Published online May 8, 2017. doi: 10.5409/wjcp.v6.i2.110
Peer-review started: January 10, 2017
First decision: February 17, 2017
Revised: March 4, 2017
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: May 8, 2017
To describe our institutional experience with conversion from intravenous (IV) fentanyl infusion directly to enteral methadone and occurrence of withdrawal in critically ill mechanically ventilated children exposed to prolonged sedation and analgesia.
With Institutional Review Board approval, we retrospectively studied consecutively admitted invasively mechanically ventilated children (0-18 years) sedated with IV fentanyl infusion > 5 d and subsequently converted directly to enteral methadone. Data were obtained on subject demographics, illness severity, daily IV fentanyl and enteral methadone dosing, time to complete conversion, withdrawal scores (WAT-1), pain scores, and need for rescue opioids. Patients were classified as rapid conversion group (RCG) if completely converted ≤ 48 h and slow conversion group (SCG) if completely converted in > 48 h. Primary outcome was difference in WAT-1 scores at 7 d. Secondary outcomes included differences in overall pain scores, and differences in daily rescue opioids.
Compared to SCG (n = 21), RCG (n = 21) had lower median WAT-1 scores at 7 d (2.5 vs 5, P = 0.027). Additionally, RCG had lower overall median pain scores (3 vs 6, P = 0.007), and required less median daily rescue opioids (3 vs 12, P = 0.003) than SCG. The starting daily median methadone dose was 2.3 times the daily median fentanyl dose in the RCG, compared to 1.1 times in the SCG (P = 0.049).
We observed wide variation in conversion from IV fentanyl infusion directly to enteral methadone and variability in withdrawal in critically ill mechanically ventilated children exposed to prolonged sedation. In those children who converted successfully from IV fentanyl infusion to enteral methadone within a period of 48 h, a methadone:fentanyl dose conversion ratio of approximately 2.5:1 was associated with less withdrawal and reduced need for rescue opioids.
Core tip: Critically ill children exposed to prolonged opioid infusions for sedation and analgesia frequently experience withdrawal symptoms when these infusions are discontinued. Conversion to intermittent opioids such as methadone may reduce such withdrawal symptoms, but published studies and guidelines vary widely in terms of dosing and timeframes for such conversions. In this pragmatic analysis of current practice in our institution, we observed wide variation in dosing conversion and timeframes. We observed that it is feasible to convert from intravenous fentanyl infusion directly to enteral methadone within a timeframe of 48 h using a methadone:fentanyl dose conversion ratio of approximately 2.5:1 to minimize withdrawal and reduce need for rescue opioids.
- Citation: Srinivasan V, Pung D, O’Neill SP. Conversion from prolonged intravenous fentanyl infusion to enteral methadone in critically ill children. World J Clin Pediatr 2017; 6(2): 110-117
- URL: https://www.wjgnet.com/2219-2808/full/v6/i2/110.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v6.i2.110
Children admitted to the pediatric intensive care unit (PICU) are often administered opioids in the form of intravenous (IV) infusions to provide consistent sedation and analgesia titrated to effect[1,2]. Tolerance and physical dependence frequently develop with prolonged opioid use resulting in an increased likelihood of developing a withdrawal syndrome when the IV opioid infusion is abruptly discontinued[3]. Withdrawal is frequently associated with neurologic, autonomic and gastrointestinal abnormalities, which can result in considerable morbidity with prolongation of PICU and hospital length of stay[4]. The risk of withdrawal increases depending on the cumulative dose exposure as well as the duration of infusion[3,4]. For example, a cumulative IV fentanyl dose of at least 1.5 milligrams per kilogram (mg/kg) or 5 d of IV infusion has been associated with a 50% risk of developing withdrawal symptoms when the IV fentanyl infusion was rapidly weaned over 2 d[4]. This risk increases to 100% when the patient has received a cumulative dose of at least 2.5 mg/kg or 9 d of continuous IV fentanyl infusion[4]. Withdrawal may be avoided or attenuated during recovery either by slowly tapering the IV infusion, or by conveniently substituting the IV opioid infusion with IV or enteral opioids that are then tapered slowly over a period of time.
Methadone is a commonly used synthetic opioid for weaning critically ill children off IV opioid infusions due to its long half-life, good oral bioavailability and low cost[5-9]. Methadone is available for administration in both IV and enteral forms. However, concerns with use of methadone include lack of pharmacokinetic data in children, significant interactions with other drugs, and increased risk of electrocardiographic abnormalities such as QTc prolongation[10-12]. Importantly, there is a lack of consensus on an optimal dosing guideline for conversion from IV fentanyl infusions directly to enteral methadone (or via IV methadone as an intermediate step). A variety of studies have documented varying methadone:fentanyl conversion ratios (ranging from 1:1 to 4:1) and time frames (ranging from 24-48 h or longer) during conversion from IV fentanyl infusion to enteral methadone[6-9,13-16]. We undertook this study to describe our institutional experience with conversion from IV fentanyl infusion directly to enteral methadone and occurrence of withdrawal in critically ill children exposed to prolonged IV fentanyl infusion for sedation and analgesia. A secondary objective of our study was to derive an optimal dose conversion ratio of methadone:fentanyl associated with minimal withdrawal when converting from IV fentanyl infusion completely and directly to enteral methadone within a 48-h timeframe.
With Institutional Review Board approval and waiver of informed consent, we retrospectively reviewed the medical records of consecutive children admitted to our PICU between November 2004 and February 2008. Patients were included if they were between 0-18 years of age, invasively mechanically ventilated via endotracheal tube or tracheostomy, on IV fentanyl infusion for more than 5 d and started on scheduled enteral methadone with the intention to wean off the IV fentanyl infusion completely. Patients with “Do not attempt resuscitation” status, burns, malignancy, chronic pain syndromes, or prior opioid use for more than 7 d in the 3 mo preceding admission to the PICU were excluded. Children undergoing cardiac surgery and neonates are cared for in other intensive care units separate from the PICU at our institution and were not eligible for this study. The pharmacy computer system database and the hospital electronic health record systems were screened for eligible subjects. Data collected included demographic information on age, weight, gender and diagnoses, severity of illness expressed as pediatric risk of mortality (PRISM III) scores[17], daily IV fentanyl and enteral methadone dosing, duration and adjustments of IV fentanyl infusion, time to conversion from IV fentanyl infusion to enteral methadone, administration of opioid rescues, and use of concomitant sedative and analgesic medications (benzodiazepines, barbiturates, clonidine, acetaminophen, non-steroidal anti-inflammatory drugs, and neuromuscular blockers).
All patients were monitored for opioid withdrawal symptoms using the Withdrawal Assessment Tool-Version 1 (WAT-1)[18]. The WAT-1 scale ranges from 0 to 12, with higher scores indicating more withdrawal symptoms. All patients were also monitored for pain during this period using pain scales depending on patient age and verbal/cognitive capacity: We used the Face, Legs, Activity, Cry, and Consolability scale in nonverbal children 0 to 6 years of age; the Individualized Numeric Rating Scale in nonverbal cognitively impaired children aged 6 years or older; and the Wong-Baker Faces Pain Scale in verbal children aged 3 years or older[19-21]. All pain scales range from 0 to 10, with higher scores indicating more pain. Data on WAT-1 scores were abstracted at 12, 24, 48, 72, 96 h and 7 d from the time of enteral methadone initiation. Data on overall pain scores were abstracted during the 7 d from initial enteral methadone initiation.
During the study period, critically ill children requiring invasive mechanical ventilation in our PICU were typically provided sedation and analgesia with IV fentanyl infusions in combination with other agents. Patients who were administered IV fentanyl infusions for prolonged periods (typically greater than 5 d) were usually switched to enteral methadone administered every 12 h during recovery to manage dependence and prevent symptoms of withdrawal. The initial dose conversion from IV fentanyl infusion to enteral methadone was determined by the clinical team based on clinical judgment and in discussion with the Clinical Pharmacist. The suggested time frame for conversion from IV fentanyl infusion to enteral methadone was usually 48 h, but was not standardized and left to attending physician discretion. After the second dose of enteral methadone, the IV fentanyl infusion was decreased by 50%. After the third dose of enteral methadone, the IV fentanyl infusion was decreased by a further 50%. After the fourth dose of enteral methadone, the IV fentanyl infusion was typically discontinued. Thereafter, the dosing of enteral methadone was adjusted by the attending physician to prevent both withdrawal symptoms as well as over-sedation.
Patients were classified into the rapid conversion group (RCG) if they were completely converted from IV fentanyl infusion to enteral methadone in 48 h or less, or the slow conversion group (SCG) if they were completely converted from IV fentanyl infusion to enteral methadone in more than 48 h. The primary outcome measure was difference in WAT-1 scores between the RCG and the SCG at 7 d from the time of enteral methadone initiation. Secondary outcome measures were differences in WAT-1 scores at 12, 24, 48, 72 and 96 h from the time of enteral methadone initiation, as well as overall WAT-1 and overall pain scores during the 7 d from enteral methadone initiation. Additional secondary outcomes included differences in ventilator free days at 28 d (VFD), PICU length of stay (LOS), and use of daily rescue opioids and concomitant medications.
Statistical analysis was performed using Stata 12.0 software (StataCorp, College Station, TX). Standard descriptive summaries were reported for baseline demographic data. The data were presented as mean ± SD if normally distributed, or median with inter-quartile range (IQR) if not normally distributed. Differences between the RCG and the SCG were compared using the t-test (in the case of continuous variables that were normally distributed) or the Mann-Whitney U test (in the case of continuous variables that were not normally distributed). Differences in categorical variables were compared using the χ2 test or Fisher’s exact test. Differences at respective paired time points for WAT-1 and pain scores between the RCG and the SCG were compared using the Mann-Whitney U test (as these were rank ordered). A P-value of less than 0.05 was considered statistically significant. Statistical methods and analysis were completed by Srinivasan V (first author of the study and trained in analytical methods via University of Pennsylvania biostatistics certificate courses).
A total of forty-two children were included in the study: 21 (50%) in the RCG and 21 (50%) in the SCG. The median time to complete conversion from IV fentanyl infusion to enteral methadone in the RCG was 25 h (IQR 19-34 h), while the median time to complete conversion in the SCG was 109 h (IQR 77-240 h, P < 0.05). Both groups were comparable with regard to baseline characteristics, including severity of illness and admitting diagnosis (Table 1). There were no significant differences in initial fentanyl infusion dose, duration of fentanyl infusion, maximum dose of fentanyl infusion or cumulative dose of fentanyl prior to conversion between the two groups. Table 2 compares the two groups during conversion from IV fentanyl infusion to enteral methadone. Compared with the SCG, the RCG required fewer rescue opioids in the first 96 h of transition per patient and fewer increases in the scheduled dose of enteral methadone. There were no significant differences between the use of concomitant sedative and analgesic medications across the groups.
| Rapid conversion groupa (n = 21) | Slow conversion groupb (n = 21) | P value | |
| Age, yr (median, IQR) | 1 (0.3-3.5) | 2 (0.8-4) | 0.95 |
| Gender, male (%) | 14 (67%) | 9 (43%) | 0.21 |
| Weight, kg (median, IQR) | 10 (5.5-14.3) | 9.6 (6.8-15.9) | 0.88 |
| PRISM III (mean ± SD) | 11.4 ± 9 | 16.1 ± 9.9 | 0.13 |
| Admitting diagnosis, n (%) | 1 | ||
| ARDS/acute lung injury | 14 (67) | 14 (67) | |
| Other (sepsis, seizures) | 7 (33) | 7 (33) | |
| Pre-existing tracheostomy, n (%) | 6 (29) | 6 (29) | 1 |
| Duration of IV fentanyl infusion prior to initiation of enteral methadone, d (median, IQR) | 9 (8-14) | 10 (8-21) | 0.48 |
| Maximum dose of IV fentanyl infusion, μg/kg per hour (median, IQR) | 6 (4-7) | 6.75 (4-9.25) | 0.41 |
| Cumulative dose of IV fentanyl infusion at time of initiation of enteral methadone, mg/kg (median, IQR) | 1.48 (1.11-1.92) | 1.64 (1.03-1.98) | 0.49 |
| Concomitant sedative and analgesic infusions | 0.61 | ||
| Benzodiazepine, n (%) | 18 (86) | 20 (95) | |
| Ketamine, n (%) | 0 (0) | 0 (0) | |
| Dexmedetomidine, n (%) | 0 (0) | 0 (0) |
| Rapid conversion groupa (n = 21) | Slow conversion groupb (n = 21) | P value | |
| Dose of IV fentanyl infusion at initiation of enteral methadone, μg/kg per hour (median, IQR) | 4 (3-4) | 4.5 (3.6-7) | 0.23 |
| Adjustments in scheduled enteral methadone dose | < 0.05 | ||
| Increase in dose | 15 | 33 | |
| Decrease in dose | 17 | 3 | |
| Opioid rescues in first 96 h of transition per patient (median, IQR) | 3 (1-7) | 12 (4-17) | < 0.05 |
| 0-24 h | 0 (0-2) | 3 (0-4) | < 0.05 |
| 24-48 h | 1 (0-2) | 2 (1-6) | 0.02 |
| 48-72 h | 0 (0-1) | 1 (1-6) | 0.01 |
| 72-96 h | 0 (0-2) | 2 (0-4) | 0.12 |
| Opioid rescues in first 96 h of transition by agent | < 0.05 | ||
| Morphine | 44 | 51 | |
| Fentanyl | 51 | 210 | |
| Concomitant medications administered in first 96 h of transition (number of administrations) | 0.6 | ||
| Benzodiazepines | 32 | 40 | |
| Clonidine | 5 | 3 | |
| Barbiturates | 2 | 8 | |
| NSAIDS | 2 | 2 | |
| Neuromuscular blockers | 4 | 6 | |
| Acetaminophen | 9 | 10 |
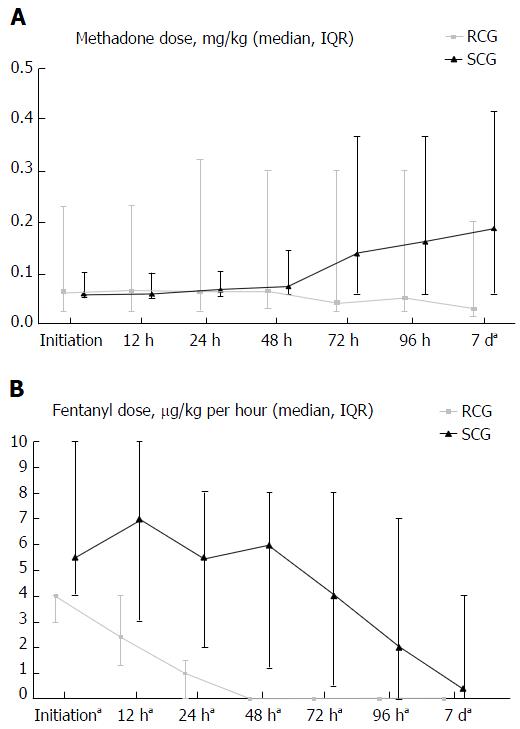
The initial daily median enteral methadone dose was 2.3 times the daily median IV fentanyl dose in the RCG, compared to 1.1 times in the SCG (P < 0.05). Both groups had similar daily doses of enteral methadone at initiation (0.064 mg/kg in the RCG vs 0.06 mg/kg in the SCG, P = 0.62) and at 48 h (0.064 mg/kg in the RCG vs 0.076 mg/kg in the SCG, P = 0.9). However, at 7 d, the RCG had a significantly lower daily dose of enteral methadone compared to the SCG (0.03 mg/kg vs 0.189 mg/kg, P = 0.02) (Figure 1A). While the RCG experienced a consistent reduction in the IV fentanyl infusion dropping to zero by 48 h, the SCG experienced an increase in the IV fentanyl infusion over the first 48 h followed by a consistent reduction thereafter (Figure 1B).
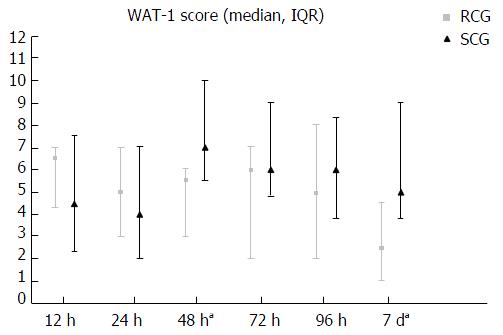
For the primary outcome measure of withdrawal at 7 d, the RCG had lower median WAT-1 scores at 7 d (2.5 vs 5, P = 0.03) (Figure 2). Secondary outcome measures differed between RCG and SCG for: Lower median WAT-1 scores at 48 h (5.5 vs 9, P = 0.04), lower overall median WAT-1 scores (5 vs 6, P = 0.03) and lower overall median pain scores (3 vs 6, P < 0.05). There were no significant differences between RCG and SCG for median WAT-1 scores at 12, 24, 72 and 96 h. Additionally, the RCG had significantly more VFD and shorter PICU LOS than the SCG (Table 3).
Critically ill children are at high risk of dependence and withdrawal after prolonged IV opioid infusion use for sedation and analgesia[3]. A significant withdrawal syndrome may occur if IV opioid infusions are abruptly discontinued resulting in considerable morbidity with prolongation of intensive care dependency. Such withdrawal may be minimized, or prevented by a variety of methods including gradually reducing the IV opioid infusion, conversion of the IV opioid infusion to intermittent IV dosing, and conversion of IV opioid infusions to enteral opioids followed by a gradual taper. In this paper, we report the results of our institutional experience with the conversion of IV fentanyl infusion directly to enteral methadone and occurrence of subsequent withdrawal in critically ill mechanically ventilated children receiving prolonged sedation. We observed wide variability in conversion from IV fentanyl infusion directly to enteral methadone and variability in withdrawal in critically ill mechanically ventilated children exposed to prolonged sedation. We also observed that in the subset of children who converted completely from IV fentanyl infusion directly to enteral methadone within a period of 48 h, a methadone:fentanyl dose conversion ratio of approximately 2.5:1 was associated with less withdrawal and reduced need for rescue opioids.
Guidelines and studies describing the conversion of IV opioid infusions such as fentanyl to enteral opioids such as methadone often differ with respect to optimal conversion dose, frequency and duration of therapy[6-9,13-16]. One such published guideline recommends a 1:1 dose conversion of IV fentanyl directly to enteral methadone over a period of 48 h to prevent withdrawal from dependence[13]. Another institutional policy recommends a 2.5:1 conversion ratio from IV fentanyl to IV methadone initially and subsequently to enteral methadone once patients tolerate oral intake[14]. These differences in formulation and dosing of methadone at the time of conversion in these studies and guidelines serve to highlight the paucity of knowledge and likely reflect differences in patient profiles, individual pharmacokinetic variation, and prescriber characteristics.
Previous studies have largely focused on transitioning from IV opioid infusions to IV methadone as an intermediate step before transitioning over to enteral methadone[6,7,9]. In contrast, the findings from our study establish that it is feasible to convert from IV fentanyl infusion directly to enteral methadone within a 48 h time period. Table 4 provides an example using data from our study to illustrate dose conversion using a methadone:fentanyl ratio of 2.5:1. Lugo et al[8] also studied such a direct conversion to enteral methadone, but employed a fixed methadone dose of 0.1 mg/kg administered enterally every 6 h for conversion regardless of IV fentanyl infusion dose at the time of conversion. A direct conversion has the advantage of reducing the need for continued IV access with the potential to decrease IV catheter infiltrates (in the case of peripheral IV catheters) and catheter-associated complications such as infections and thrombosis (in the case of central IV catheters). Additionally, such a strategy can favorably influence hospital admission costs by reducing the overall duration of PICU and hospital LOS as further weaning of enteral medications can take place either on the general floor or even at home[22].
| A 10-kg child is receiving IV fentanyl infusion of 5 mcg/kg per hour. The total daily fentanyl dose is 5 μg/kg per hour × 24 h = 1.2 mg/d |
| Dose conversion ratio - methadone:fentanyl = 2.5 (rounded up from 2.3 observed in rapid conversion group in the present study that converted from IV fentanyl infusion directly to enteral methadone within 48 h) based on potency, half-life and enteral bioavailability |
| Total daily dose of enteral methadone = 2.5 × 1.2 mg/d = 3 mg/d administered in 2 divided doses, i.e., 1.5 mg dosed every 12 h |
| Following the second dose of enteral methadone, the IV fentanyl infusion is decreased by 50% to 2.5 mcg/kg per hour |
| Following the third dose of enteral methadone, the IV fentanyl infusion is decreased again by 50% to 1.25 mcg/kg per hour |
| Following the fourth dose of enteral methadone, the IV fentanyl infusion is discontinued |
In the present study, we included critically ill children with a high likelihood of opioid dependence from exposure to IV fentanyl infusion for greater than 5 d who were converted directly to enteral methadone. Methadone is a commonly used synthetic enteral opioid to prevent opioid withdrawal in our unit due to its long mean elimination half-life in children (19 ± 14 h, range 4-62 h), good oral bioavailability (70% to 100%), low cost and ease of tapering[5-9]. Even though the suggested time frame for conversion was 48 h, we observed that only half the patients (RCG) were converted within this time period (in the absence of a unit specific protocol). This variation may have been due to patient factors such as intercurrent illness or perception of pain that may have influenced the conversion. Additionally, prescribing providers might have been anxious about possible withdrawal symptoms due to the perceived rapid time frame for conversion. Consequently, it is possible that in the RCG patients, providers preferentially used a higher dose of enteral methadone during conversion from IV fentanyl infusions to alleviate such concerns. In the other half of patients (SCG), the approximately 1:1 dose conversion required a longer time frame for conversion (median time of 109 h) raising the possibility that the dose of methadone was inadequate to prevent withdrawal. However, several confounding factors could have delayed the conversion in this group, including co-morbidities and intercurrent procedures that we were unable to adjust for in our analyses due to the small sample size. The SCG group was also observed to have an increase in the IV fentanyl infusion within the first 48 h of conversion, and ultimately ended up with a higher daily median methadone dose compared with the RCG (0.189 mg/kg vs 0.03 mg/kg, P = 0.02) which could possibly reflect an attempt to “catch-up” with withdrawal and pain symptoms.
Importantly, when compared to the SCG patients, the RCG patients were observed to have more ventilator free days and shorter PICU length of stay. While association does not imply causation, it is possible that the “higher” initial dose conversion in the RCG minimized withdrawal during conversion and allowed for subsequent progressive weaning of the enteral methadone. In contrast, the SCG which started out with a “lower” initial dose conversion ended up with higher doses of enteral methadone later on that might have resulted in over sedation and prolonged needs for intensive care dependency. However, this observation requires further prospective study in future trials.
The current study has several limitations. This was a retrospective review of patients admitted to the PICU and therefore subject to bias from incorrect or missing documentation in the patient charts. We attempted to overcome this limitation with rigorous definitions and integrity of data abstraction that we established a priori. Though it is possible that patients in the RCG happened to experience less withdrawal symptoms and were easier to wean compared to those in the SCG, both groups were well balanced with regard to age, illness severity, diagnoses, and extent of exposure to IV fentanyl infusion (Table 1). The small sample size precluded us from adjusting for confounding factors between the groups to study the independent association of methadone dosing in relation to other clinically relevant outcomes such as ventilator free days and PICU length of stay. The findings from this study cannot be extrapolated to conversions other than from IV fentanyl infusion to enteral methadone. The dosing of enteral methadone was subject to the discretion of the attending physician and the time frame for conversion from the IV infusion, though intended to be 48 h, was variable. This study did not take into account withdrawal symptoms from discontinuation of other medications such as benzodiazepines and barbiturates. Though sedative and analgesic regimens with ketamine and dexmedetomidine could theoretically lower the risk for withdrawal[23,24], none of our patients received either of these medications. The findings of this single center study may not be generalizable to other institutions. Incomplete cross-tolerance could have also complicated assessment of our findings. This phenomenon, in which exposure to one opioid could result in some degree of tolerance with exposure to another opioid, can be partial or complete[25].
Though methadone is commonly prescribed to facilitate conversion from prolonged IV opioid infusion use and minimize opioid withdrawal in the PICU, concerns exist that methadone may be more potent than suggested when using equianalgesic dose conversion ratios from opioids such as morphine[26]. This is particularly notable as a function of prior opioid dose and tends to increase with higher doses with consequent risk for oversedation and toxicities. A recent systematic review by Johnson et al[27] did not find differences between weight-based and formula-based approaches to initial methadone dosing in critically ill children. However, this review did note that children receiving a formula-based approach to dosing tended to experience more instances of oversedation. The authors concluded that the most prudent course is to start with the lowest possible dose and titrate based on clinical response to avoid complications[27].
In recent years, efforts to develop pathways and protocols have emerged as rational approaches to reduce intensive care dependency and rein in healthcare costs by decreasing variations in treatment styles. Our results are similar to the findings of the quality improvement study by Abdouni et al[14] who observed that employing a standardized treatment protocol to convert from IV fentanyl infusion to intermittent methadone dosing using a dose conversion ratio of 2.5:1 reduced the length of opioid exposure and minimized withdrawal symptoms. By observing the feasibility of a direct conversion from IV fentanyl infusion to intermittent enteral methadone, our study provides additional support to further refine such clinical pathways and ultimately improve clinical outcomes for critically ill children.
In our institutional experience, we observed wide variation in clinician practice during conversion from IV fentanyl infusion to enteral methadone and variability in withdrawal in critically ill mechanically ventilated children exposed to prolonged sedation. In those children who converted successfully from IV fentanyl infusion to enteral methadone within a period of 48 h, a methadone:fentanyl dose conversion ratio of approximately 2.5:1 appeared to minimize withdrawal with less need for rescue opioids. Further prospective studies are needed to examine the optimum methadone:fentanyl dosing conversion to reduce withdrawal and improve clinical, economic and patient satisfaction outcomes.
Critically ill children are at high risk of dependence and withdrawal after prolonged intravenous (IV) opioid infusion use for sedation and analgesia which can result in considerable morbidity with prolongation of intensive care dependency. The optimal strategy to minimize such withdrawal remains controversial.
Direct rapid conversion of IV opioid infusions to enteral medications are ideal to minimize withdrawal as well as enhance patient satisfaction. An additional benefit of such a strategy is to facilitate rapid transition from intensive care to home with decrease in healthcare costs.
In contrast to most previous studies that examined conversion from IV opioid infusions to IV methadone, this study demonstrates the feasibility of a direct conversion from IV fentanyl infusion to enteral methadone within a 48-h time frame with minimal withdrawal in critically ill children.
In practical terms, a direct conversion from IV fentanyl infusion to enteral methadone over a 48-h timeframe appears to be feasible in a ratio of methadone:fentanyl of 2.5:1 with minimal withdrawal and less need for rescue opioids.
FLACC: Face, Legs, Activity, Cry and Consolability; IQR: Inter-quartile range; IV: Intravenous; LOS: Length of stay; PICU: Pediatric intensive care unit; PRISM: Pediatric Risk of Mortality; RCG: Rapid conversion group; SCG: Slow conversion group; WAT-1: Withdrawal Assessment Tool-version 1.
The findings are very valuable and should be shared with the scientific community. The corrections are shown as green highlight in the manuscript.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Krishnan T, Sangkhathat S S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Anand KJ, Arnold JH. Opioid tolerance and dependence in infants and children. Crit Care Med. 1994;22:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Tobias JD. Sedation and analgesia in the pediatric intensive care unit. Pediatr Ann. 2005;34:636-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Crit Care Med. 2008;36:2427-2432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22:763-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 209] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Tobias JD, Schleien CL, Haun SE. Methadone as treatment for iatrogenic narcotic dependency in pediatric intensive care unit patients. Crit Care Med. 1990;18:1292-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Robertson RC, Darsey E, Fortenberry JD, Pettignano R, Hartley G. Evaluation of an opiate-weaning protocol using methadone in pediatric intensive care unit patients. Pediatr Crit Care Med. 2000;1:119-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Meyer MM, Berens RJ. Efficacy of an enteral 10-day methadone wean to prevent opioid withdrawal in fentanyl-tolerant pediatric intensive care unit patients. Pediatr Crit Care Med. 2001;2:329-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Lugo RA, MacLaren R, Cash J, Pribble CG, Vernon DD. Enteral methadone to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy. 2001;21:1566-1573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Siddappa R, Fletcher JE, Heard AM, Kielma D, Cimino M, Heard CM. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth. 2003;13:805-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33:485-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14:747-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122-2132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Abdouni R, Reyburn-Orne T, Youssef TH, Haddad IY, Gerkin RD. Impact of a Standardized Treatment Guideline for Pediatric Iatrogenic Opioid Dependence: A Quality Improvement Initiative. J Pediatr Pharmacol Ther. 2016;21:54-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Bowens CD, Thompson JA, Thompson MT, Breitzka RL, Thompson DG, Sheeran PW. A trial of methadone tapering schedules in pediatric intensive care unit patients exposed to prolonged sedative infusions. Pediatr Crit Care Med. 2011;12:504-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage. 2009;38:409-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1185] [Cited by in F6Publishing: 1228] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 18. | Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9:573-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Merkel S, Voepel-Lewis T, Malviya S. Pain assessment in infants and young children: the FLACC scale. Am J Nurs. 2002;102:55-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Solodiuk J, Curley MA. Pain assessment in nonverbal children with severe cognitive impairments: the Individualized Numeric Rating Scale (INRS). J Pediatr Nurs. 2003;18:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Wong DL, Baker CM. Smiling faces as anchor for pain intensity scales. Pain. 2001;89:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Tobias JD, Deshpande JK, Gregory DF. Outpatient therapy of iatrogenic drug dependency following prolonged sedation in the pediatric intensive care unit. Intensive Care Med. 1994;20:504-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010;125:e1208-e1225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Golding CL, Miller JL, Gessouroun MR, Johnson PN. Ketamine Continuous Infusions in Critically Ill Infants and Children. Ann Pharmacother. 2016;50:234-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Choe CH, Smith FL. Sedative tolerance accompanies tolerance to the analgesic effects of fentanyl in infant rats. Pediatr Res. 2000;47:727-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Lawlor PG, Turner KS, Hanson J, Bruera ED. Dose ratio between morphine and methadone in patients with cancer pain: a retrospective study. Cancer. 1998;82:1167-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 27. | Johnson PN, Boyles KA, Miller JL. Selection of the initial methadone regimen for the management of iatrogenic opioid abstinence syndrome in critically ill children. Pharmacotherapy. 2012;32:148-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |