Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.524
Peer-review started: June 29, 2016
First decision: August 5, 2016
Revised: August 26, 2016
Accepted: September 7, 2016
Article in press: September 8, 2016
Published online: November 6, 2016
To evaluate the lower-limb muscle oxygenation in hemodialysis (HD) patients and identify the factors associating with muscle oxygenation.
Sixty-seven HD patients (53 men and 14 women; mean age, 67.1 ± 1.2 years; mean HD duration, 5.6 ± 0.9 years) were recruited. In addition, 15 healthy individuals (nine men and six women; mean age, 38.2 ± 4.6 years) were recruited as the control group. Lower-limb muscle regional saturation of oxygen (rSO2) was monitored on the lateral side of the gastrocnemius muscle before HD using an INVOS 5100C (Covidien Japan, Tokyo, Japan), which utilizes near-infrared spectroscopy. Here, we evaluated the association between lower-limb muscle rSO2 and clinical parameters.
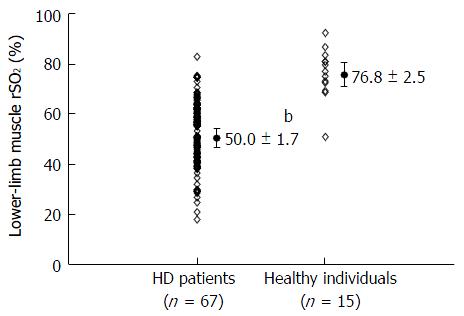
The rSO2 values were significantly lower in patients undergoing HD than in healthy individuals (50.0% ± 1.7% vs 76.8% ± 2.5%, P < 0.001). Lower-limb muscle rSO2 showed significant positive correlations with diastolic blood pressure, blood urea nitrogen concentration, serum creatinine concentration, serum potassium concentration, serum inorganic phosphate concentration, and serum albumin concentration as well as negative correlation with HD duration. We conducted a multiple linear regression analysis using parameters that were significantly correlated with the lower-limb muscle rSO2 in a simple linear regression analysis. Multiple regression analysis demonstrated that lower-limb muscle rSO2 was independently associated with serum inorganic phosphate (standardized coefficient: 0.27) and serum albumin concentrations (standardized coefficient: 0.24). In addition, there were no differences in lower-limb muscle rSO2 between diabetic and non-diabetic HD patients. This study has several limitations. Firstly, its sample size was relatively small. Secondly, we could not evaluate the association between lower-limb muscle rSO2 and calculated nutritional markers, including normalized protein catabolic rate and body mass index, anthropometric measurements representing nutritional status, and the severity of protein-energy wasting. Finally, we did not routinely examine the arterial vascular status of HD patients without symptoms of peripheral artery disease. As such, it is possible that some HD patients with subclinical peripheral artery disease may have been included in this study.
In HD patients, the oxygenation of lower-limb muscle tissue was associated with serum inorganic phosphate and albumin concentrations, both of which represent nutritional status.
Core tip: Sarcopenia, defined by reduced muscle mass and peripheral arterial disease, is common in patients undergoing hemodialysis (HD). Therefore, muscle status, including muscle oxygenation, would deteriorate; however, no muscle status evaluation method has been established and remains under debate. Here we investigated the tissue oxygenation of lower-limb muscles using near-infrared spectroscopy in HD patients. Values of regional saturation of oxygen in the lower-limb muscles were significantly lower in HD patients than in healthy controls and independently associated with serum inorganic phosphate and albumin concentrations, both of which represent nutritional status.
- Citation: Miyazawa H, Ookawara S, Ito K, Yanai K, Ishii H, Kitano T, Shindo M, Ueda Y, Kaku Y, Hirai K, Hoshino T, Tabei K, Morishita Y. Factors associating with oxygenation of lower-limb muscle tissue in hemodialysis patients. World J Nephrol 2016; 5(6): 524-530
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/524.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.524
According to the increase in numbers of older and diabetic patients undergoing hemodialysis (HD), protein-energy wasting, which includes abnormal serum biochemistry, reduced body mass, reduced muscle mass, and decreased dietary intake, has been a concern in the clinical setting[1]. In particular, sarcopenia refers to a state of reduced muscle mass[2] and is reportedly an independent predictor of mortality in HD patients[3]. However, a standard muscle status evaluation method has not been established and remains under debate. Furthermore, peripheral artery disease is common in HD patients[4]; therefore, lower-limb muscle oxygenation would deteriorate as a result of a decreased oxygen supply induced by impaired microcirculation.
Near-infrared spectroscopy (NIRS) is recently reported as a way to evaluate the regional oxygen saturation (rSO2), a tissue oxygenation marker[5,6], especially including cerebral oxygenation in various conditions. Then, rSO2 value indicates changes in tissue oxygen metabolisms between oxygen supply from arterial blood flow and tissue oxygen consumption[7-10]. Furthermore, an adequate measurement of tissue oxygenation of muscle mass using NIRS has been previously reported[11] and this method was used to evaluate muscle status[12-14]. However, few reports have examined lower-limb muscle oxygenation using NIRS much less the correlation between these values and clinical parameters in HD patients. Therefore, this study aimed to: (1) monitor lower-limb muscle rSO2 before HD; and (2) clarify the association influencing the value of lower-limb muscle oxygenation in HD patients.
This was performed as a single-center observational study and included HD patients who met the following criteria: (1) end-stage renal disease and receiving intermittent HD; (2) agreement for the purpose of this study; and (3) presence of arteriovenous fistula as a vascular access for HD. The exclusion criteria were: (1) coexisting disease including chronic obstructive pulmonary disease, apparent neurological disorder, and chronic hypotension (defined as systolic blood pressure < 100 mmHg); and (2) symptomatic ischemia and receiving lower leg amputation. Sixty seven HD patients were recruited (53 men and 14 women; mean age, 67.1 ± 1.2 years; mean HD duration, 5.9 ± 0.9 years). The causes of chronic renal failure were diabetes mellitus (DM; 38 patients), chronic glomerulonephritis (14 patients), nephrosclerosis (four patients), polycystic kidney disease (three patients), and other (eight patients). Each patient received maintenance HD two or three times a week, and each session was 3 or 4 h in duration. The patients’ general characteristics are summarized in Table 1. Furthermore, the proportions of patients using phosphate binder and vitamin D metabolites were 71.6% (n = 48) and 37.3% (n = 25), respectively. The HD dialysate comprised 140 mEq/L Na+, 2.0 mEq/L K+, 114.5 mEq/L Cl-, 2.5 mEq/L Ca2+, 1.0 mEq/L Mg2+, 25 mEq/L HCO3-, and 150 mg/dL glucose. The dialyzer included polysulfone membrane (three patients; 1.0 m2, five patients; 1.3 m2, four patients; 1.5 m2, six patients; 1.6 m2, thirteen patients; 1.8 m2, nine patients; 2.0 m2, sixteen patients; 2.1 m2, four patients; 2.3 m2) and cellulose triacetate membrane (two patients; 1.3 m2, five patients; 1.5 m2). The dialysate flow rate in all patients was consistently 500 mL/min and the blood flow rate was 183 ± 1.7 mL/min in this study. The dialysate purification process followed the recommendations in the Japanese Society for Dialysis Therapy guidelines[15]. The dialysate bacteria and endotoxin concentration in this study were less than 0.1 CFU/mL and 0.001 EU/mL respectively. All participants provided informed consent to participate in this study. This study was approved by the Institutional Review Board of Saitama Medical Center, Jichi Medical University, Japan (No. RIN13-39), and conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo in 2004). In addition, 15 healthy volunteers (nine men and six women; mean age, 38.2 ± 4.6 years) were recruited as the control group.
| Mean ± SE | P values | r | |
| Total number of patients (male/female) | 67 (53/14) | ||
| Age (yr) | 67.1 ± 1.2 | 0.09 | -0.21 |
| Disease | |||
| Diabetes mellitus | 38 | ||
| Chronic glomerulonephritis | 14 | ||
| Nephrosclerosis | 4 | ||
| Polycystic kidney disease | 3 | ||
| Others | 8 | ||
| HD duration (yr) | 5.6 ± 0.9 | 0.02 | -0.28 |
| Systolic blood pressure (mmHg) | 144 ± 3 | 0.97 | -0.01 |
| Diastolic blood pressure (mmHg) | 75 ± 2 | 0.04 | 0.26 |
| pH | 7.39 ± 0.02 | 0.41 | -0.10 |
| pCO2 (mmHg) | 36.9 ± 0.5 | 0.14 | 0.18 |
| pO2 (mmHg) | 85.6 ± 2.0 | 0.80 | -0.03 |
| HCO3- (mEq/L) | 21.9 ± 0.3 | 0.70 | 0.05 |
| SpO2 (%) | 95.4 ± 0.3 | 0.88 | -0.02 |
| Hb (g/dL) | 9.9 ± 0.1 | 0.30 | 0.13 |
| Aterial O2 content (mL/dL) | 12.9 ± 0.2 | 0.27 | 0.13 |
| BUN (mg/dL) | 51.4 ± 2.2 | 0.03 | 0.27 |
| Cr (mg/dL) | 8.4 ± 0.3 | 0.02 | 0.28 |
| Na (mEq/L) | 137.0 ± 0.4 | 0.19 | 0.16 |
| K (mEq/L) | 4.2 ± 0.1 | 0.02 | 0.30 |
| Ca (mg/dL) | 8.7 ± 0.1 | 0.91 | -0.01 |
| P (mg/dL) | 4.5 ± 0.2 | < 0.01 | 0.31 |
| Total protein (g/dL) | 6.1 ± 0.1 | 0.72 | 0.04 |
| Serum albumin (g/dL) | 3.2 ± 0.1 | 0.02 | 0.29 |
| Serum osmolality (mOsm/kg•H2O) | 301 ± 1 | 0.09 | 0.21 |
| Plasma glucose (mg/dL) | 156 ± 8 | 0.52 | -0.08 |
| Kt/V urea | 1.18 ± 0.04 | 0.65 | -0.06 |
| Urea reduction ratio (%) | 61.3 ± 1.6 | 0.94 | 0.01 |
Lower-limb muscle rSO2 was monitored at right side lower-limb on the lateral side of the gastrocnemius muscle with an INVOS 5100C saturation monitor (Covidien Japan, Tokyo, Japan), which is based on the NIRS technology. This monitor utilizes a light-emitting diode that generates near-infrared light at two wavelengths (735 and 810 nm) as well as two photodiodes constructed by silicon, which function as light detectors; these data are interpreted as a single numerical value that expresses the rSO2 value[5,6]. All data taken by this monitor were stored in sequence with immediate and automatic manner. Interobserver variance for this monitor, which means a reproducibility of rSO2 monitoring, is considered acceptable based on the study reported previously[11]. Thus, rSO2 values would be reliable in the estimation of the lower-limb muscle oxygen metabolism.
Before HD, included patients in this study lied supine for 10 min to avoid the influence of change in posture. An rSO2 monitoring probe was attached to the patients’ gastrocnemius muscle to take the measurement during resting in the supine. Then, rSO2 value was monitored for 5 min, and we evaluated the mean rSO2 for 5 min as a lower-limb muscle oxygenation marker.
Blood samples were drawn from arteriovenous fistula before HD under ambient air. Samples drawn from the radial artery or those from an arterial line at the arteriovenous fistula were previously reported to present similar values during evaluation of the clinical parameters of oxygen status, including pH, oxygen pressure (pO2), and oxygen saturation (SpO2)[16]. Thus, we took all blood samples including blood gas analysis from the arterial site of arteriovenous fistula in each patient prior to HD.
We calculated arterial O2 content (CaO2) and serum osmolality (sOsm) using following equations:
CaO2 (mL/dL) = 1.34 × Hb × SpO2/100 + (0.0031 × pO2)[17]
sOsm (mOsm/kg•H2O) = (2 × Na) + PG/18 + BUN/2.8[18]
Where Hb indicates the hemoglobin concentration (g/dL), SpO2 indicates the oxygen saturation (%), and pO2 indicates the oxygen pressure (mmHg). Na indicates the serum sodium concentration (mEq/L), PG indicates the plasma glucose level (mg/dL), and BUN indicates the blood urea nitrogen concentration (mg/dL). Furthermore, for evaluating the efficacy of HD, Kt/V by using Daugirdas II formula[19] and urea reduction ratio[20] were calculated in each patient. The rSO2 in healthy individuals was monitored for 5 min during resting in the supine position according to the same manner of rSO2 measurement in HD patients.
Data are expressed as mean ± standard error. The Shapiro-Wilk test was used to confirm that all data were normally distributed. Student’s t-test was used to compare non-paired values between the 2 values. Correlations between the 2 values were evaluated by Pearson’s correlation coefficient and linear regression analysis. Parameters that were significantly correlated with lower-limb muscle rSO2 in a simple linear regression analysis were included in a multiple linear regression analysis in order to identify factors associating with lower-limb muscle rSO2 in patients undergoing HD. A difference with P < 0.05 was considered statistically significant.
Lower-limb muscle rSO2 values in patients undergoing HD were significantly lower than those of healthy individuals (HD patients: 50.0% ± 1.7%; healthy individuals: 76.8% ± 2.5%; P < 0.001; Figure 1).
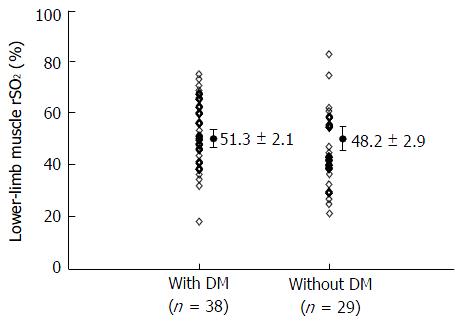
Table 1 shows the patients’ characteristics as well as correlations between lower-limb muscle rSO2 and clinical parameters. According to a simple linear regression analysis, lower-limb muscle rSO2 showed significant positive correlations with diastolic blood pressure, BUN, serum creatinine, serum potassium, serum inorganic phosphate, and serum albumin as well as negative correlation with HD duration. We conducted a multiple linear regression analysis using parameters that were significantly correlated with lower-limb muscle rSO2 in a simple linear regression analysis (Table 2). As a result, lower-limb muscle SO2 was independently associated with serum inorganic phosphate (standardized coefficient: 0.27) and serum albumin (standardized coefficient: 0.24). In addition, we examined the relationship between presence of DM and lower-limb muscle rSO2 values, and found no differences in these values between patients with and those without DM (P = 0.38; Figure 2).
| Parameters | Coefficient (95%CI) | Standardized coefficient | P values |
| P | 3.02 (0.38-5.65) | 0.27 | 0.025 |
| Serum albumin | 6.26 (0.15-12.37) | 0.24 | 0.045 |
In the present study, lower-limb muscle rSO2 values in HD patients were significantly lower than those of healthy individuals. Furthermore, multiple linear regression analysis indicated that serum inorganic phosphate and albumin concentrations, both of which represent nutritional status, were independently associated with the lower-limb muscle rSO2.
Regarding the association between tissue oxygenation and HD therapy, cerebral rSO2 values in HD patients were significantly lower than those of healthy individuals[21,22]. These values were maintained during HD and not influenced by blood volume reduction[22], whereas intradialytic hypotension might be associated with a decrease in cerebral rSO2[23]. Therefore, these findings indicate the importance of monitoring systemic tissue oxygenation, particularly cerebral oxygenation as a part of systemic oxygenation, in HD patients.
According to the increasing number of elderly patients on maintenance HD, malnutrition and loss of muscle mass are considered common in the clinical setting of dialysis therapy. The age-related loss of muscle mass and function was termed sarcopenia[2] and the European working group on sarcopenia in older people defined sarcopenia as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcome such as physical disability, poor quality of life, and death”[24]. Therefore, muscle mass condition has been a concern in patients undergoing HD, and here we focused on muscle oxygenation as a method to define muscle condition. The measurement of muscle rSO2 using NIRS at the lower extremities was reportedly applicable as a tool for evaluating tissue oxygenation because of the good reproducibility and inter-subject variability of its measurements[11]. Clinically, in patients with acute compartment syndrome of the leg, the tissue oxygenation decrease of injured leg was reported to reflect the decrease of perfusion pressure at the injured compared to uninjured leg[25]. Furthermore, in patients with community-acquired pneumonia, values of forearm muscle rSO2 at intensive care unit admission and those at 24 h after admission were independently associated with mortality[14]. However, to date, tissue oxygenation at lower extremities in patients undergoing HD has rarely been reported.
The reduction of the lower-limb muscle rSO2 values in HD patients included in this study was similar to the result of cerebral rSO2 comparison between HD patients and healthy controls[21,22]. Although this result is inconclusive because the ages and sexes were not completely matched between the groups, De Blasi et al[12] reported that the tissue oxygen saturation values at the gastrocnemius muscle are lower in patients undergoing HD with and without DM compared to healthy controls, even though the differences are not significant (tissue oxygen saturation: In HD patients with and without DM, 49.2% ± 16.6% and 51.5% ± 21.4%; in healthy controls, 62.3% ± 7.3%). HD patients with symptomatic lower-limb ischemia who were diagnosed with peripheral artery disease by angiography and/or magnetic resonance imaging were excluded from the study. The prevalence of subclinical peripheral artery disease in HD patients is approximately 20%-25%[4]. We did not use methods such as the ankle-brachial pressure index to investigate the presence of peripheral artery disease in asymptomatic patients. Therefore, while we have excluded patients with symptomatic peripheral artery disease from the present study, we may have inadvertently included some with subclinical disease. In these patients, muscle rSO2 might be reduced via the decrease in oxygen supply caused by the microcirculatory impairment due to subclinical peripheral artery disease. Thus, it would be necessary to perform arterial vascular examinations in future studies so that such patients can be excluded.
In the fields of chronic kidney disease and dialysis therapy, the concept of protein-energy wasting has recently been proposed[1]. Furthermore, protein-energy wasting itself was one of the strongest predictors of mortality in patients with chronic kidney disease[26]. Therefore, to prevent the progression of protein-energy wasting in patients undergoing HD, the initiation of oral nutritional supplementation, including 1.2 g/kg per day of dietary protein intake, is recommended[27]. On the other hand, there was a significant positive correlation between dietary protein intake and phosphorus intake as well as between phosphorus intake and pre-dialysis serum inorganic phosphate concentration[28]. Based on these results, serum inorganic phosphate concentration might be positively associated with dietary protein intake. In this study, lower-limb muscle rSO2 values were positively and significantly associated with serum inorganic phosphate concentration. The increase in its concentration would reflect the nutritional status improvement via increased dietary protein intake; therefore, lower-limb muscle oxygenation might be associated with the change in serum inorganic phosphate concentration, which could be influenced by the nutritional status. However, although serum inorganic phosphate concentration is one of several nutritional markers, hyperphosphatemia may contribute to worsening vascular calcification and a greater risk of cardiovascular morbidity[29,30]; therefore, further studies will be necessary to confirm the association between lower-limb muscle rSO2 and other nutritional markers such as normalized protein catabolic rate[31], which represents the dietary protein intake in anuric HD patients.
Regarding the association between serum albumin concentration and lower-limb muscle rSO2, serum albumin was reportedly a prognostic marker of survival in patients undergoing HD similar to nutritional status[32,33]. Serum albumin reduction is included in the criteria in the diagnosis of protein-energy wasting[1], leading to prognostic worsening in patients undergoing HD. Furthermore, serum albumin itself, which associates with the formation of plasma colloid osmotic pressure, influences the preservation of microcirculation via the fluid movement from the interstitial space into vessels at small systemic blood vessels. Therefore, serum albumin itself might be associated with muscle oxygenation through the preservation of muscle microcirculation, in addition to the nutritional status and prognosis in HD patients. Furthermore, in this study, lower-limb muscle rSO2 values were not significantly different between patients with or those without DM, although cerebral rSO2 values revealed significant decrease in patients undergoing HD with than those without DM[21]. Differences in tissue oxygenation associated with DM itself between the brain and the muscle is very interesting; however, its precise mechanism remains uncertain.
This study has several limitations. Firstly, its sample size was relatively small. Secondly, we could not evaluate the association between lower-limb muscle rSO2 and calculated nutritional markers, including normalized protein catabolic rate and body mass index, anthropometric measurements representing nutritional status, and the severity of protein-energy wasting. Finally, we did not routinely examine the arterial vascular status of HD patients without symptoms of peripheral artery disease. As such, it is possible that some HD patients with subclinical peripheral artery disease may have been included in this study. Thus, additional studies are necessary to fully clarify the correlation between lower-limb muscle rSO2 and various clinical parameters.
In conclusion, lower-limb muscle rSO2 values in patients undergoing HD were significantly lower than those in healthy individuals. Furthermore, the oxygenation of lower-limb muscle tissue was associated with serum inorganic phosphate and albumin concentrations, both of which represent nutritional status.
We thank the study participants and the clinical engineers, Takayuki Uchida, Masaya Kofuji, and Hideyuki Hayasaka, for their day-to-day clinical care about hemodialysis.
The incidence of protein-energy wasting in patients undergoing maintenance hemodialysis (HD) is high. In particular, sarcopenia characterized by skeletal muscle atrophy and decrease in muscle strength and function correlates with mortality in HD patients. In addition, peripheral arterial disease, including critical limb ischemia, is a major risk factor for amputation and death in HD patients. Therefore, a method for evaluating muscle is required, especially in the lower-limb.
Recently, near-infrared spectroscopy (NIRS) has been used as a way to measure the regional saturation of oxygen (rSO2), which is a marker of tissue oxygenation. NIRS is a non-invasive method for measuring tissue oxygenation continuously and mainly used for monitoring brain tissue oxygen saturation during cardiac surgery. In this study, the authors used NIRS to measure the lower-limb muscle rSO2 in HD patients.
Lower-limb muscle rSO2 values were significantly lower in HD patients than in healthy individuals, and independently associated with serum inorganic phosphate and albumin concentrations.
Serum inorganic phosphate and albumin concentrations are markers of nutritional status. Therefore, the authors can conclude that lower-limb muscle oxygenation may be associated with nutritional status in HD patients.
NIRS technology uses a light-emitting diode which transmits near-infrared light at 2 wavelengths, as well as 2 silicon photodiodes which act as light detectors. Each result is read as a single numerical value representing the rSO2.
The authors performed a very interesting and well conducted study aimed at evaluating the factors influencing regional muscular oxygenation in patients underwent hemodialysis. The adopted statistical approach is convincing and the conclusions are supported by the findings reported.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caliandro P, Friedman EA, Hammes M, Stavroulopoulos A S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1218] [Cited by in F6Publishing: 1224] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 2. | Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S-991S. [PubMed] [Cited in This Article: ] |
| 3. | Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, Amodeo C, Cuppari L, Kamimura MA. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30:1718-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Liu JH, Chen JY, Lin SY, Lin HH, Ting IW, Liang CC, Wang IK, Kuo HL, Chang CT, Huang CC. Comparing Survival between peritoneal dialysis and hemodialysis patients with subclinical peripheral artery disease: a 6-year follow-up. Int J Med Sci. 2013;10:434-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 634] [Cited by in F6Publishing: 597] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 7. | Parnia S, Nasir A, Ahn A, Malik H, Yang J, Zhu J, Dorazi F, Richman P. A feasibility study of cerebral oximetry during in-hospital mechanical and manual cardiopulmonary resuscitation*. Crit Care Med. 2014;42:930-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, Brown C, Katz NM, Grams ME, Hogue CW. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med. 2013;41:464-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | McCusker K, Chalafant A, de Foe G, Gunaydin S, Vijay V. Influence of hematocrit and pump prime on cerebral oxygen saturation in on-pump coronary revascularization. Perfusion. 2006;21:149-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Calderon-Arnulphi M, Alaraj A, Amin-Hanjani S, Mantulin WW, Polzonetti CM, Gratton E, Charbel FT. Detection of cerebral ischemia in neurovascular surgery using quantitative frequency-domain near-infrared spectroscopy. J Neurosurg. 2007;106:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Boezeman RP, Kelder JC, Waanders FG, Moll FL, de Vries JP. In vivo measurements of regional hemoglobin oxygen saturation values and limb-to-arm ratios of near-infrared spectroscopy for tissue oxygenation monitoring of lower extremities in healthy subjects. Med Devices (Auckl). 2015;8:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | De Blasi RA, Luciani R, Punzo G, Arcioni R, Romano R, Boezi M, Menè P. Microcirculatory changes and skeletal muscle oxygenation measured at rest by non-infrared spectroscopy in patients with and without diabetes undergoing haemodialysis. Crit Care. 2009;13 Suppl 5:S9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Pipili C, Grapsa E, Tripodaki ES, Ioannidou S, Manetos C, Parisi M, Nanas S. Changes in skeletal muscle microcirculation after a hemodialysis session correlates with adequacy of dialysis. Int J Nephrol Renovasc Dis. 2015;8:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Claverias L, Marí M, Marín-Corral J, Magret M, Trefler S, Bodí M, García-España A, Yébenes JC, Pascual S, Gea J. The prognostic value of muscle regional oxygen saturation index in severe community-acquired pneumonia: a prospective observational study. J Intensive Care. 2016;4:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kawanishi H, Akiba T, Masakane I, Tomo T, Mineshima M, Kawasaki T, Hirakata H, Akizawa T. Standard on microbiological management of fluids for hemodialysis and related therapies by the Japanese Society for Dialysis Therapy 2008. Ther Apher Dial. 2009;13:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Nielsen AL, Thunedborg P, Brinkenfeldt H, Hegbrant J, Jensen HA, Wandrup JH. Assessment of pH and oxygen status during hemodialysis using the arterial blood line in patients with an arteriovenous fistula. Blood Purif. 1999;17:206-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276:H438-H445. [PubMed] [Cited in This Article: ] |
| 18. | Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310:102-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 203] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205-1213. [PubMed] [Cited in This Article: ] |
| 20. | Owen WF, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 939] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 21. | Ito K, Ookawara S, Ueda Y, Goto S, Miyazawa H, Yamada H, Kitano T, Shindo M, Kaku Y, Hirai K. Factors affecting cerebral oxygenation in hemodialysis patients: cerebral oxygenation associates with pH, hemodialysis duration, serum albumin concentration, and diabetes mellitus. PLoS One. 2015;10:e0117474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, Kaku Y, Hirai K, Nabata A, Mori H. Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract. 2014;126:57-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Miyazawa H, Ookawara S, Tabei K. Aggravation of Cerebral Oxygenation due to Intradialytic Hypotension Induced by Blood Volume Reduction During Hemodialysis: A Case Report. Ther Apher Dial. 2015;19:525-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6987] [Cited by in F6Publishing: 7599] [Article Influence: 542.8] [Reference Citation Analysis (0)] |
| 25. | Shuler MS, Reisman WM, Kinsey TL, Whitesides TE, Hammerberg EM, Davila MG, Moore TJ. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92:863-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Kalantar-Zadeh K, Cano NJ, Budde K, Chazot C, Kovesdy CP, Mak RH, Mehrotra R, Raj DS, Sehgal AR, Stenvinkel P. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7:369-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Hruska KA, Saab G, Mathew S, Lund R. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007;20:309-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 30. | Achinger SG, Ayus JC. Left ventricular hypertrophy: is hyperphosphatemia among dialysis patients a risk factor? J Am Soc Nephrol. 2006;17:S255-S261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Depner TA, Daugirdas JT. Equations for normalized protein catabolic rate based on two-point modeling of hemodialysis urea kinetics. J Am Soc Nephrol. 1996;7:780-785. [PubMed] [Cited in This Article: ] |
| 32. | Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000;15:953-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 533] [Cited by in F6Publishing: 495] [Article Influence: 20.6] [Reference Citation Analysis (0)] |