Copyright
©2014 Baishideng Publishing Group Inc.
World J Pharmacol. Dec 9, 2014; 3(4): 120-139
Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.120
Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.120
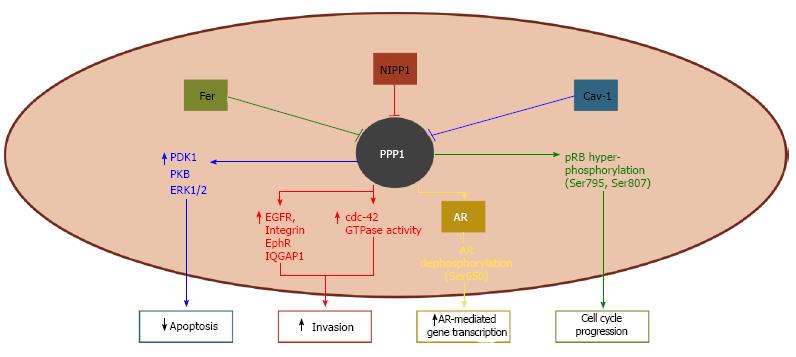
Figure 1 Phosphoprotein phosphatase-1 complexes described in human prostate cancer.
Phosphoprotein phosphatase-1 (PPP1) dephosphorylates pRb at Ser795 and Ser807 and contributes to its hypophosphorylated and activated state. The activity of Fer tyrosine kinase leads to the inhibition of PPP1 and consequent hyperphosphorylation of pRb, which culminate in poor G1-S transition control. The inhibition of PPP1 by the nuclear inhibitor of protein phosphatase 1 (NIPP1) increases the expression of epidermal growth factor receptor (EGFR), integrin, epherin receptor (EphR), and Ras GTPase-activating-like protein IQGAP1, as well as enhances the activity of cdc-42 GTPase. This, in turn, promotes invasiveness of tumor cells. Caveolin-1 (Cav-1) inhibits PPP1 and potentiates the activity of phosphoinositide-dependent kinase-1 (PDK1), protein kinase B (PKB), and extracellular signal-regulated kinase 1/2 (ERK1/2), increasing cell survival. PPP1 specifically dephosphorylates androgen receptor (AR) at Ser650, thus inhibiting AR nuclear export and enhancing AR-mediated gene transcription.
- Citation: Felgueiras J, Fardilha M. Phosphoprotein phosphatase 1-interacting proteins as therapeutic targets in prostate cancer. World J Pharmacol 2014; 3(4): 120-139
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/120.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.120