Published online Mar 9, 2022. doi: 10.5409/wjcp.v11.i2.93
Peer-review started: April 25, 2021
First decision: June 17, 2021
Revised: July 4, 2021
Accepted: February 25, 2022
Article in press: February 25, 2022
Published online: March 9, 2022
Nitrous oxide is one of the most commonly used inhalational anesthetic agents used in practice. It is a cost-effective, pleasant, safe, and versatile anesthetic agent with many desirable properties like good quality analgesia, decreased awareness, accelerated induction and recovery from anesthesia, and reduced utilization of other expensive inhalational agents with potential cost savings. The use of nitrous oxide has been questioned by a lot of studies and case reports perceiving its adverse systemic, hematological, immune, and neurologic adverse effects. However, the literature in the recent past has tried to resolve the controversies related to its use. The concerns over an increase in cardiovascular complications and mortality following nitrous oxide use have been negated by recent data. However, its use in certain vulnerable populations like children with cobalamin and folate deficiency or defects in their metabolic pathways remains a cause of concern for its toxic effects. In this narrative review, we aim to discuss the pharmacological properties of nitrous oxide, the potential advantages and drawbacks of the use of nitrous oxide in children, address the neurodevelopmental and other systemic effects, and throw light on the evidence regarding the safety of nitrous oxide use and its current role in pediatric procedural sedation and anesthesia practice. The literature related to its use in the pediatric population for painful procedures and surgeries has been summarized.
Core Tip: The literature is insufficient presently to advise either the routine use or complete elimination of nitrous oxide, and further research is needed to fully establish its role in pediatric anesthesia practice. No major adverse effects have been reported in large trials on the use of nitrous oxide in children despite the prevailing concerns over its safety in this population. A reasonable and balanced approach should be adopted to individualize its use considering its risks and benefits as related to a particular case.
- Citation: Gupta N, Gupta A, Narayanan M R V. Current status of nitrous oxide use in pediatric patients. World J Clin Pediatr 2022; 11(2): 93-104
- URL: https://www.wjgnet.com/2219-2808/full/v11/i2/93.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i2.93
Nitrous oxide has been a part of the routine anesthetic practice for over 15 decades. From being the fad of recreational use at parties, nitrous oxide has evolved to hold an important place in contemporary practice of anesthesia[1]. It was first synthesized by Joseph Priestly in 1772, and 7 years later Humphrey Davy established its analgesic and psychotropic potential. However, Davy’s suggestion on using it as an anesthetic did not gain popularity until 1844 when Gardner Colton demonstrated its analgesic properties and Horace Wells demonstrated the first use of nitrous oxide for analgesia for painless tooth extraction. From the year 1868, the commercial availability of compressed nitrous oxide cylinders led to its universal adoption as an ether adjunct. Consequently, it was widely used for general procedural sedation in dentistry, obstetric analgesia, and during general anesthesia with other anesthetic agents. Its additive use with ether provided smoother induction, reduced ether requirements, cardiorespiratory stability, and faster emergence.
While its advantages were being appreciated, various concerns about its metabolic and other adverse effects begin to be recognized in the middle of the nineteenth century, including reports of fatalities from the faulty delivery systems, which led to an ongoing debate on whether it should be abandoned. Results of a few large-scale trials further fueled the debate and challenged its continued use in anesthesia practice. Nitrous oxide can also have a direct environmental impact as it is a major contributor of greenhouse gases. This has questioned its role in sustainable and eco-friendly anesthetic practice. However, the anesthetic use of nitrous oxide contributes to only 2% of the nitrous oxide source in the atmosphere.
The use of nitrous oxide continues to be a vacillation for many anesthesiologists due to the inconclusiveness of the currently available data. In this review, we discuss the present status of nitrous oxide in pediatric anesthesia practice. We will go through the pharmacological properties of nitrous oxide followed by the pros and cons of using nitrous oxide, addressing the neurodevelopmental and other systemic effects. The conclusions of the landmark trials regarding nitrous oxide will be summarized followed by the literature related to its use in pediatric procedural sedation and surgeries.
Studies published prior to August 2019 were retrieved from the electronic databases (Google Scholar, Cochrane Central Register of Controlled Trials on The Cochrane Library, PubMed and EMBASE), and their references were additionally scrutinized for any further relevant articles that investigated nitrous oxide. The literature search was done by independent authors, and the following search terms were used in various combinations using Boolean operators (such as AND, OR, NOT): Pediatric patients, pediatric, children, neonates, infants, adolescents, nitrous oxide, laughing gas, N2O, sedation, conscious sedation, procedural sedation, pain, analgesia, anesthesia, homocysteine, methionine synthase, teratogenic, teratogen, teratogens, teratogenesis, postoperative nausea and vomiting, postoperative nausea and/or vomiting (PONV), postoperative vomiting, postoperative nausea, postoperative emesis, environmental effects, ozone depletion, occupational, occupation, exposure, hazard, anesthesia dental, emergency service, post-traumatic stress disorder, chronic postsurgical pain, and CPSP. We got 779 results, and after eliminating duplication, adult trials, and articles in languages other than English, 137 articles were found suitable and were studied.
Nitrous oxide occurs as a colorless, odorless gas at room temperature and pressure. Though the exact mechanism of action is not known, it is postulated to act on dopaminergic, Gamma aminobutyric acid, alpha 2, and N-methyl-d-aspartate (NMDA) receptors to produce sedation and analgesia. However, nitrous oxide does not produce skeletal muscle relaxation. After inhalation, nitrous oxide is primarily excreted via the lungs unchanged. Nitrous oxide is the least potent volatile agent with a minimum alveolar concentration of 105%. Nitrous oxide has a blood gas partition coefficient of 0.47, which confers it low solubility.
Use of nitrous oxide in combination with other inhalational agents provides an additive anesthetic action since the minimum alveolar concentration of nitrous oxide is directly additive to theirs. Nitrous oxide in 60%-70% concentration equals a minimum alveolar concentration value of around 0.55-0.65[1,2]. It accelerates the time of anesthetic induction when used in conjunction with poorly soluble inhalational agents. Nitrous oxide as a component of anesthesia has shown to reduce the utilization of inhalational agents, propofol, and opioids[2,3]. During inhalational induction with mask in children, high concentration of nitrous oxide facilitates a faster loss of consciousness by concentration effect and second gas effect. The use of nitrous oxide during induction has proven to increase the mask acceptance in children and lower incidence of airway related complications. However, nitrous oxide favors the incidence of excitatory phenomena with sevoflurane during inhalational induction. It has been seen that adding up nitrous oxide to other inhalational anesthetic agents decreases the occurrence of hemodynamic suppression as compared to use of equipotent doses of volatile agents alone[2].
Nitrous oxide is a cheap anesthetic agent and reduces the utilization of other potent volatile agents and opioids. Therefore, the overall expenses and associated adverse effects are lowered. Along with the additive action with other inhalational agents, the major advantage of nitrous oxide is that it provides good amnesia and hence prevents awareness. Nitrous oxide has been a popular agent for use in pediatric anesthesia during surgical procedures as a constituent of anesthetic gas mixture in addition to other volatile agents and opioids. In addition, it has been used for providing procedural sedation in the emergency room and for various urological procedures and ontological procedures. Nitrous oxide also has been used for mild sedation and analgesia in children undergoing dental procedures, upper gastrointestinal endoscopy, fiberoptic bronchoscopy, and venipuncture procedures. Nitrous oxide has been shown to significantly reduce chronic postsurgical pain (CPSP) in recent studies due to its antagonist action on NMDA receptors, which have been purported to have a role in central sensitization and establishment of CPSP[4].
Nevertheless, nitrous oxide has numerous detrimental effects that may limit its overall clinical application. These consist of an increased risk of PONV, neurologic and hematologic complications, diffusion hypoxia, its property of expanding closed spaces, ozone depletion potential, and recent concerns of adverse consequences on the developing brain[5,6]. There were also concerns of immunosuppression and impairment of wound healing due to inhibition of mononuclear cell proliferation and neutrophil chemotaxis[5-7]. The advantages and disadvantages of nitrous oxide have been summed up in the Table 1. Some of the disadvantages quoted are controversial as discussed later in the chapter.
| Advantages | Disadvantages |
| Analgesia | Low potency |
| Reduced awareness | Risk of diffusion hypoxia |
| Colorless and odorless | PONV [risk ratio 1.21 (CI: 1.04-1.40); P = 0.014]2 |
| Inexpensive (Rs 50/patient)1 | Ability to expand air filled cavities |
| Faster onset and emergence (elimination half-life 5 min) | Increases cuff pressure of ETT and LMA |
| Minimal metabolism (< 0.004%) | Hematological/neurological toxicity |
| Cardiorespiratory stability | Immune deficiency? |
| Prevents CPSP | Reproductive effects |
| Treatment-resistant refractory depression | Myocardial ischemia? |
| Greenhouse gas | |
| Apoptosis in developing brains |
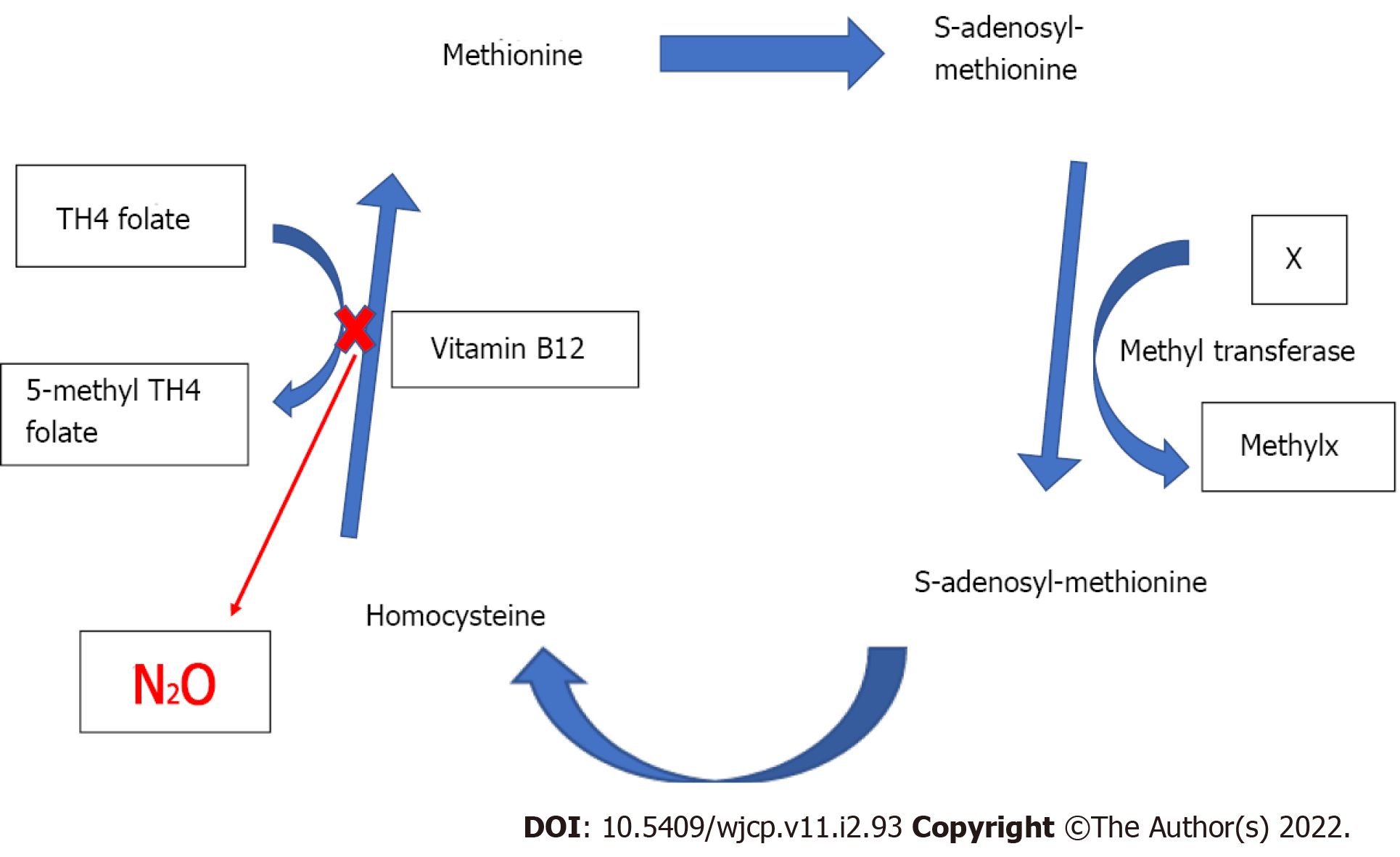
The systemic effects of nitrous oxide are summarized in the table below (Table 2). Nitrous oxide oxidizes the cobalt atom of the enzyme methionine synthetase and thereby permanently inactivates it, which in turn interferes with the metabolism of vitamin B12 and folate (Figure 1). Hence, the transformation of homocysteine to S-adenosylmethionine is impaired, which is a substrate for the chemical reaction involving tetrahydrofolate and thymidine during DNA synthesis. A short nitrous oxide exposure of only 30 min was found to decrease the methionine synthetase enzyme activity by 50% in rats, while it became almost untraceable after 6 h[8].
| Respiratory system | Decreases tidal volume and respiratory rate |
| Reduced ventilatory response to carbon dioxide and hypoxia | |
| Central nervous system | Loss of awareness |
| Analgesia | |
| Increased cerebral blood flow and intracranial pressure | |
| (Concentration > 70%) | |
| Cardiovascular system | Sympathomimetic |
| Direct myocardial depression | |
| Hemodynamic effects | Combination with other inhalational agents reduce the incidence of hypotension when compared to administration of the agents alone |
Acute neurologic signs and pancytopenia were seen in an infant after nitrous oxide anesthesia, and vitamin B12 supplementation treated the symptoms[9]. The problem would be magnified in patients having preexisting methionine synthase deficiency where nitrous oxide exposure can precipitate pernicious anemia (manifesting as spinal cord subacute combined degeneration and megaloblastic anemia), psychomotor delay, growth retardation, and neurological symptoms[10,11].
Nitrous oxide has also been noticed to increase blood homocysteine levels. Similarly, nitrous oxide facilitated reduction in methionine synthase enzyme activity in patients with Type-III Homocystinuria (due to a defect in methylene tetrahydrofolate reductase), can complicate into myelopathy, macrocytic anemia, and death. A report described a cataclysmic event in a child who was anesthetized with nitrous oxide and developed convulsions and apneic episodes postoperatively and later succumbed[11].
A preliminary study on metabolic effects of repeated exposure to nitrous oxide concluded that homocysteine levels did not consistently correlate with cumulative nitrous oxide exposure and children predisposed to metabolic and nutritional disturbance[12]. Though this finding is reassuring, considering the gravity of consequences, nitrous oxide should be used with caution in children with congenital deficiency or defective enzymes that are involved in the pathway to DNA synthesis or in patients at risk of vitamin B12 deficiency (e.g., pernicious anemia, post-illeal resection surgery, vegetarians, malnourished children, and infants on complete breast feeds).
Postoperative nausea and vomiting: Nitrous oxide administration is considered an independent risk factor for PONV. Nitrous oxide heightens the risk of PONV by up to 20% in adults[13]. Notwithstanding, nitrous oxide did not increase the incidence of PONV in children when used as an adjuvant to other volatile agents[14]. The incidence and severity of PONV did not vary between those receiving 70% nitrous oxide during anesthesia as compared to those who did not[15]. Nonetheless, in combination with propofol it did increase the occurrence of PONV[15].
Environmental and occupational exposure safety: The National Institute of Occupational Safety and Health has set an upper limit for safe workplace exposure to nitrous oxide of 25 ppm. However, the environmental levels may reach up to 2000 ppm in the absence of scavenging, and many grave problems like neurological, hematologic, genotoxic, and reproductive may develop in exposed team[16,17]. Pediatric anesthesiologists may be at the highest risk because of exposure to nitrous oxide and other inhalation agents at high concentrations and flows during the inhalation induction process and during anesthesia. In addition, nitrous oxide has been implicated in ozone destruction in the atmospheric stratosphere[18]. However, all clinical applications of nitrous oxide combined amount to < 2% of pollution related to its use and is probably of little significance, if any.
Neurodevelopmental effects: Similar to other inhalational agents, there has been a concern of nitrous oxide in accelerating apoptosis in the developing brain leading to neurotoxicity[6,7,19]. The human brain continues to develop after birth for several years undergoing synaptogenesis where new synaptic connections are formed by neuronal rearrangement. At the same time, unwanted neurons undergo apoptosis. It has been proposed that nitrous oxide along with many other anesthetics may hasten neuronal apoptosis and lead to cerebral toxicity and behavioral and learning impairments later in life.
Animal studies have observed that high dose or repeated exposure to NMDA antagonists such as nitrous oxide can lead to irreversible brain damage[19,20]. Intriguingly, one rat study revealed that use of nitrous oxide alone did not increase apoptosis, but its use in combination with isoflurane considerably enhanced neuronal cell death[19]. Another rat study demonstrated that nitrous oxide with isoflurane and midazolam given for 6 h led to widespread apoptosis as well as memory and learning disability[20]. In contrast, xenon, which is an inert gas with anaesthetic properties, has been found to mitigate isoflurane related apoptosis in rat brain[21]. However, at present no human data has proven its role for harmful neurodevelopmental effects. Therefore, at present, the literature does not advocate its complete exclusion from practice of pediatric anesthesia due to this concern. However, recently the United States Food and Drug Administration released a safety alert on the risk of potential neurotoxicity of general anesthetic drugs (including nitrous oxide) in children < 3 years, and the use of general anesthesia will remain under scrutiny until the risk is categorically ruled out in the future by robust evidence.
Nitrous oxide is 30 times more blood soluble than nitrogen (air) despite being a relatively insoluble agent otherwise. The blood gas partition coefficient of nitrous oxide is 0.47 as opposed to 0.015 of nitrogen. So, nitrous oxide diffuses quickly into a closed gas space resulting in significant clinical consequences. Expansion of the airspace can cause distension of expansible spaces and increased pressure in non-expansible spaces.
It has been shown that due to the high blood flow in lungs, 75% nitrous oxide can double the volume of a pneumothorax in 10 min and triple in 30 min. Nitrous oxide can cause increased middle ear pressure, intraocular pressure, and intracranial pressure. However, it is not necessary to stop nitrous oxide prior to dura closure in craniotomy[22]. Use of nitrous oxide in bowel surgeries can increase the bowel gas causing over distension, increasing abdominal pressure, and compromising respiration[23]. The risk of venous air embolism is increased with administration of nitrous oxide by decreasing the lethal dose of volume of air embolism. Whenever venous air embolism is diagnosed, nitrous oxide administration should be halted[24]. The air-filled cuffs of endotracheal tubes and laryngeal mask airways are also susceptible to expansion with the use of nitrous oxide. The increased cuff pressure can lead to surrounding mucosal ischemia due to impaired perfusion[25].
Hence, it is advisable to avoid the use of nitrous oxide in laparoscopic, bowel, middle ear, and vitreo-retinal surgeries and to use with caution in neurosurgeries.
The present review identified the literature explaining why the usage of nitrous oxide has been under constant scrutiny, the current role of nitrous oxide in contemporary pediatric anesthesia, procedural sedation, and exploring its potential novel benefits like prevention of CPSP in the pediatric population.
Many large-scale studies and meta-analyses have been conducted to study the unfavorable effects of nitrous oxide[26-30]. The results of these trials and meta-analyses highlight why the usage of nitrous oxide have been contentious despite its remarkably safe journey of over one and a half centuries in anesthesia and its multiple advantages as a component of balanced anesthesia. A summary of the most landmark articles exploring the effects of use of nitrous oxide as a component of anesthesia have been complied in Table 3. These trials have been labelled as ‘landmark’ trials for nitrous oxide because of the vast magnitude of data studied and since they turned out to be trailblazers in the history of nitrous oxide use and had a direct influence on the worldwide practice of nitrous oxide anesthesia.
| Trial | Ref. | Main findings |
| ENIGMA Trial | Myles et al[26], 2007 | Increased rates of major complications (OR: 0.71; 95%CI: 0.56-0.89; P = 0.003) myocardial infarction, stroke, pneumonia, pulmonary embolism, wound infection, severe PONV (OR: 0.40; 95%CI: 0.31-0.51; P < 0.001), and death. |
| ENIGMA II Trial | Myles et al[27], 2014 | Risk of death at 1 year, cardiovascular complications (combined RR for death and cardiovascular complications was 0.96, 95%CI: 0.83-1.12; P = 0.64) or surgical-site infection in the nitrous oxide group not increased (P = 0.61). Risk of PONV was reduced by one third in the patients not exposed to nitrous oxide (P < 0.0001), but the absolute risk reduction was only 4%. |
| A large retrospective analysis of registries | Turan et al[28], 2013 | Patients receiving nitrous oxide had 40% lower risk of pulmonary complication (OR: 95% Bonferroni-adjusted CI: 0.59, 0.44-0.78) and death (OR: 97.5%CI: 0.67, 0.46-0.97; P = 0.02), while cardiovascular complications were comparable. |
| Cochrane review on complications with use of nitrous oxide | Sun et al[29], 2015 | Nitrous oxide increased the incidence of pulmonary atelectasis (OR: 1.57, 95%CI: 1.18-2.10, P = 0.002) but had no effects on the rates of in-hospital mortality, pneumonia, myocardial infarction, stroke, venous thromboembolism, wound infection, or length of hospital stay. |
| Cochrane review on accidental awareness with use of nitrous oxide | Hounsome et al[30], 2016 | Despite the inclusion of 3520 participants, only three awareness events were reported by two studies. In one study the event was due to technical failure. Due to the low quality of evidence, the authors could not determine whether the use of nitrous oxide in general anesthesia increases, decreases, or has no effect on the risk of accidental awareness. |
The ENIGMA trial by Myles et al[26] was the first major trial that recruited 2050 patients and compared no nitrous oxide (80% oxygen with 20% nitrogen) and nitrous oxide-based anesthesia (70% N2O and 30% oxygen). The primary endpoint of this trial was the length of hospital stay. The secondary outcomes comprised of the length of intensive care unit stay and the incidence of postsurgical complications including death within 30 d of surgery. This trial set up a major controversary as use of nitrous oxide as a part of anesthetic gas mixture led to an increased incidence of cardiopulmonary complications, stroke, wound infection, and even mortality in the nitrous oxide cohort. This trial questioned the use of nitrous oxide and was followed by a period of nitrous oxide free anesthesia almost globally.
However, the authors countered their own findings in their next multicentric randomized study with a larger sample size of 7112 patients who had a history of coronary artery disease and were undergoing any major non-cardiac surgery[27]. They assessed the effect of the use of nitrous oxide on the incidence of mortality and any cardiovascular complication (e.g., stroke, myocardial infarction, pulmonary embolism, or cardiac arrest) that occurred within 30 d of undergoing surgery. They found that the risk of cardiovascular complications, surgical-site infection, or death at 1 year were not found to be increased in the nitrous oxide group, and the risk of PONV was found to be only mildly increased[27].
To the great relief of proponents of nitrous oxide, a large trial by Turan et al[28], which evaluated 49016 patients who underwent noncardiac surgery, evaluated the relationship between intraoperative nitrous oxide use and 30d mortality and major postoperative complications. They documented a reduction in pulmonary complications and mortality rates with the use of nitrous oxide, while cardiac risk was not found to be increased.
A Cochrane review further substantiated the fact that use of nitrous oxide was not associated with an increased risk of pneumonia, acute myocardial infarction, stroke, wound infection, venous thromboembolic phenomenon, or increased length of hospital stay or in-hospital mortality[29]. The effect of nitrous oxide on intraoperative awareness is also contentious with some studies reporting increased incidence while others finding a protective effect of nitrous oxide. A recent Cochrane review by Hounsome et al[30] assessed the effect of nitrous oxide on the risk of accidental awareness under anesthesia in 5-year-old and older patients. However, despite the inclusion of 3520 patients, they found only three awareness events and could not come to a definitive conclusion regarding this.
Nitrous oxide is frequently used for procedural pain relief (e.g., bone marrow aspiration, intercostal drain insertion, venipuncture, lumber puncture, wound sutures, dental extraction, etc.)[31-35]. If used with proper precautions, no major adverse effects have been reported with nitrous oxide use for sedation[36-39].
The use of nitrous oxide in concentrations up to 50% with oxygen during pediatric procedures is an effective substitute for parenteral sedation in minor surgical procedures as it provides pain and anxiety alleviation, maintains protective airway reflexes, and is safe[31-33]. Entonox, which is a mixture of 50% nitrous oxide with 50% oxygen in equal proportions, is a good analgesic agent described in pediatric minor procedures like wound and burn dressing, suturing and suture removal, urinary catheterization, change of gastrostomy tube, synovial fluid and bone marrow aspiration, acute trauma, fracture reduction, lumbar puncture, and minor dental procedures. However, there is evidence on safe administration of nitrous oxide in delivered concentrations of 20%-70% in children without any major reported adverse events, and hence the cut-off value for procedural sedation should not be arbitrarily limited to 50% for fear of complications[33].
At present there is limited evidence regarding the efficacy of nitrous oxide in infants and neonates. In one prospective cohort trial, nitrous oxide was successfully utilized for sedation during tracheal intubation in preterm infants undergoing surfactant therapy[34]. In another randomized trial, the use of nitrous oxide in combination with lignocaine/prilocaine 5% ointment was found to have significantly lower pain scores when compared to topical cream or nitrous alone for injection in infants[35].
A French multicentric prospective survey assessed the side-effects among 35942 data sheets (mainly pediatric) where Entonox was used as a sole agent for procedural pain[36]. Overall, 4.4% adverse effects were reported, with the commonest being neuropsychiatric and gastrointestinal complaints (86%). Others were PONV and agitation or euphoria.
The rapid psychomotor recovery with nitrous oxide enables quicker patient discharge and removes the need for a patient to be escorted. In a French survey by Annequin et al[37] that assessed 1025 pediatric procedures describing the use of Entonox, Entonox alone provided unsatisfactory pain relief. Crying and physical restraint was required in many children < 3 years of age. Notwithstanding, the use of nitrous oxide was observed to have better effectiveness compared to oral midazolam for sedation during skin suturing in children[38].
Nitrous oxide is frequently used in pediatric dental procedures, and > 90% children undergoing a dental extraction procedure effectively completed the procedure under nitrous oxide sedation[32]. Nitrous oxide and midazolam were compared with the combination technique for moderate (conscious) sedation to decrease fear and anxiety associated with dental procedures in a systematic review and meta-analysis that included 534 participants[39]. Their main findings were that the combination of the two agents provides the best features and lead to fewer adverse effects due to midazolam by reducing the total dose while also facilitating better acceptance of the nitrous oxide inhalation technique and improving the recovery time.
The American Academy of Pediatric Dentistry released Guidelines in 2009 stating that the use of oxygen saturation monitoring with pulse oximetry was not mandatory for children getting only nitrous oxide for sedation in dental procedures. Similarly, guidelines from the British Dental Society did not recommend preoperative fasting before its administration. In general, the risk of aspiration during use of nitrous oxide for sedation is low, even among the non-fasted children[40-42]. However, most anesthesia-related guidelines would still recommend the standard 2 h of fasting with clear fluids before nitrous oxide sedation as there is a lack of literature directly assessing airway patency during nitrous oxide sedation and the fasting requirements.
In the majority of trials for procedural sedation and analgesia in children, nitrous oxide has been found to be favored as a combination technique in addition to use of topical creams, other sedatives, or both agents, while data is insufficient for its use as a sole agent[43-49]. The summary of various trials on procedural sedation and analgesia have been summed up in Table 4.
| Ref. | Main study objective | Setting/procedures | Number of children; Age | Findings |
| Babl et al[43], 2008 | Depth of sedation and incidence of adverse effects with various N2O concentrations | Pediatric ER procedures | 762; 1-17 yr | N2O in high concentration (70%) and continuous flow was found to be a safe agent for procedural sedation and analgesia in toddlers and older children |
| Babl et al[44], 2010 | Sedation practices and the associated adverse events profile | Procedural sedation and analgesia from registry database at the largest Australian pediatric ER of a children’s hospital | 2002; 1-17 yr | N2O was used in majority cases (81%), and incidence of serious adverse events was low. (desaturation, n = 2; seizures, n = 2, and chest pain, n = 1) |
| Brown et al[45], 2009 | Evaluate the PediSedate (a N2O delivery system combined with an interactive video component) for reducing children’s behavioral distress | Children who received the PediSedate before invasive procedures | 40; 3-9 yr | PediSedate is an effective system for procedural sedation in children |
| Ekbom et al[46], 2011 | To find out whether oral midazolam or 50% N2O, or 10% N2O; along with lidocaine/prilocaine ointment is most effective in gaining IV access in obese or growth retarded children | Children and adolescents undergoing IV access at a Children’s Hospital in Stockholm, Sweden | 90; 5-18yr | 50% N2O resulted in an improved rate of IV access, a shorter procedure time, and a better experience for these children |
| Jimenez et al[47], 2012 | Comparison of N2O and hematoma block with and without trans-mucosal fentanyl for sedation and analgesia in the reduction of radioulnar fractures. | Retrospective, observational study, in children with radioulnar fractures in a pediatric ER | 81; 4-15 yr | The combination of all 3 agents in pediatric ER improved analgesia compared with only N2O and hematoma block combination |
| Lee et al[48], 2012 | Comparison of the sedaoanalgesia profile of N2O vs IV ketamine | Prospective, randomized study at ER of a single academic center in children undergoing primary repair of a laceration wound | 32; 3-10 yr | N2O was found preferable to ketamine because it provides a faster recovery, is safe, and maintains a suitable safe plane of sedation |
| Srinivasan et al[49], 2013 | Determine the effectiveness and safety of procedural sedation performed using ketamine (0.5-1 mg/kg) or N2O (50%-70%). | Retrospective review and analysis of a quality improvement database for procedural sedations performed at St Louis Children’s Hospital undergoing sedation by pediatric hospitalists | 8870; 7 mo to 4 yr | Combination of ketamine and N2O provides lowest rates of complications. Respiratory and cardiovascular events occurred more frequently with ketamine, whereas NV, sedation level not achieved, and procedure not completed were more frequent with N2O |
There is not much data on the chronic use of nitrous oxide for procedural sedation in burn victims for procedures such as burn dressings and other chronic conditions demanding repeated exposures. Few studies have reported its use in burns but have not specifically reported that data for better analysis. Recently, nitrous oxide has gained attention for its role in treatment-resistant refractory depression[50]. A recent study has elucidated its mechanism to be mediated through neuronal nitric oxide synthase activation in the medial prefrontal cortex[51]. However, there is no pediatric literature in this regard. Considering the recent evidence, the Food and Drug Administration alert on anesthesia related neurotoxicity in young children, and the risk of its metabolic toxicity on repeated exposures, caution should be employed while considering its use for pain and sedation for chronic conditions[52].
The proposed mechanism of action of nitrous oxide is by acting as a NMDA receptor antagonist, and nitrous oxide anesthesia has a potential preventive action on the development of CPSP, though it is still not proven and there is limited evidence in the pediatric subpopulation. A follow-up study of the ENIGMA-II trial at 3 mo found that use of nitrous oxide decreased the incidence of CPSP and documented that a history of severe postoperative pain in the first week of surgery, any wound related complication, and having an abdominal incision were the factors associated with increased risk of CPSP[53].
The same group of investigators later evaluated the ENIGMA-II trial participants at 12 mo of exposure to nitrous oxide and concluded that its administration had no overall benefit on CPSP, but potential benefits were found in Asian patients and patients with specific polymorphisms of the tetrahydrofolate reductase gene[54]. It was proposed that these phenotypes were more susceptible to the inhibitory effects of nitrous oxide, thereby resulting in reduced DNA synthesis. This culminated in an impaired gene expression thereby leading to impaired neuronal plasticity and neuro-inflammation.
There are several drugs being used presently as supplements to general anesthesia that have the potential to reduce the incidence of intraoperative awareness like benzodiazepines, opioids, and alpha2 adrenoceptor agonists. Nevertheless, none of these would offer comparable amnesia, analgesia and cardiovascular stability of the same degree provided by nitrous oxide[20,27,33,36,54]. Recently, xenon, which is an inert gas, has been proposed as a suitable alternative to nitrous oxide. Xenon has profound analgesic properties and superior cardiovascular stability than nitrous oxide. Furthermore, its use has not been associated with harmful neurodevelopmental consequences on developing brain. Hence, it can be considered an attractive option to nitrous oxide in pediatric anesthesia in the future[21]. Presently, its clinical value has been limited mainly by its expense.
The present narrative review summarized the data related to usage of nitrous oxide in pediatric patients. At present there is insufficient evidence to support or refute its continued usage in pediatric practice. Though several new anesthetic agents have been developed, an alternative as flexible and cost-effective as nitrous oxide is yet to be discovered. Certain adverse effects of nitrous oxide like diffusion hypoxia, its ability to expand closed airspaces, increased risk of PONV, ozone depletion, hematologic and neurologic complications, adverse effects on developing brain, and immunosuppression remain a concern to pediatric anesthesiologists. At clinically used concentrations and duration, its use does not appear to be related to hematologic complications and neurobehavioral effects on the developing brain. Its use in children seems justified as a constituent of anesthetic gas mixture and for procedural sedation in the pediatric population for light to moderate pain procedures barring its well-recognized contraindications. Combination techniques utilizing nitrous oxide in addition to topical local anesthetics and/or other sedatives have been found to be most effective for procedural sedation, and no major adverse effects reported from even large-scale trials. An individualized approach weighing the risks and benefits of nitrous oxide would be optimal in a particular case. Future perspectives include large-scale research into its specific long-term adverse effects on the developing brain in children in different conditions of administrations, research to fill the gaps in knowledge related to procedural sedation and exploring its potential novel benefits like prevention of CPSP in the pediatric subpopulation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: AIIMS.
Specialty type: Anesthesiology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Mapesa WA, Kenya; Mondardini MC, Italy S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008;55:124-130; quiz 131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 2. | Kihara S, Yaguchi Y, Inomata S, Watanabe S, Brimacombe JR, Taguchi N, Komatsuzaki T. Influence of nitrous oxide on minimum alveolar concentration of sevoflurane for laryngeal mask insertion in children. Anesthesiology. 2003;99:1055-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Davidson JA, Macleod AD, Howie JC, White M, Kenny GN. Effective concentration 50 for propofol with and without 67% nitrous oxide. Acta Anaesthesiol Scand. 1993;37:458-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Inada T, Inada K, Kawachi S, Takubo K, Tai M, Yasugi H. Haemodynamic comparison of sevoflurane and isoflurane anaesthesia in surgical patients. Can J Anaesth. 1997;44:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Lehmberg J, Waldner M, Baethmann A, Uhl E. Inflammatory response to nitrous oxide in the central nervous system. Brain Res. 2008;1246:88-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Tramèr MR. Do we need to know whether nitrous oxide harms patients? Lancet. 2014;384:1407-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Munson ES. Complications of nitrous oxide anesthesia for ear surgery. Anesth Clin North Am. 1993;11:559-572. [DOI] [Cited in This Article: ] |
| 8. | Nunn JF. Clinical aspects of the interaction between nitrous oxide and vitamin B12. Br J Anaesth. 1987;59:3-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Mosca VS. Letter to the JPO editors re: article by Andreacchio et al entitled "lateral column lengthening as treatment for planovalgus foot deformity in ambulatory children with spastic cerebral palsy"(J Pediatr Orthop 2000;20:501-505). J Pediatr Orthop. 2006;26:412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ilniczky S, Jelencsik I, Kenéz J, Szirmai I. MR findings in subacute combined degeneration of the spinal cord caused by nitrous oxide anesthesia--two cases. Eur J Neurol. 2002;9:101-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Chen H, Lovell M, Baines D. Metabolic effects of repeated exposure to nitrous oxide: a preliminary report. Pediatr Anesth. 2010;20:365-366. [DOI] [Cited in This Article: ] |
| 12. | Selzer RR, Rosenblatt DS, Laxova R, Hogan K. Adverse effect of nitrous oxide in a child with 5,10-methylenetetrahydrofolate reductase deficiency. N Engl J Med. 2003;349:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Fernández-Guisasola J, Gómez-Arnau JI, Cabrera Y, del Valle SG. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta-analysis. Anaesthesia. 2010;65:379-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Bortone L, Picetti E, Mergoni M. Anesthesia with sevoflurane in children: nitrous oxide does not increase postoperative vomiting. Paediatr Anaesth. 2002;12:775-779. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Crawford MW, Lerman J, Sloan MH, Sikich N, Halpern L, Bissonnette B. Recovery characteristics of propofol anaesthesia, with and without nitrous oxide: a comparison with halothane/nitrous oxide anaesthesia in children. Paediatr Anaesth. 1998;8:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Krajewski W, Kucharska M, Wesolowski W. Occupational exposure to nitrous oxide: the role of scavenging and ventilation systems in reducing the exposure level in operating rooms. Int J Hygiene Environ Health. 2007;210:133-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Perić M, Vranes Z, Marusić M. Immunological disturbances in anaesthetic personnel chronically exposed to high occupational concentrations of nitrous oxide and halothane. Anaesthesia. 1991;46:531-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2921] [Cited by in F6Publishing: 2898] [Article Influence: 193.2] [Reference Citation Analysis (0)] |
| 19. | Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1380] [Cited by in F6Publishing: 1283] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 20. | Ghoneim MM, Dhanaraj J, Choi WW. Comparison of four opioid analgesics as supplements to nitrous oxide anesthesia. Anesth Analg. 1984;63:405-412. [PubMed] [Cited in This Article: ] |
| 21. | Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Steffey EP, Johnson BH, Eger EI 2nd, Howland D Jr. Nitrous oxide: effect on accumulation rate and uptake of bowel gases. Anesth Analg. 1979;58:405-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Pasternak JJ, Lanier WL. Is nitrous oxide use appropriate in neurosurgical and neurologically at-risk patients? Curr Opin Anaesthesiol. 2010;23:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Rodgers L, Dangel-Palmer MC, Berner N. Acute circulatory and respiratory collapse in obstetrical patients: a case report and review of the literature. AANA J. 2000;68:444-450. [PubMed] [Cited in This Article: ] |
| 25. | Mosby EL, Schelkun PM, Vincent SK. Nitrous oxide use and endotracheal tube rupture. Anesth Prog. 1988;35:14-16. [PubMed] [Cited in This Article: ] |
| 26. | Myles PS, Leslie K, Chan MT, Forbes A, Paech MJ, Peyton P, Silbert BS, Pascoe E; ENIGMA Trial Group. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology. 2007;107:221-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 27. | Myles PS, Leslie K, Chan MT. ANZCA Trials Group for the ENIGMA-II investigators. The safety of addition of nitrous oxide to general Anesthesia in at-risk patients having major noncardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet. 2014;384:1446-1454. [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Turan A, Mascha EJ, You J, Kurz A, Shiba A, Saager L, Sessler DI. The association between nitrous oxide and postoperative mortality and morbidity after noncardiac surgery. Anesth Analg. 2013;116:1026-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Sun R, Jia WQ, Zhang P, Yang K, Tian JH, Ma B, Liu Y, Jia RH, Luo XF, Kuriyama A. Nitrous oxide-based techniques versus nitrous oxide-free techniques for general anaesthesia. Cochrane Database Syst Rev. 2015;CD008984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Hounsome J, Greenhalgh J, Schofield-Robinson OJ, Lewis SR, Cook TM, Smith AF. Nitrous oxide-based vs. nitrous oxide-free general anaesthesia and accidental awareness in surgical patients: an abridged Cochrane systematic review. Anaesthesia. 2018;73:365-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Bruce E, Franck L. Self-administered nitrous oxide (Entonox®) for the management of procedural pain. Paediatric Nursing. 2000;12:15-19. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Foley J. A prospective study of the use of nitrous oxide inhalation sedation for dental treatment in anxious children. Eur J Paediatr Dent. 2005;6:121-128. [PubMed] [Cited in This Article: ] |
| 33. | Buhre W, Disma N, Hendrickx J, DeHert S, Hollmann MW, Huhn R, Jakobsson J, Nagele P, Peyton P, Vutskits L. European Society of Anaesthesiology Task Force on Nitrous Oxide: a narrative review of its role in clinical practice. Br J Anaesth. 2019;122:587-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Milesi C, Pidoux O, Sabatier E, Badr M, Cambonie G, Picaud JC. Nitrous oxide analgesia for intubating preterm neonates: a pilot study. Acta Paediatr. 2006;95:1104-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Carbajal R, Biran V, Lenclen R, Epaud R, Cimerman P, Thibault P, Annequin D, Gold F, Fauroux B. EMLA cream and nitrous oxide to alleviate pain induced by palivizumab (Synagis) intramuscular injections in infants and young children. Pediatrics. 2008;121:e1591-e1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Onody P, Gil P, Hennequin M. Safety of inhalation of a 50% nitrous oxide/oxygen premix: a prospective survey of 35 828 administrations. Drug Saf. 2006;29:633-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Annequin D, Carbajal R, Chauvin P, Gall O, Tourniaire B, Murat I. Fixed 50% nitrous oxide oxygen mixture for painful procedures: A French survey. Pediatrics. 2000;105:E47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Luhmann JD, Kennedy RM, Porter FL, Miller JP, Jaffe DM. A randomized clinical trial of continuous-flow nitrous oxide and midazolam for sedation of young children during laceration repair. Ann Emerg Med. 2001;37:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Sivaramakrishnan G, Sridharan K. Nitrous Oxide and Midazolam Sedation: A Systematic Review and Meta-Analysis. Anesth Prog. 2017;64:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Babl FE, Puspitadewi A, Barnett P, Oakley E, Spicer M. Preprocedural fasting state and adverse events in children receiving nitrous oxide for procedural sedation and analgesia. Pediatr Emerg Care. 2005;21:736-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Babl FE, Grindlay J, Barrett MJ. Laryngospasm With Apparent Aspiration During Sedation With Nitrous Oxide. Ann Emerg Med. 2015;66:475-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Tsze DS, Mallory MD, Cravero JP. Practice Patterns and Adverse Events of Nitrous Oxide Sedation and Analgesia: A Report from the Pediatric Sedation Research Consortium. J Pediatr. 2016;169:260-5.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Babl FE, Oakley E, Seaman C, Barnett P, Sharwood LN. High-concentration nitrous oxide for procedural sedation in children: adverse events and depth of sedation. Pediatrics. 2008;121:e528-e532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Babl FE, Belousoff J, Deasy C, Hopper S, Theophilos T. Paediatric procedural sedation based on nitrous oxide and ketamine: sedation registry data from Australia. Emerg Med J. 2010;27:607-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Brown SC, Hart G, Chastain DP, Schneeweiss S, McGrath PA. Reducing distress for children during invasive procedures: randomized clinical trial of effectiveness of the PediSedate. Paediatr Anaesth. 2009;19:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Ekbom K, Kalman S, Jakobsson J, Marcus C. Efficient intravenous access without distress: a double-blind randomized study of midazolam and nitrous oxide in children and adolescents. Arch Pediatr Adolesc Med. 2011;165:785-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 47. | Jimenez A, Blazquez D, Cruz J. Use of combined transmucosal fentanyl, nitrous oxide, and hematoma block for fracture reduction in a pediatric emergency department. Pediatr Emerg Care. 2012;28:676-679. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Lee JH, Kim K, Kim TY, Jo YH, Kim SH, Rhee JE, Heo CY, Eun SC. A randomized comparison of nitrous oxide versus intravenous ketamine for laceration repair in children. Pediatr Emerg Care. 2012;28:1297-1301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Srinivasan M, Carlson DW. Procedural sedation by pediatric hospitalists: analysis of the nature and incidence of complications during ketamine and nitrous oxide sedation. Hosp Pediatr. 2013;3:342-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Lew V, McKay E, Maze M. Past, present, and future of nitrous oxide. Br Med Bull. 2018;125:103-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Liu W, Li Q, Ye B, Cao H, Shen F, Xu Z, Du W, Guo F, Liu J, Li T, Zhang B, Liu Z. Repeated Nitrous Oxide Exposure Exerts Antidepressant-Like Effects Through Neuronal Nitric Oxide Synthase Activation in the Medial Prefrontal Cortex. Front Psychiatry. 2020;11:837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | US FDA. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women 2018. Available from: https://www.fda.gov/Drugs/DrugSafety/ ucm532356.htm. [DOI] [Cited in This Article: ] |
| 53. | Chan MTV, Wan ACM, Gin T, Leslie K, Myles PS. Chronic postsurgical pain after nitrous oxide anesthesia. Pain. 2011;152:2514-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Chan MT, Peyton PJ, Myles PS, Leslie K, Buckley N, Kasza J, Paech MJ, Beattie WS, Sessler DI, Forbes A, Wallace S, Chen Y, Tian Y, Wu WK; and the Australian and New Zealand College of Anaesthetists Clinical Trials Network for the ENIGMA-II investigators. Chronic postsurgical pain in the Evaluation of Nitrous Oxide in the Gas Mixture for Anaesthesia (ENIGMA)-II trial. Br J Anaesth. 2016;117:801-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |