncol Stem Cell Ther. 2020;. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Takaishi K, Muto T, Mimura N, Takiguchi J, Nagao Y, Oshima-Hasegawa N, Tsukamoto S, Takeda Y, Mitsukawa S, Takeuchi M, Ohwada C, Ota S, Iseki T, Nakaseko C, Sakaida E. Long-term complete remission following tandem autologous stem cell transplantation and consolidative radiotherapy for refractory mediastinal gray-zone lymphoma. Int J Hematol. 2018;108:452-455. [PubMed] [DOI] [Cited in This Article: ] |