Published online Nov 6, 2016. doi: 10.5527/wjn.v5.i6.531
Peer-review started: May 11, 2016
First decision: June 14, 2016
Revised: August 2, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: November 6, 2016
To avoid desensitization protocols and ABO incompatible kidney transplantation (KT) due to high costs and increased risk of infections from intense immunosuppression.
We present institutional ethical review board - approved study of single center 6-way kidney exchange transplantation. The participants comprised ABO incompatibility (n = 1); positive cross-match and/or presence of donor specific antibody (n = 5). The average time required from registration in kidney paired donation (KPD) registry to find suitable donors was 45 d and time required to perform transplants after legal permission was 2 mo.
Graft and patient survival were 100%, and 100%, respectively. One patient had biopsy-proven acute borderline T cell rejection (Banff update 2013, type 3). Mean serum creatinine was 0.8 mg/dL at 9 mo follow-up. The waiting time in KPD was short as compared to deceased donor KT.
We report first non-simultaneous, single center, 6-way kidney exchange transplantation from India. Our experience will encourage other centers in India to undertake this practice.
Core tip: We report first non-simultaneous single center 6-way kidney exchange transplantation from India which has the potential to expand the living donor pool and increases kidney transplant opportunity for immunologically sensitized patients. Simultaneous transplant surgery is an accepted standard practice in kidney paired donation and should be encouraged. Non-simultaneous paired exchange transplants should be cautiously performed in carefully selected donors/recipient pairs with “due diligence” and legal permission from institutional ethical review board and written informed consent from the donors/recipients. Counseling to understand the risks and benefits of this procedure is mandatory.
- Citation: Kute VB, Patel HV, Varyani UT, Shah PR, Modi PR, Shah VR, Rizvi SJ, Pal BC, Shah PS, Wakhare PS, Ghodela VA, Shinde SG, Trivedi VB, Patel MH, Trivedi HL. Six end-stage renal disease patients benefited from first non-simultaneous single center 6-way kidney exchange transplantation in India. World J Nephrol 2016; 5(6): 531-537
- URL: https://www.wjgnet.com/2220-6124/full/v5/i6/531.htm
- DOI: https://dx.doi.org/10.5527/wjn.v5.i6.531
As per Indian chronic kidney disease (CKD) registry only 2% of end stage renal disease (ESRD) patients underwent kidney transplantation (KT). Due to economic constrains 61% patients did not receive any form of renal replacement therapy; 35% patients were on maintenance hemodialysis while only 5% on continuous ambulatory peritoneal dialysis. Economic constrains resulting in non-adherence to dialysis therapy leads to higher morbidity and mortality. In absence of national healthcare insurance scheme in India and in other South Asian countries, 75% of CKD patients were referred late in stage 5 CKD. Of these, 90% die within a month of diagnosis and majority (66%) of patients are unable to continue dialysis therapy beyond the first 3 mo due to financial reasons[1]. Every year 175000 new patients develop ESRD in India. Kidney transplantation rate is 3.25 per million populations (pmp). India does not have a robust deceased donor kidney transplant program (0.08 pmp); 90% of KT was from living donors.
Our center in Ahmedabad, India has pioneered kidney paired donation (KPD) transplantation over the last 10 years[2-6]. Two way single center KPD has the inherent limitation to increase transplant rate[2-4]. Domino paired donation (DPD) would increase the number of kidney exchange transplants by about 20%, depending on the size of the donor pool[7]. Herein, we report six ESRD patients who underwent the non-simultaneous single center 6-way kidney exchange transplantation. We believe this is the first of its’ kind in India.
The KPD allocation procedure was facilitated by a nephrologist supervised by ethical review board ensuring equitable allocation. In manual KPD allocation, we took into account the following: Bonus points were given to previous history of positive cross-match more than twice, history of failure to desensitization protocol, history of previous KT, dialysis time, HLA match, pediatric patients, O group patients, waiting time, and failure of vascular access for dialysis. All patients were matched with donors of similar age group. The difference between kidney exchange donors should be ≤ 10 years. All the patients exchanged kidney of similar quality (anatomical, functional and immunological similarity). There should be single renal artery and vein on side of donor nephrectomy, measured glomerular filtration rate ≥ 90 mL/min per 1.73 m2 using Tc99m DTPA renal scan should be considered as an acceptable level of kidney function for kidney donation.
We demonstrated absence of the donor specific antibody (DSA) in the each recipient to mismatched antigens of intended donor using data of blood groups, 14 points HLA antigens of donors and recipients and recipients’ HLA antibody specificities (by one Lambda single antigen bead assay). Thereafter, actual testing of complement-dependent cytotoxicity (CDC)-lymphocyte cross-match (LCM), flow cross match (FCM), were performed for every patient with the intended donor in transplant workup. It was mandated that all three test (LCM, FCM and DSA) should be negative prior to assigning the final KPD donor.
Induction immunosuppression consisted of methyl prednisolone 500 mg for 3 d and rabbit thymoglobulin (1-2 mg/kg) and maintenance immunosuppression consisted of tacrolimus (target level 8-10 ng/mL in first 3 mo and 4-7 ng/mL thereafter), prednisolone 20 mg/d and tapered to 10 mg at 3 mo and thereafter) and mycophenolate sodium 1080-1440 mg/d.
The clinical study has been reviewed by the ethics committee according to international standards of Good Clinical Practice as well as according to local laws and regulations (transplant human organ act, India). We also abide by the Declaration of Helsinki and Declaration of Istanbul principles. The average monthly family income of the 6 ESRD patients was approximately 100 USD. The cost of kidney transplant in our transplant center is approximately 5000 USD. Two patients received 100% economic support and four patients received 50% economic support from the Government funds for the transplantation. Transplants were done irrespective of the recipient’s financial status. All the kidney donors were family members (spouse or parents) and there did not receive any compensation for kidney donation.
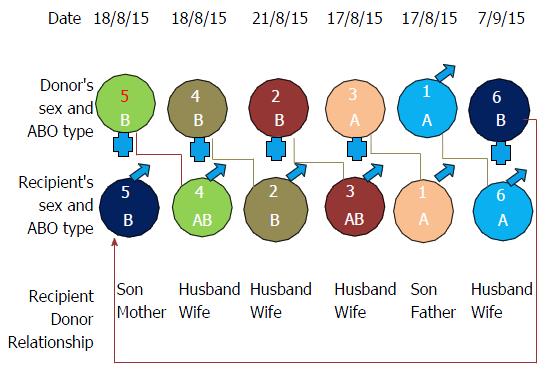
Figure 1 shows variables of the 6-way kidney exchange transplantation. Table 1 shows the demographics of 6 ESRD patients. All were male recipient, 5 donors were females and one donor was male. This gender imbalance in recipient and donors is common in our transplant scenario and not limited to KPD. Reasons for enrollment of donor recipient pair (DRP) in this program included ABO incompatibility (n = 1); positive cross-match and/or DSA (n = 5). The average time required from registration in our single center KPD registry to find KPD donor was 45 d and time required to perform actual KT after legal permission was 2 mo. Table 2 shows our desensitization protocol and their outcome. Initially, 3 patients who were sensitized were not willing to participate in KPD so we attempted desensitization protocol. Unfortunately, they did not responded to desensitization protocol and were willing to participate in KPD. Two patients joined KPD without undergoing desensitization therapy as due to the high cost. We initially planned simultaneous KT surgery of 6 pairs which was postponed twice; due to respiratory infection in patient no 1, 3 and on another occasion due to respiratory infection in patient no 2, 6. Later with written informed consent of the 6 pairs, we performed first non-simultaneous KT on 4 different days as shown in Figure 1.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| Age (yr) | 17 | 38 | 45 | 40 | 25 | 45 |
| Gender | Male | Male | Male | Male | Male | Male |
| Original disease | CGN | HT | HT | ADPKD | HT | DM |
| ABO group | A | B | AB | AB | B | A |
| Dialysis | 12 mo | 8 mo | 7 mo | 18 mo | 7 mo | 3 mo |
| Weight (kg) | 40 | 44 | 59 | 68 | 43 | 55 |
| Original donor relation | Father | Wife | Wife | Wife | Mother | Wife |
| Reason for joining KPD | ||||||
| Sensitized | Yes | Yes | Yes | Yes | Yes | No |
| ABO incompatible | Yes | |||||
| Time from KPD registration to find donor | 2 wk | 1 d | 2 wk | 6 mo | 4 wk | 1 mo |
| Time from donor to KT | 58 d | 60 d | 55 d | 75 d | 48 d | 60 d |
| With original donor | ||||||
| LCM (%) | 80/80/90 | 5/5/7 | 5/0/5 | 80/80/90 | 12/7/25 | |
| FCM (MCS) | 260/298 | Neg/236 | 80/159 | 150/250 | N/N | |
| DSA class 1 (MFI) | A1 = 8000 | No DSA | B44 = 4188 | A11 = 10000 | BW4 = 5000 | |
| B57 = 6200 | A31 = 1100 | B57 = 12645 | ||||
| BW4 = 12645 | ||||||
| DSA class 2 (MFI) | DQ7 = 1600, DQ9 = 2200 | DQ2 = 9000 | No DSA | No DSA | DR13 = 7000 | |
| With cross donor | ||||||
| LCM (%) | Neg/neg/neg | 15/15/20 | Neg/neg | Neg/Neg/Neg | 5/12/2010 | Neg/Neg/Neg |
| FCM (MCS) | Neg/neg | Neg/200 | Neg/neg | Neg/Neg | Neg/Neg | Neg/Neg |
| DSA class 1 | No DSA | No DSA | No DSA | No DSA | No DSA | No DSA |
| DSA class 2 | No DSA | No DSA | No DSA | No DSA | No DSA | No DSA |
| Desensitization | Yes | Yes | Yes | No | No | No |
| State | Gujarat | Rajasthan | Gujarat | Gujarat | Rajasthan | Rajasthan |
| Patient 1 | Patient 2 | Patient 3 | |
| Before desensitization with original donor | |||
| LCM/DTT/AHG (%) | 80/80/90 | 5/5/7 | 5/0/5 |
| T/B FCM (MCS) | 260/298 | Neg/236 | 80/159 |
| DSA class 1 | A1 = 8000, B57 = 6200 | No DSA | B44 = 4188, A31 1100 |
| DSA class 2 | DQ7 = 1600, DQ9 = 2200 | DQ2 = 9000 | No DSA |
| DP | 8TPE + 8Bort/MP/IVIG + TAC/MMF | 9TPE + 6Bort/MP/IVIG + R + TAC/MMF | 4TPE + 4Bort/MP/IVIG + TAC/MMF |
| After desensitization with original donor | |||
| LCM | 80/80/90 | 17/15/20 | 5/0/7 |
| FCM | 254/333 | Neg/504 | Neg/129 |
| DSA class 1 | A1 = 2000, B57 = 5500 | No DSA | B44 = 4622, A31 = 1400 |
| DSA class 2 | DQ7 = 2000, DQ9 = 2000 | DQ2 = 12000 | No DSA |
| Side effects | Tuberculosis | Graft nephrectomy | Tuberculosis |
Table 3 showed HLA data of 6 DRP. Table 4 showed the demographic data of 6 donors. All donors underwent uneventful left laparoscopic nephrectomy.
| A | B | Bw | Cw | DR B1 | DR B3,4,5 | DQ B1 | ||||||||
| P1 | 2 | 29 | 44 | - | 4 | - | 7 | 15 | 4 | 15 | 51 | 53 | 4 | 6 |
| D1 | 1 | 29 | 44 | 57 | 4 | - | 6 | 15 | 7 | 15 | 51 | 53 | 6 | 9 |
| P2 | 2 | 33 | 40 | 44 | 4 | 6 | 7 | 15 | 10 | 15 | 51 | - | 5 | 6 |
| D2 | 33 | - | 8 | 58 | 4 | 6 | 3 | 7 | 17 | - | 52 | - | 2 | - |
| P3 | 2 | 11 | 35 | 52 | 4 | 6 | 12 | - | 4 | 15 | 51 | 53 | 6 | 8 |
| D3 | 31 | 33 | 44 | 52 | 4 | - | 7 | 12 | 4 | 15 | 51 | 53 | 6 | 8 |
| P4 | 24 | 32 | 18 | 35 | 6 | - | 4 | 7 | 11 | 15 | 51 | 52 | 6 | 7 |
| D4 | 11 | 32 | 8 | 57 | 4 | 6 | 6 | 7 | 13 | 15 | 51 | 52 | 6 | - |
| P5 | 24 | 33 | 8 | 35 | 6 | - | 4 | 7 | 17 | - | 52 | - | 2 | - |
| D5 | 24 | 32 | 8 | 49 | 4 | 6 | 7 | - | 13 | 17 | 52 | - | 2 | 6 |
| P6 | 11 | 24 | 13 | 56 | 4 | 6 | 3 | 4 | 15 | 17 | 51 | 52 | 2 | 5 |
| D6 | 1 | 68 | 75 | 70 | 6 | 0 | 7 | 8 | 10 | 11 | 52 | 0 | 5 | 7 |
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | Donor 6 | |
| Age (yr) | 46 | 38 | 40 | 38 | 45 | 35 |
| Gender | Male | Female | Female | Female | Female | Female |
| Weight (kg) | 44 | 50 | 50 | 50 | 60 | 82 |
| ABO group | A1 | B | A | B | B | B |
| GFR (R/L) | 54/61 | 50/50 | 47/55 | 55/55 | 56/50 | 52/49 |
| Creatinine (mg/dL) | 0.8 | 0.7 | 0.5 | 0.6 | 0.6 | 0.8 |
| Renal vessel (R/L) | 2A2V/1A1V | 1A1V/1A1V | 1A1V/1A1V | 1A1V/2A1V | 1A2V/1A1V | 1A1V/1A1V |
| LDN side | L | L | L | L | L | L |
Table 5 showed the surgical details and outcome. Mean anastomosis time was 27 min, 3 patients underwent robotic KT and 3 underwent open KT. Graft and patient survival was 100% and 100%, respectively, with mean serum creatinine of 0.8 mg/dL at 9 mo follow-up. The allograft biopsy-proven acute borderline T cell rejection (Banff update 2013, type 3) was noted in a single patient who received lower dose of thymoglobulin due to pre-transplant pulmonary infection. We routinely did not perform protocol allograft biopsy. However, rejection episode responded well to methyl prednisolone (500 mg × 3 doses) with stable graft function at 9 mo follow-up. Two of the 3 patients who underwent pre-transplant desensitization developed more infections [tuberculosis (n = 2), urinary tract infection (n = 2)] and new onset diabetes after transplantation vs patients who did not underwent pre-transplant desensitization.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
| WIT (s) | 113 | 120 | 137 | 97 | 136 | 85 |
| CIT (min) | 50 | 150 | 85 | 136 | 73 | 60 |
| AT (min) | 31 | 27 | 24 | 40 | 25 | 14 |
| UO (mL) | 1100 | 1200 | 600 | 700 | 1000 | 950 |
| Robotic KT | Yes | Yes | Yes | |||
| Open KT | Yes | Yes | Yes | |||
| LDN | Yes | Yes | Yes | Yes | Yes | Yes |
| KT date (2015) | 17-Aug | 21-Aug | 17-Aug | 18-Aug | 18-Aug | 7-Sep |
| Thymoglobulin (mg) | No | 50 | 25 | 75 | 75 | 75 |
| Creatinine (mg/dL) | 0.7 | 0.7 | 1 | 0.7 | 1 | 1.1 |
As of August 2015, all the KPD transplants (103 two-way and 9 three-way exchanges in 233 patients) in our center have been performed simultaneously to eliminate the risk of reneging by the intended donor of the recipient who had already received a kidney from a matched donor. We have performed 27 patients in 3 way simultaneous KPD transplants as DRP were not willing for the non-simultaneous KT and this will also avoid the risk of donor reneging. However, from our previous experience of 10 KPD transplants (5 pairs of 2-way exchanges) in our single center in single day (on world kidney day 2013); our surgical team proposed that simultaneous KT of more than five-way exchange in KPD transplants is very difficult to perform logistically in our single center[6]. In addition, patients developed infections leading to break in simultaneous KT twice.
It is difficult to do simultaneous living donor kidney transplantation (LDKT) beyond the 2-way exchange in the Indian setting due to shortage of surgical staff. Single center non-simultaneous KPD or multicenter simultaneous KPD are the two options to overcome this problem. The later way may involve donor travel or shipping of kidney. When cold ischemia time (CIT) is < 8 h, it has no impact on long term graft survival[8,9]. Therefore, transport of living donor kidney may be a feasible alternative to donor travel in multicenter simultaneous KPD program where CIT < 8 h[8,9]. Australian program preferred to ship donor kidneys rather than the donor despite prolonged CIT for interstate exchanges[10]. In India, there is only one report of multicenter simultaneous KPD of 5 DRP[11]. Therefore, single center non-simultaneous KT is the alternative way to overcome this problem. The practical solution to perform long chain KPD transplant is non-simultaneous KT after written informed consent of pairs and proper selection of patients and donors. There are two possible problems in doing non-simultaneous KT; donor reneging and if recipient develops a medical issue, which could jeopardize the chain. We have done proper counseling for the pairs to avoid donor reneging and one can have the option of standard criteria young deceased donor KT without waiting time in case of donor reneging. If donor of a patient donated kidney and that patient becomes un-transplantable due to any reason (infection or heart disease) or patient expires, this would be quite unfair and may lead to legal and ethical problems. The morbidity and mortality on maintenance hemodialysis is high in the Indian scenario, and therefore, all the patients were kept in-house before KT.
Simultaneous transplant surgery is an accepted standard practice in KPD and should be encouraged. Performing non-simultaneous surgeries in a closed loop KPD has the risk of one donor reneging and a recipient missing out on a kidney transplant, when their original donor has already donated a kidney. Non-simultaneous paired exchange transplants should be cautiously performed in carefully selected donors/recipient pairs with “due diligence” after legal permission from institutional ethical review board and written informed consent from the donors/recipients and proper counseling about risk and benefits is mandatory.
While non-simultaneous KPD chain transplantation is routine in North America and Europe, but uncommon in India. Apex Swap Transplant registry (ASTRA), Mumbai performed Indian first multicenter simultaneous KPD chain transplantation surgery of 5 DRP on the 25th of June 2013 in three hospitals in Mumbai with team work after legal permission from the Maharashtra government[11]. The waiting time between patient enrollment and transplantation was 2 years due to long time required in taking the legal permission and the second attempt[11]. The first attempt resulted in failure and collapse of the chain due to the death of a patient. In the second attempt, they required 8 mo to rebuild a new chain (only one DRP was common). If the DPD transplant had not been carried out, some of them would have had to wait indefinitely and the others, for a long time. ASTRA has reported 30 two-way exchanges and 3 domino chain KT. This successful domino chain KT opens a new avenue to many more such dominos across the country giving an opportunity of getting a well-matched kidney to the patients who would otherwise land up on dialysis.
Matching at the single-center KPD program would eliminate need of donor travel, donor separation from patient, close family members and familiar transplant team, increased cold ischemia time associated with transport of kidneys, the need for harmony and cooperation between different transplant centers, standardization of protocols between center for medical selection of donor and patients, privacy and legal concerns.
Domino paired donation would facilitate more transplants by contributing to KPD programs than 2-way KPD. The advantages are expand blood type distribution of donors, increase number of transplants, better quality of transplants (HLA match, age of donor/recipient, waiting time, hard to transplant patients), and relaxes the reciprocity requirement of KPD. DPD would increase the number of transplants by about 20%, depending on the size of the pool[12]. DPD is ideal in balancing the principles of utility and justice[12,13]. A non-directed donor into non-simultaneous extended chain is not superior to DPD when segment lengths are limited to three[14].
Even in KPD programs in which mathematical optimization are applied, more than 50% of the incompatible pairs in the pool remain unmatched. In many cases, pools of incompatible donor-recipient pairs have a high proportion of patients with blood types that are hard to match and those with HLA sensitization[15].
In one South Korean center, 179 LDKT were performed, with 70 domino chains initiated by an altruistic living non-directed donor. One-year and 5-year patient and graft survival rates were 97.2% and 90.8%, and 98.3% and 87.7%, respectively, with a median follow-up of 46 mo. Multicenter domino KPD increases access to LDKT, with patients and graft survival rates similar to conventional KPD[16].
Long time is required to take legal permission from different state governments when 2 pairs are from different state. This is the biggest hurdle for multicenter KPD expansion in India. Transplantation of Human Organs Act (THOA) and rules in India were amended in 2011 to promote organ transplantation, including KPD and KPD from near relatives is legal in India[17]. According to THOA 2013, cases of swap donation referred to under subsection 3A of section 9 of the act shall be approved by authorization committee of hospital or district or state in which transplantation is proposed to be done and donation of organs shall be permissible only from near relatives of the swap recipients. This is new hope to reduce waiting time required to take legal permission from different state authorization committee. It will also increase the participation of compatible pairs to increase transplant quality like better HLA matching in spousal donors and young donor. In our study we have taken permission of 2 different state governments before KT.
The published literature on desensitization and outcome are from single-center studies with small sample size, short term follow-up and variable outcome[18-20]. Multi-centric, prospective, randomized and controlled studies are needed for the evaluation of the efficacy and safety of desensitization protocols. Patients who underwent KT after desensitization therapy are often complicated by high rates of early acute humoral rejection and late antibody mediated injury due to low titers of DSA. Despite acceptable short-term patient and graft survivals, acute rejection rate and acute antibody-mediated rejection rate (36%, 28%, respectively), were significantly higher than in non-sensitized patients.
In our domino chain, 3 patients did not responded to desensitization protocols and 2 developed tuberculosis. Patients who are difficult in desensitization protocols (high PRA, high-strength DSA) and difficult in KPD [non-O donor (like AB), O recipient] can be considered for the combination of desensitization and KPD to increase match rate[20]. CDC T and B cell cross-match-positive patients should not be considered for desensitization if they have more than three DSA and one DSA with MFI values > 5000.
In conclusion, we report first successful non-simultaneous single center 6-way kidney exchange transplantation from India. This procedure has the potential to expand the living donor pool and increases transplant opportunity for the sensitized patients.
Kidney paired donation (KPD) is routinely practiced in the Western countries, but is now being slowly introduced in India. The authors avoid desensitization protocols and ABO incompatible kidney transplantation (KT) due to high costs and increased risk of infections from intense immunosuppression.
It is difficult to do simultaneous living donor KT beyond the 2-way exchange in the Indian setting due to shortage of surgical staff. Single center non-simultaneous KPD or multicenter simultaneous KPD are the two options to overcome this problem. This successful non-simultaneous single center 6-way kidney exchange transplantation from India opens a new avenue to many more such KPD chain across the country giving an opportunity of getting a well-matched kidney to the patients who would otherwise land up on dialysis.
While non-simultaneous KPD chain transplantation is routine in North America and Europe, but uncommon in India.
Simultaneous transplant surgery is an accepted standard practice in kidney paired donation and should be encouraged. Non-simultaneous paired exchange transplants should be cautiously performed in carefully selected donors/recipient pairs. Counseling to understand the risks and benefits of this procedure is mandatory. Their experience will encourage other centers in India to undertake this practice.
They report first non-simultaneous single center 6-way kidney exchange transplantation from India which has the potential to expand the living donor pool and increases kidney transplant opportunity for immunologically sensitized patients.
In general, the draft is interesting and had been good written.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chang CC, Friedman EA, Taheri S S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Abraham G. The challenges of renal replacement therapy in Asia. Nat Clin Pract Nephrol. 2008;4:643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 247] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Kute VB, Patel HV, Shah PR, Vanikar AV, Trivedi HL. National kidney paired donation programme in India: Challenges, solution, future direction. Nephrology (Carlton). 2015;20:442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Kute VB, Shah PS, Vanikar AV, Gumber MR, Patel HV, Engineer DP, Shah PR, Modi PR, Shah VR, Rizvi SJ. Increasing access to renal transplantation in India through our single-center kidney paired donation program: a model for the developing world to prevent commercial transplantation. Transpl Int. 2014;27:1015-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Modi PR, Trivedi HL. Facilitators to national kidney paired donation program. Transpl Int. 2013;26:e38-e39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Kute VB, Gumber MR, Patel HV, Shah PR, Vanikar AV, Modi PR, Shah VR, Patel MP, Trivedi HL. Outcome of kidney paired donation transplantation to increase donor pool and to prevent commercial transplantation: a single-center experience from a developing country. Int Urol Nephrol. 2013;45:1171-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Engineer DP, Modi PR, Rizvi SJ, Shah VR, Modi MP. Ten kidney paired donation transplantation on World Kidney Day 2013: raising awareness and time to take action to increase donor pool. Ren Fail. 2013;35:1269-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gentry SE, Montgomery RA, Swihart BJ, Segev DL. The roles of dominos and nonsimultaneous chains in kidney paired donation. Am J Transplant. 2009;9:1330-1336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Segev DL, Veale JL, Berger JC, Hiller JM, Hanto RL, Leeser DB, Geffner SR, Shenoy S, Bry WI, Katznelson S. Transporting live donor kidneys for kidney paired donation: initial national results. Am J Transplant. 2011;11:356-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 9. | Simpkins CE, Montgomery RA, Hawxby AM, Locke JE, Gentry SE, Warren DS, Segev DL. Cold ischemia time and allograft outcomes in live donor renal transplantation: is live donor organ transport feasible? Am J Transplant. 2007;7:99-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Allen R, Pleass H, Clayton PA, Woodroffe C, Ferrari P. Outcomes of kidney paired donation transplants in relation to shipping and cold ischaemia time. Transpl Int. 2016;29:425-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Travasso C. Five patients benefit from India’s first “domino” kidney swap. BMJ. 2013;347:f4260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ashlagi I, Gilchrist DS, Roth AE, Rees MA. Nonsimultaneous chains and dominos in kidney- paired donation-revisited. Am J Transplant. 2011;11:984-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Montgomery RA, Gentry SE, Marks WH, Warren DS, Hiller J, Houp J, Zachary AA, Melancon JK, Maley WR, Rabb H. Domino paired kidney donation: a strategy to make best use of live non-directed donation. Lancet. 2006;368:419-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Gentry SE, Segev DL. The honeymoon phase and studies of nonsimultaneous chains in kidney-paired donation. Am J Transplant. 2011;11:2778-2779; author reply 2780-2781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA. 2005;293:1883-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 326] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 16. | Lee YJ, Lee SU, Chung SY, Cho BH, Kwak JY, Kang CM, Park JT, Han DJ, Kim DJ. Clinical outcomes of multicenter domino kidney paired donation. Am J Transplant. 2009;9:2424-2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Agarwal SK, Srivastava RK, Gupta S, Tripathi S. Evolution of the Transplantation of Human Organ Act and law in India. Transplantation. 2012;94:110-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Marfo K, Lu A, Ling M, Akalin E. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Lemy A, Toungouz M, Abramowicz D. Bortezomib: a new player in pre- and post-transplant desensitization? Nephrol Dial Transplant. 2010;25:3480-3489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Montgomery RA, Lonze BE, Jackson AM. Using donor exchange paradigms with desensitization to enhance transplant rates among highly sensitized patients. Curr Opin Organ Transplant. 2011;16:439-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |