Published online Aug 28, 2013. doi: 10.5319/wjo.v3.i3.58
Revised: July 10, 2013
Accepted: July 17, 2013
Published online: August 28, 2013
Millions of people worldwide are exposed to harmful levels of noise daily in their work and leisure environment. This makes noise-induced hearing loss (NIHL) a major occupational health risk globally. NIHL is the second most common form of acquired hearing loss after age-related hearing loss and is itself a major contributing factor to presbycusis. Temporary threshold shifts, once thought to be relatively harmless and recoverable, are now known to cause permanent cochlear injury leading to permanent loss of hearing sensitivity. This article reviews the current understanding of the cellular and molecular pathophysiology of NIHL with latest findings from animal models. Therapeutic approaches to protect against or to mitigate NIHL are discussed based on their proposed action against these known mechanisms of cochlear injury. Successes in identifying genes that predispose individuals to NIHL by candidate gene association studies are discussed with matched gene knockout animal models. This links to exciting developments in experimental gene therapy to replace and regenerate lost hair cells and post-noise otoprotective therapies currently being investigated in clinical trials. The aim is to provide new insights into current and projected future strategies to manage NIHL; bench to bedside treatment is foreseeable in the next 5 to 10 years.
Core tip: Noise-induced hearing loss (NIHL) affects millions of people worldwide irrespective of age, sex, and race. Hearing aids and cochlear implants are currently the only available interventions. This review article summarizes the cellular and molecular mechanisms of NIHL to-date. Significant milestones in uncovering genetic predisposition to NIHL in humans, experimental gene therapies and post-noise otoprotective strategies to reduce the impact of NIHL are reviewed.
- Citation: Wong ACY, Froud KE, Hsieh YSY. Noise-induced hearing loss in the 21st century: A research and translational update. World J Otorhinolaryngol 2013; 3(3): 58-70
- URL: https://www.wjgnet.com/2218-6247/full/v3/i3/58.htm
- DOI: https://dx.doi.org/10.5319/wjo.v3.i3.58
Noise-induced hearing loss (NIHL) is a major health problem indiscriminately affecting people of all ages, sex, or race worldwide[1]. A single traumatic exposure to loud sound, such as gun-shot or fireworks, or prolonged or repeated exposure to excessive sound over the acceptable daily exposure (85 dBA for 8 h, a guideline set by the National Institute for Occupational Safety and Health), cause sensorineural damage to the cochlea. This damage leads to either immediate hearing loss (impulse noise) or chronic progressive NIHL. Besides traditional hazardous exposure to occupational noise in industrial (construction, mining, forestry, aircraft, agricultural) and military settings, recreational exposure is equally accountable, since many leisure activity venues (clubs, discos, gyms, sport arenas) exceed recommended sound levels. Further, the Action on Hearing Loss (United Kingdom) has issued a serious warning that approximately two-thirds of 18- to 30-year olds are exposed to dangerously high-intensity sounds (> 85 dB) which can cause hearing damage, through personal listening devices[2]. NIHL causes social isolation, impaired communication with family and coworkers, lost productivity, decreased self-esteem, depression and cognitive decline. With an aging population and the global expectation to delay retirement age, the compounding socioeconomic impact of NIHL and age-related hearing loss (ARHL) is set to become even more significant. Despite this, hearing aids and cochlear implants are the only currently available management strategies for NIHL. It is therefore crucial to develop pharmacological and molecular therapies for NIHL that can ameliorate or repair injury to the cochlea and reduce the impact of hearing loss. This paper reviews the current knowledge of the cellular and molecular mechanisms of NIHL as well as genetic predisposition to NIHL in humans and matched animal models. Significant research milestones and treatment avenues including gene therapies and post-noise otoprotective strategies achieved in recent years are discussed.
Sound detection by the cochlea is made possible by its sensorineural cellular elements, namely sensory hair cells and supporting cells. Outer hair cells (OHC) are electromotile and contract upon depolarization (reverse-transduction). These cells mechanically enhance the vibration of a narrow region of the basilar membrane to improve detection sensitivity (approximately 40-60 dB)[3] and frequency selectivity of the organ of Corti through cochlear amplification. The mechanical vibration is then transduced by inner hair cells (IHC), the classical sensory receptor cells, into auditory neurotransmission. This transduction is achieved through electrochemical coupling to its postsynaptic auditory afferent neurons, the spiral ganglion neurons (SGN). The structural organization of the cochlea is maintained by supporting cells lining the sensory epithelium and lateral wall tissues, the stria vascularis and spiral ligament. The supporting cells are also critical in maintaining endolymph ion homeostasis and cochlea blood supply.
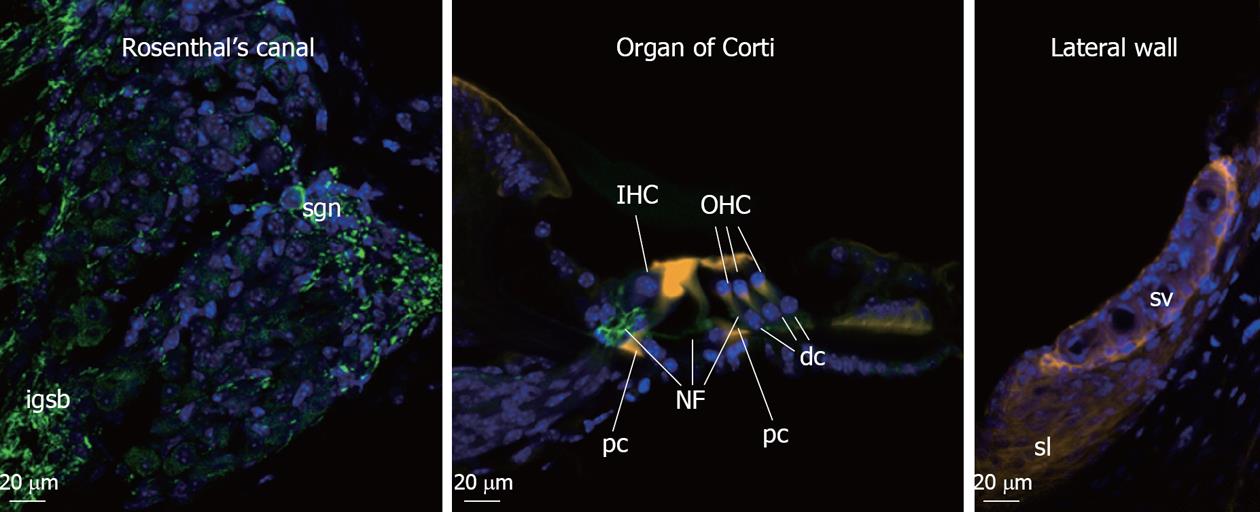
The classical features of NIHL at the cellular level include damaged hair cell stereocilia, hair cell loss, swelling of afferent dendrites and SGN in Rosenthal’s canal. The organ of Corti is compressed as result of damages to the supporting pillar cells, strial shrinkage, and loss of fibrocytes in the spiral limbus and spiral ligament. The cellular architecture of the high-frequency encoding basal region of the organ of Corti is more vulnerable to noise injury compared to the low-frequency apical region. This is consistent with the “half-octave shift” phenomenon[4,5] whereby the largest noise-induced threshold shifts are observed at the frequency approximately one-half octave above the stimulus frequency. This is especially true with pure tone and higher-level noise exposure, since the OHC are more prone to noise induced damage affecting their cochlear amplifier function. This sensorineural tissue damage is irreversible in the mammalian cochlea since the hair cells, which provide trophic support to the SGN, cannot regenerate. Figure 1 shows the cochlear cell types affected in NIHL.
Research using animal models of NIHL suggests two routes of cochlear damage following noise exposure. The first is that intense noise causes direct mechanical disruption of the hair cell stereocilia and direct damage to supporting and sensory cells leading to hair cell loss[6,7]. The other route is metabolic damage through various biochemical pathways that converge and cumulatively trigger hair cell death through either apoptosis or necrosis[8,9].
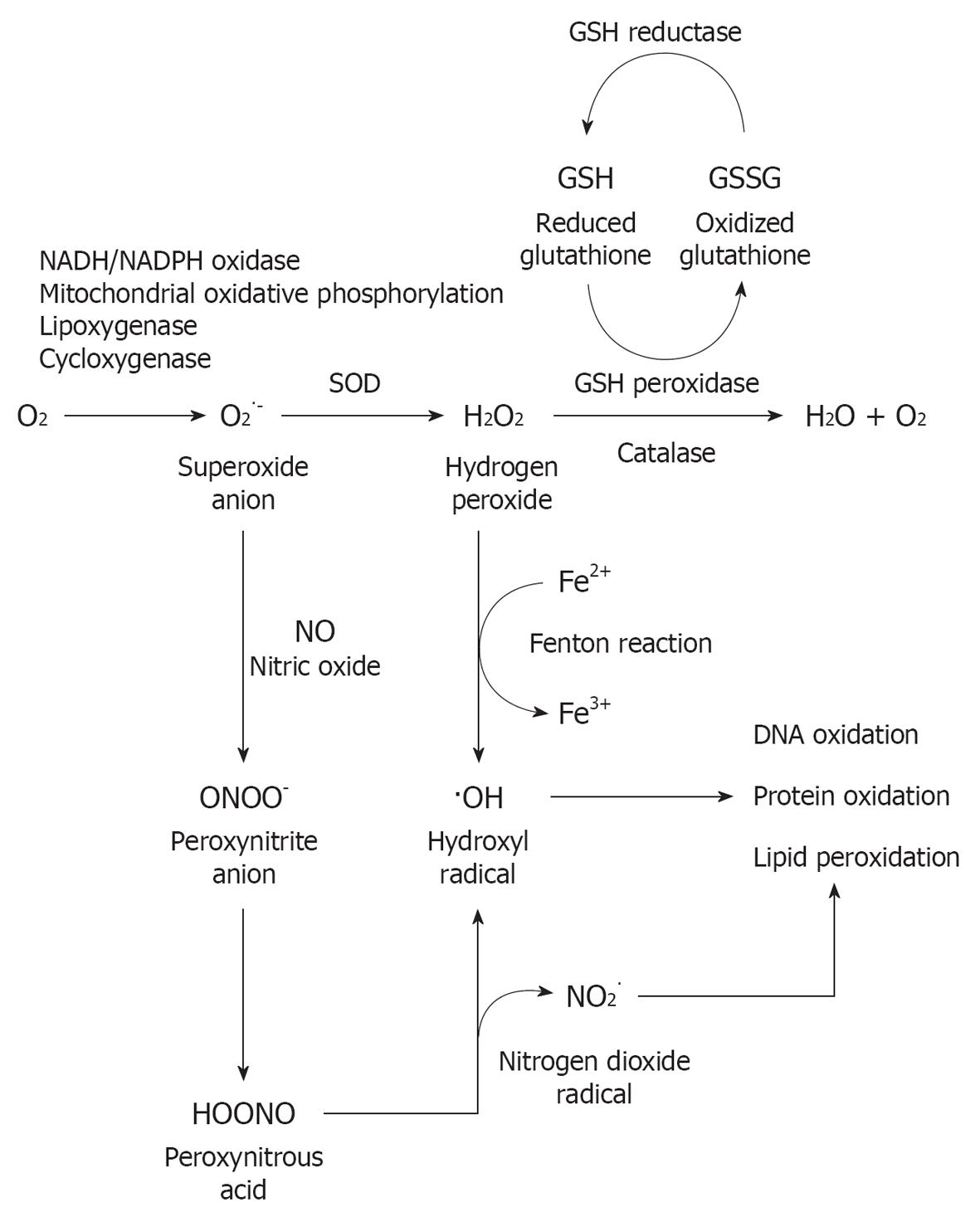
Current theories of metabolic damage focus on oxidative stress, which includes excessive generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the cochlea triggered by exposure to loud sound, followed by caspase-mediated cell death by apoptosis[8,10-12]. ROS have been detected in cochlear tissue immediately after noise exposure[13] and seen to persist for 7-10 d after, spreading from the basal end of the organ of Corti to the apical turn; the RNS product peroxynitrite (ONOO-), generated by the combination of nitric oxide (NO) and superoxide has also been found[14]. This prolonged oxidative stress is proposed to induce the delayed and continued cochlear injury. This time might, therefore, provide a “window of opportunity” for post-noise otoprotective interventions to ameliorate or repair injury to the cochlea and reduce the impact of hearing loss. Apoptosis-inducing factor and EndoG are also released by mitochondria into the cytosol of cochlear cells following noise exposure[15]. Translocation of these pro-apoptotic factors into the nucleus triggers apoptosis. Activation of the c-Jun N-terminal kinase/mitogen-activated protein kinase (JNK/MAPK) signaling pathway is also implicated in OHC apoptosis in response to oxidative stress[16].
Free radicals (ROS and RNS) can cause damage by reacting with DNA, proteins, cytosolic molecules, cell surface receptors, and breaking down membrane lipids. ROS produced by the mitochondria induce lipid peroxidation in the cochlea through the formation of malondialdehyde and 4-hydroxynonenal byproducts[14]. This overloads the cochlear antioxidant enzyme system, including superoxide dismutase, catalase (CAT), glutathione peroxidase and glutathione reductase, and depletes glutathione, the endogenous antioxidant. Figure 2 provides an overview of oxidative stress pathways and the production of free radicals. In addition to apoptosis, ROS generation also leads to inflammation, and production of the pro-inflammatory cytokines interleukin-6 (IL-6)[17] and tumor necrosis factor α[18]. The presence of vasoactive lipid peroxidation products such as isoprostanes potentially also lead to the reduced cochlear blood flow associated with excessive noise[19-21]. Noise-induced ischemia and subsequent re-perfusion further potentiate the generation of ROS. A recent study has implicated the NO synthase/cGMP-dependent protein kinase (Prkg-1) signaling pathway, normally involved in vasodilation, in NIHL[22]. Treatment with the phosphodiesterase type 5 inhibitor vardenafil (Levitra) almost completely prevented NIHL in the rat model.
Excessive noise also leads to an increase in free Ca2+ in cochlear hair cells immediately post-noise[23]. This increase can be caused by Ca2+ entry through ion channels, such as L-type Ca2+ channels and P2X2 ATP receptor subunit, and lead to further release of Ca2+ from intracellular stores[24]. Elevated Ca2+ levels in the cochlea may link to ROS production as well as triggering apoptotic and necrotic cell death pathways independent of ROS formation[24]. In knock-out mice lacking expression of the canonical transient receptor potential channel subtype 3 (TRPC3 channel), a non-selective cation-permeable receptor expressed in sensorineural cochlear tissue[25,26], cochlear hair cells displayed approximately 40% reduction in Ca2+ re-entry following intracellular calcium depletion. The TRPC knockout mice have hyperacusis at frequencies tonotopically encoded by mid-apical basilar membrane, a region highly reliant on OHC cochlear amplification[27]. The consequence of disrupted calcium homeostasis on noise susceptibility is also demonstrated in plasma membrane Ca2+-ATPase isoform 2 (Pmca2 or Atp2b2) mutant mice. The C-terminally truncated PMCA2a is the only isoform detected in the stereocilia of hair cells[28]. Pmca2 null mice are deaf while their heterozygous littermates have significant hearing loss[29]. People carrying a homozygous mutation in cadherin 23 (CDH23) and a heterozygous, hypofunctional variant in PMCA2 have exaggerated hearing loss compared to those having CDH23 mutation alone[30].
An established mechanism of NIHL damage is the excess release of the excitatory neurotransmitter glutamate at the IHC afferent synapse. Glutamate excitotoxicity resulting from excessive glutamate release following noise overstimulation leads to an influx of cations such as Ca2+ across the post-synaptic membrane. The osmotic imbalance results in swelling of the postsynaptic afferent dendrites. Secondary to this cellular degeneration is calcium-dependent caspase-mediated apoptosis by intrinsic (mitochondria-mediated) pathway[31-33]. This may lead to degeneration of type 1 SGN weeks and months after a noise exposure[34]. The inhibitory neurotransmitter γ-amino butyric acid (GABA) is also associated with the regulation of auditory function[35]. Mice lacking the GABAB1 receptor subunit have elevated hearing thresholds but increased resistance to permanent acoustic injury[35].
A theory much revisited recently is the role of intrinsic feedback pathways providing endogenous cochlear tissue protection against noise damage. Purinergic signaling through ATP activation of the ATP-gated ion channel P2X2 receptor subunit within the cochlea is known to modulate cochlear function through regulating ion homeostasis[36-38]. In a recent study, Housley et al[39] have shown that ATP is released into the cochlear partition upon sound exposure, activating P2X2 receptors, which reduce the sensitivity of the hair cells through K+ shunting. This purinergic regulation of hearing sensitivity was revealed by the absence of noise-induced temporary threshold shift (TTS) in P2X2 receptor knockout mice. P2X2 receptor knockout mice also showed higher threshold shifts in response to moderate noise exposure and more substantial permanent loss of hearing sensitivity compared to their wild-type littermates, supporting the protective role of P2X2 receptor signaling pathway in NIHL[40].
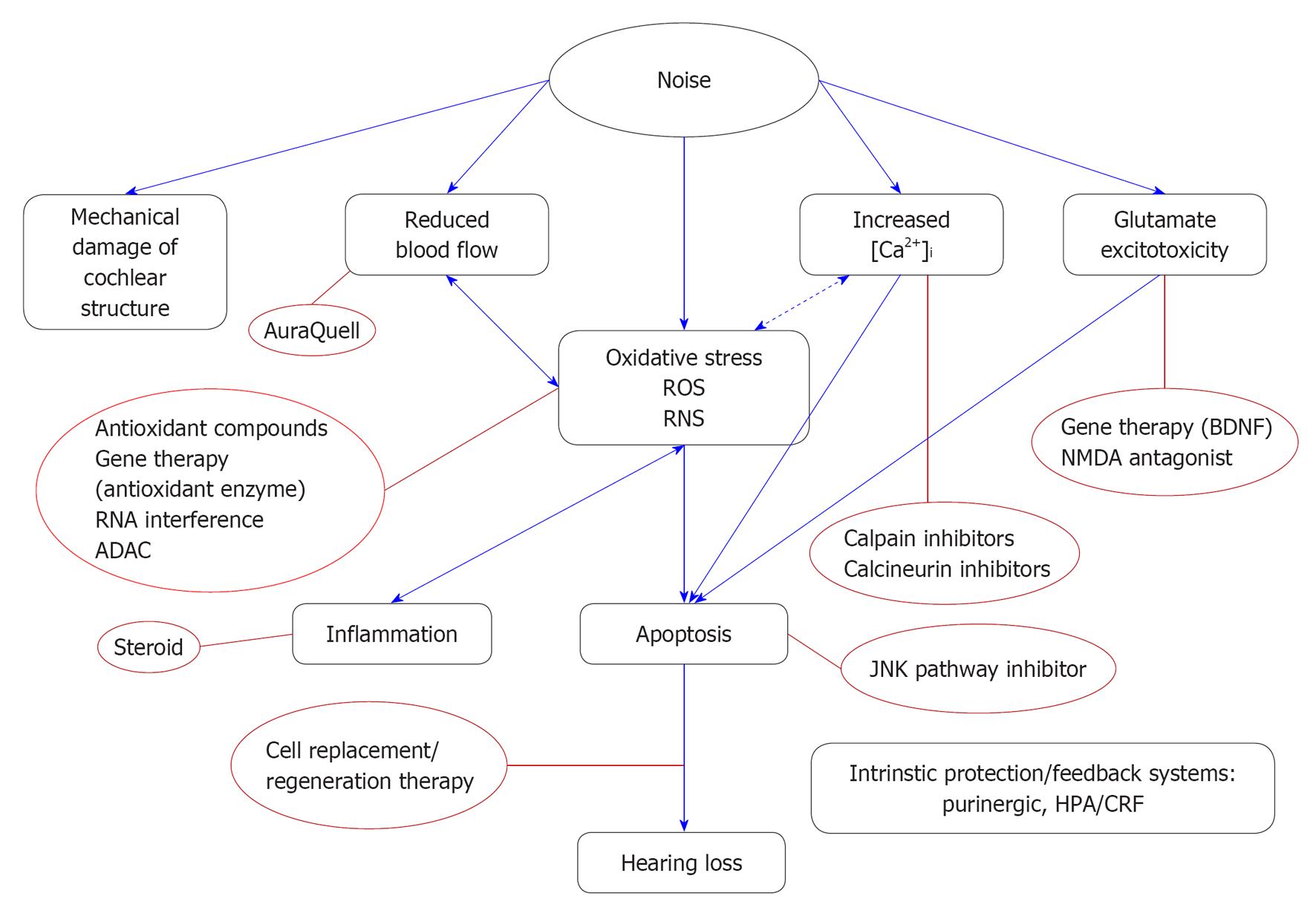
Noise causes psychological stress. The hypothalamic-pituitary-adrenal (HPA) axis can be activated by noise stress and directly modulate the sensitivity of the auditory system[41-43]. Glucocorticoid receptors are expressed in human and rodent cochlea[44-46]. Systemic glucocorticoids or steroid hormones are widely used to treat sudden hearing loss with variable success[47-49]. For example, dexamethasone decrease the auditory thresholds in mice subjected to a moderate acoustic trauma, while the pre-treatment with glucocorticoid receptor antagonists exacerbates threshold shifts[50]. The corticotropin-releasing factor (CRF) involved in the activation of the HPA axis also modulates hearing sensitivity. CRF receptor-1 knockout mice showed elevated auditory thresholds, while CRF receptor-2 knockout mice exhibits lower auditory thresholds than wild type mice, but increased susceptibility to acoustic trauma[51,52]. Figure 3 summarizes the mechanisms of NIHL discussed.
NIHL is a complex condition caused by the interaction of genetic and environmental factors. Therefore, individual vulnerability to NIHL is highly variable. Understanding the genetic makeup of people susceptible to NIHL will assist in early interventions and may lead to personalized therapies. Knockout mouse studies have implicated deficits in genes involved in antioxidative pathways or the structure of the cochlea to increase susceptibility to acoustic overstimulation. These include genes encoding proteins of the hair cell (Cdh23[53], Pmca2[29]), oxidative stress (Sod1[54]; Gpx1[55]), stress-activated heat shock factor (Hsf1[56,57]) and potassium recycling[58,59]. In contrast, until recently little was known about the genetic factors that influence NIHL in humans. The advance in high-throughput DNA sequencing technologies, or next-generation sequencing (reviewed in Metzker[60]) has greatly accelerated understanding of human NIHL genetic predisposition. Genes shown to be associated with oxidative stress and cochlear function in mice are obvious candidate genes for human studies.
Some original linkage studies on oxidative stress genes apparently showed a link between NIHL and mutations in these genes. Glutathione S-transferase Mu 1 and theta 1 (GSTM1 and GSTT1) deletion polymorphisms were found in 58 noise-exposed workers[61] and deletion polymorphisms of antioxidant genes paraoxonase 1, paraoxonase 2 and superoxide dismutase 2 (SOD2) were seen in 94 noise-exposed male workers[62]. However, these studies need to be interpreted with caution due to sample size and conflicting results from repeated studies with larger populations[63] (Swedish workers, 103 susceptible to noise and 114 resistant to noise). Association with the CAT gene was revisited by Konings et al[64] in two large independent populations (Swedish and Polish). In their study, additional single nucleotide polymorphisms (SNPs) were investigated to cover most of the common genetic variants. Interactions between noise exposure and genotypes and their effect on NIHL were also analyzed. Konings’ study confirmed that two SNPs in CAT have associations with NIHL susceptibility, but only when noise exposure levels are taken into account. Konings et al[65] extended their study in the two populations and analyzed 644 SNPs in 53 candidate genes. Positive associations were shown for protocadherin 15 (PCDH15) and myosin 14 (MYH14). These are of great importance to hearing function since cadherins 23 and PCDH15 form hair cell tip links to convey force to mechanotransduction (MET) channels in sensory hair cells[66] and patients with MYH14 mutations are affected by autosomal dominant hearing impairment (DFNA4)[67].
Hair cell stereocilia are bathed in endolymph with high K+ content, which provides the driving force for mechanosensory transduction. K+ enters the hair cells through MET channels, exits through basolateral K+ channels, and is recycled back to the endolymph through the outer sulcus cells, Reissner’s membrane, spiral ligament and spiral limbus[68]. Mutations in genes involved in K+ recycling, including GJB2, GJB3, GJB6, KCNE1, KCNQ1, and KCNQ4 cause both syndromic and nonsyndromic hearing loss (detailed in the Hereditary Hearing loss Homepage http://hereditaryhearingloss.org). Indeed, three SNPs in KCNE1 have been shown to have significant associations with NIHL[69] and the D85N polymorphism variant, when expressed in cell culture model, showed faster channel opening and larger K+ entry current. The same KCNE1 SNPs and one KCNQ4 SNP was confirmed to associate with NIHL in a later study[70].
In their seminal papers, Kujawa et al[71] have demonstrated that early-life exposure to noise exacerbates ARHL and that SGN are initially unharmed but dramatically degenerate 2 years after exposure to noise levels that cause TTS[34]. Their studies provided insight into the synergy between NIHL and ARHL, and also reinforced the importance of TTS in the development of progressive NIHL. A recent study of a rare heterozygous allele, P2X2 c.178G>T (p.V60L), presented in the DFNA41 type of progressive sensorineural hearing loss, in two unrelated large Chinese families has demonstrated neatly how environment and genetic predisposition interplay leading to NIHL[40]. DFNA41 family members heterozygous for the mutated ATP-gated P2X2 receptor (P2X2) exhibited elevated hearing thresholds in their 20 s. Mutation carriers with history of occupational noise exposure as young adults have increased threshold shifts of 10-20 dB in the 2-
8 kHz range compared to carriers with no previous noise exposure. On par with their human counterparts, p2rx2-null mice showed aggravated high-frequency hearing loss following continuous exposures to moderate noise from birth (8-16 kHz at 75 dB SPL). Patch-clamping and the use of fluorescent probes for membrane permeability analysis of transfected cells expressing P2X2 p.V60L showed abolished P2X2 receptor ion channel activity, suggesting impaired channel function in the mutant allele carriers. P2X2 receptors are expressed in the sensory hair cells and supporting cells of the organ of Corti and the afferent SGN[38,72]. Sustained noise exposure causes up-regulation of the p2rx2 transcripts and P2X2 protein[73,74]. Noise induced ATP release into the endolymphatic compartment (the scala media) activates P2X2 receptors, producing a cation shunt across the cochlear partition that reduces the driving force for both inner and OHC-mediated sound transduction[38,75,76]. The collective findings suggest the cochlear P2X2 receptor/ATP-gated ion channel signaling pathway confers protection from NIHL and the absence or mutation of P2X2 receptor increases susceptibility to NIHL and presbyacusis.
Given the vital need for therapeutic options for NIHL and the known genetic influences on individual susceptibility as discussed above, gene therapy is clearly an attractive prospect. The inner ear has an anatomical advantage for gene therapy; its relative isolation in the temporal bone encapsulated in the bony labyrinth minimizes unwanted effects of the introduced gene into other tissues. Also, as a fluid-filled organ, transfection reagent can access all functionally important cells. Several different gene therapy approaches, including those focused on neurotrophic or antioxidant support and cellular regeneration, have been explored.
Neurotrophic factors and their receptors have crucial roles in the development and maintenance of SGN, and so increasing their endogenous expression by gene therapy has been widely explored to treat NIHL[77]. Experimental viral vector delivery of neurotrophic genes to the cochlea to induce endogenous expression of the gene product, including the secretion of glial cell line-derived neurotrophic factor, hepatocyte growth factor, and brain-derived neurotrophic factor (BDNF)[78], has shown promise in preserving SGN following ototoxic and noise-induced cochlear damage (reviewed in Hildebrand et al[79]). Alternative routes of BDNF gene delivery include the use of cochlear implants to deliver fibroblasts transduced with BDNF gene cassette giving rise to BDNF secretion[80]. In addition, the grafting of transfected BDNF-secreting NIH3T cells to the posterior semicircular canals of the adult mouse inner ear has been found to elevate BDNF production[81].
Given the importance of antioxidant enzymes in curbing noise-induced free radical damage[82], gene therapy to over-express antioxidant enzymes in the cochlea may provide improved efficacy over systemic antioxidant delivery. Antioxidant gene therapy has been tested in cochlear injury induced by ototoxic drugs. Kawamoto et al[83] have shown a protective effect of adenovirus-mediated delivery of CAT and the SOD1 and SOD2 superoxide dismutase genes against aminoglycoside-induced cochlear injury in a guinea pig model.
Gene silencing through antisense oligonucleotides, microRNA and siRNA has been explored for otoprotection against cisplatin-induced hearing loss. Round window membrane delivery of siRNA against the transient receptor potential vanilloid 1 and transtympanic injection of siRNA against the NADPH oxidase NOX3 have shown to offer protection against cisplatin ototoxicity[84,85].
A recent advance in gene therapy is to regenerate hair cells in the adult organ of Corti. A potential strategy is to stimulate supporting cells of the organ of Corti to transdifferentiate into hair cells by the forced expression of the transcription factor Atoh1 (also known as Math 1). Izumikawa et al[86] showed that transfer of adenoviral vectors expressing Atoh1 resulted in the formation of “hair cell like” cells in the guinea pig organ of Corti 5 wk post-inoculation in ototoxic drug deafened cochleae. However, there are caveats in the study such as the number of new hair cells was not clear and that these cells could not be traced back to their precursors, making it difficult to delineate from hair cells that had recovered from the trauma. Further, although transdifferentiation of supporting cells to hair cells is possible, such has only been demonstrated in prenatal and neonatal preparations where both cell types are still developing[87-89]. Other studies have shown inhibition of Notch signaling to increase hair cell differentiation from stem cells in the otic placode. This mechanism is also dependent on Atoh1 activation, since silencing the transcription factor in the γ-secretase inhibitor-treated stem cells prevented the induction of hair cell fate[90]. A recent study has shown that post-noise application of a potent γ-secretase inhibitor to inhibit Notch signaling upregulates Atoh1, and leads to transdifferentiation of supporting cells into functional hair cells and improved ABR thresholds[91].
Perhaps even more innovative is the intensive research into cell-based therapy through transplantation of cells into the inner ear (reviewed by Hildebrand et al[79] and Shi et al[92]). Efforts include the generation of neurons from pluripotent embryonic stem cells and bone marrow-derived stem cells to replace or supplement auditory neurons in afferent innervation compromised by NIHL. Targeted delivery of the progenitor cells to the sensory epithelium and long-term survival and differentiation of stem cells into sensorineural cochlear tissue thus holds promise to ameliorate NIHL.
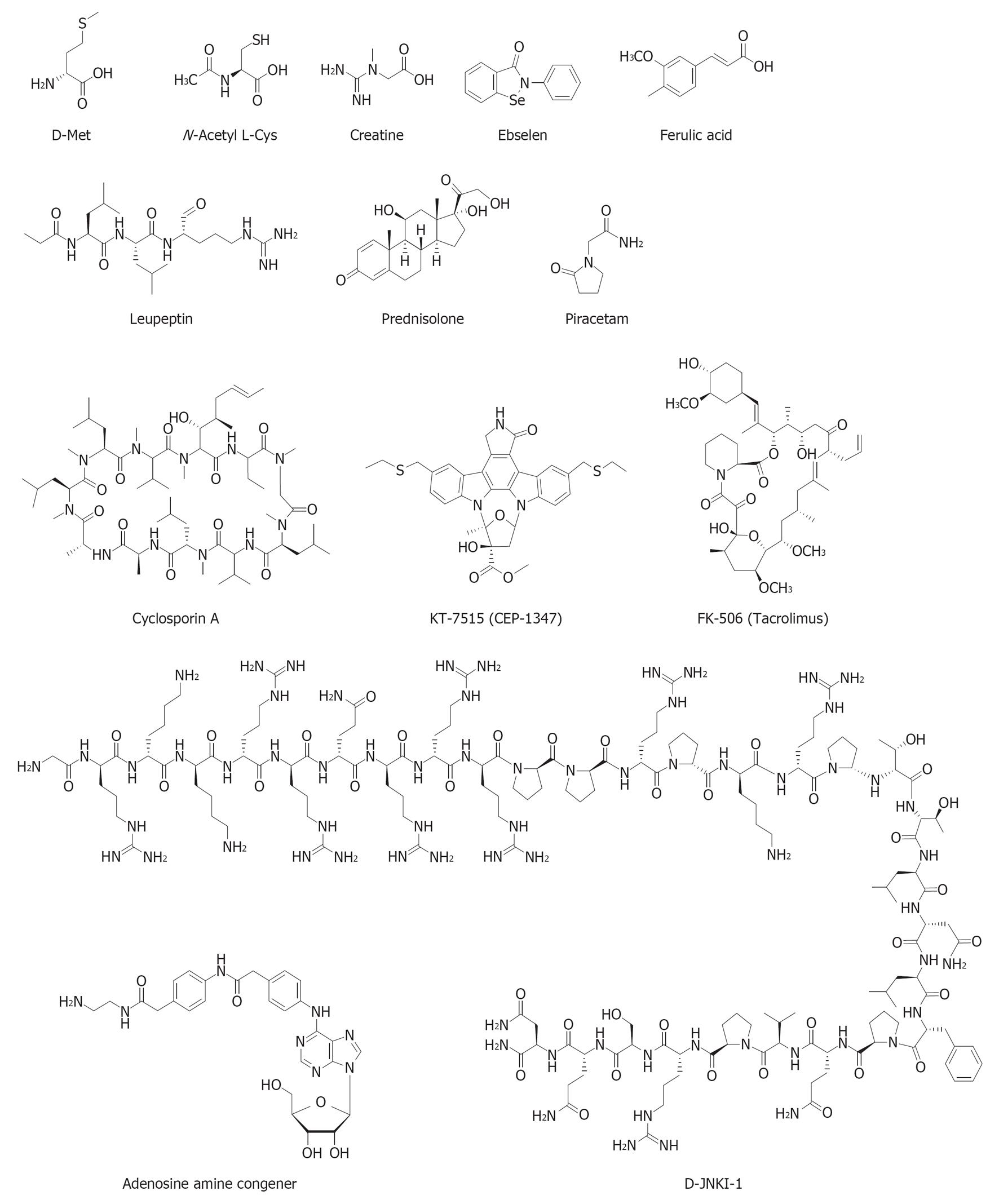
Several drugs and dietary supplements are currently in preclinical development against NIHL. The chemical structures of these otoprotective compounds are shown in Figure 4. Drug interventions in clinical trials, as depicted in the clinical database http://www.clinicaltrials.gov maintained by the National Library of Medicine at the National Institutes of Health, are included where appropriate.
Compounds that can prevent NIHL by inhibiting glutamate excitotoxicity and apoptosis include glutamate receptor (N-methyl-D-spartate) antagonists[93] and JNK/MAPK inhibitors[16,94]. The JNK group of cytoplasmic MAPKs mediate oxidative stress-induced apoptosis and are activated by environmental stress, pro-inflammatory cytokines, and excitotoxicity[95]. CEP-1347 (KT7515) is a mixed lineage kinase (upstream regulators of MAPK kinases) inhibitor, which shows promising protection from hair cell death induced by neomycin and noise[96]. Studies using a specific inhibitor of JNK, D-JNK1-I peptide, have also demonstrated protection against NIHL and aminoglycoside-induced hair cell loss when delivered directly into the scala tympani or locally to the round window membrane of the cochlea within 24 h of noise exposure[16,94]. Developed under the name of AM-111 (Xigen/Auris Medical), a Phase 2b clinical trial has recently been completed (November, 2012) in three European countries and has shown promise for the treatment of acute sensorineural hearing loss (ClinicalTrials.gov Identifier: NCT00802425).
Direct manipulation of intracellular Ca2+ levels pharmacologically is not practical, but an alternative route to minimize calcium-mediated apoptosis by blocking downstream cell death pathways has been attempted. Calpain is a family of calcium-dependent cysteine proteases ubiquitously expressed in mammalian cells. Calpain immunolabeling in the cochlea is upregulated upon noise exposure, particularly in the synaptic region of the OHCs and the nerve fibers projecting to the organ of Corti[97]. Cochlear perfusion with leupeptin, a potent calpain inhibitor, prior to noise exposure reduces noise-induced hair cell loss[97]. Another drug target is calcineurin, a serine-threonine phosphatase activated by calcium-dependent calpain activation. Increased calcineurin immunoreactivity was found at the cuticular plate of hair cells immediately after noise exposure[98]. Local delivery of calcineurin inhibitors cyclosporine A and FK506 to the cochlear perilymph using an osmotic mini-pump prior to and after noise exposure reduced noise-induced OHC death and hearing loss[98,99].
Ameliorating oxidative stress and buffering mitochondrial overproduction of free radicals is becoming an attractive avenue for the treatment of NIHL[8,100]. The potential for these therapies is highlighted by mutant mouse models. Mice with homozygous deletion of Cu/Zn superoxide dismutase 1 (sod1 knockout)[54,101], the endogenous antioxidant enzyme that catalyzes the conversion of superoxide into oxygen and hydrogen peroxide, or homozygous deletion of glutathione peroxidase 1 (Gpx1 knockout; the enzyme reducing hydrogen peroxide to water)[55], have increased noise vulnerability and noise-induced hair cell loss.
All the agents aforementioned require intra-cochlear or round window administration to be effective, and most of them are used prophylactically. The surgical administration route is obviously less attractive compared to oral intake against periodic noise exposure. Therefore, orally administered antioxidant supplements with low risk of side effects constitute the majority of otoprotective therapies in preclinical development[8,82,102-104]. N-acetylcysteine (NAC), Ebselen, D-methionine, and ACE Mg (AuraQuell, a combination of β-carotene, vitamins C and E plus magnesium) are amongst the most studied dietary antioxidant supplements approaching different phases of clinical trials for noise injury protection. NAC is a substrate for the antioxidant glutathione synthesis, activated upon de-acetylation to L-cysteine by the liver and local tissues. It is Food and Drug Administration (FDA)-approved for respiratory disease and for reversing acute hepatoxicity following acetaminophen overdose. NAC has previously been administered either intraperitoneally, or locally through the round window membrane to prevent acute acoustic trauma[105-107]. A recent double-blind study conducted on male employee of a steel manufacturing company has found oral NAC administration to be prophylactic to TTS, particularly in subjects with susceptibility to NIHL due to their deletion polymorphism for glutathione S-transferases (GSTM1 null, GSTT1 null, and GSTP1 Ile(105)/Ile(105))[108].
D-methionine is currently funded by the United States Department of Defense and approved by the FDA for Phase 3 clinical trial for treatment of permanent threshold shift (PTS) (Clinicaltrials.gov Identifier: NCT01345474). The amino acid D-methionine can be converted to cysteine through the intermediate homocysteine. Racemic methionine (D- and L-isoforms) is FDA-approved to acidify urine and is well tolerated when administered at doses ranging from 500 to 1000 mg/d. Like NAC, D-methionine can be administered orally, by systemic injection, or by direct application to the round window[109-112]. Ebselen is a mimic of glutathione peroxidase and has strong activity against the peroxynitrite anion (ONOO-)[113]. Ebselen was protective against PTS and TTS when tested in guinea pigs and rats[114-116]. Ebselen in oral capsule (200-600 mg) is also approaching Phase 2 clinical trials for TTS (Clinicaltrials.gov Identifier: NCT01444846; Sound Pharmaceuticals).
Creatine is another dietary supplement with potential for noise-injury prevention. Catalyzed by the enzyme creatine kinase, which is present in the mitochondria, brain and muscle tissue in different isoforms, creatine and phosphocreatine engage in phosphate buffering to provide rapid regeneration of adenosine-5’-triphosphate (ATP) in tissue with high metabolic energy demand, including cochlear hair cells and stria vascularis[117,118]. The creatine transporter controls cellular availability of creatine and mutations in its gene, SLC6A8, lead to creatine deficiency and X-linked syndromes showing mental retardation, developmental delay, epilepsy, speech and language delay, and bilateral sensorineural hearing loss[119-121]. Creatine kinase and the creatine transporter are both expressed in the sensory hair cells, SGN, supporting cells and in the lateral wall of the organ of Corti[117,122]. A high creatine diet has been found to reduce noise-induced TTS and PTS and hair cell loss in guinea pigs[123]. Clinical trials of creatine as a single drug or adjuvant against neurodegenerative diseases (Huntington’s, Parkinson’s, Amyotrophic lateral sclerosis) and bipolar depression have also been carried out. These trials are based on evidence that creatine can be neuroprotective by relieving oxidative stress, and that creatine can also inhibit apoptotic neuronal death through its inhibitory action on the mitochondrial transition pore[124,125].
Mice exposed to noise and treated with the anti-IL-6 antibody MR16-1 show improved ABR thresholds, reduced SGN loss and a reduction in the number of activated cochlear macrophages[17]. Combined treatment with the steroid prednisolone and the nootropic drug piracetam may rescue subjects from gun-shot noise damage[126]. In spite of the lack of a control group, results look promising. A larger number of patients recovered when treatment was given within the first hour after the acute trauma compared to those receiving treatment 1-16 h after, and only 13% recovered when treatment was given after 24 h or more.
AuraQuell, developed by OtoMedicine, is a combination of antioxidant vitamins (β-carotene, and vitamins C and E) and the mineral magnesium. The magnesium acts in part as a vasodilator and in part as an antioxidant. AuraQuell is currently in Phase 2-3 clinical trial for prevention of NIHL (ClinicalTrials.gov Identifier: NCT00808470).
Adenosine amine congener (ADAC), a selective A1 adenosine receptor agonist, has been shown to mitigate noise-induced threshold shifts, reduce oxidative stress, and facilitate hair cell survival when applied 24 h post-exposure to noise-exposed rats (8-12 kHz band noise for 2 to 24 h at 110 dB SPL)[127]. ADAC provides neuroprotection in experimental animal models of cerebral ischemia and Huntington’s disease[128-130]. Adenosine receptors are expressed in the cochlea in most cell types[131,132]. Prophylactic administration of the broadly selective A1 adenosine receptor agonist R-phenylisopropyladenosine through the round window membrane can also reduce noise-induced cochlear damage[133,134], and post-exposure administration of the selective A1 adenosine receptor agonist CCPA provides partial recovery of hearing loss[135]. Yet ADAC has advantages over other adenosine A1 receptor agonists, as it causes minimal peripheral side effects such as bradycardia, hypotension and hypothermia, and it is able to cross the blood-brain-barrier when applied systemically[136]. Other agents that have been shown to attenuate NIHL post exposure include D-methionine[109], ferulic acid[137], and a combination of salicylate and trolox[138].
NIHL is a preventable condition. However, even temporary hearing loss can incur cochlear injury that eventuate to permanent damage and hearing loss. Noise management and hearing loss prevention remain the principal strategies for reducing the burden of NIHL on the society and individuals. On the bright side, signifiant milestones have been reached in understanding the underlying cellular and molecular mechanisms of NIHL. The elucidation of oxidative stress as a major cause of NIHL has opened up therapeutic avenues, which was previously limited to electrical interventions of cochlear implants and hearing aids. Orally administered otoprotective compounds with antioxidant actions to protect against NIHL and “hearing pill” for post-exposure rescue will likely be available within the next decade. Advance in decoding the genetic predisposition for NIHL will facilitate early screening and will aid the development of personalized NIHL prevention and treatment strategies. Synergistically, advances in gene and stem cell therapy in animal models provide a promising path to remedy these genetic defects, and to regenerate sensory cells in the inner ear to restore hearing. These interventions would have been unthinkable until recently and these novel developments will likely change the face of NIHL research in the 21st century and reduce the impact of this sensory disability on global health.
We thank Dr. Srdjan Vlajkovic and Dr. Jennie Cederholm for their helpful comments in the manuscript preparation.
P- Reviewer Coate TM S- Editor Wen LL L- Editor A E- Editor Zheng XM
| 1. | Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23:146-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Smith PA, Davis A, Ferguson M, Lutman ME. The prevalence and type of social noise exposure in young adults in England. Noise Health. 2000;2:41-56. [PubMed] [Cited in This Article: ] |
| 3. | Ryan A, Dallos P. Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature. 1975;253:44-46. [PubMed] [Cited in This Article: ] |
| 4. | Ramamoorthy S, Nuttall AL. Half-octave shift in mammalian hearing is an epiphenomenon of the cochlear amplifier. PLoS One. 2012;7:e45640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Cody AR, Johnstone BM. Acoustic trauma: single neuron basis for the “half-octave shift”. J Acoust Soc Am. 1981;70:707-711. [PubMed] [Cited in This Article: ] |
| 6. | Slepecky N. Overview of mechanical damage to the inner ear: noise as a tool to probe cochlear function. Hear Res. 1986;22:307-321. [PubMed] [Cited in This Article: ] |
| 7. | Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear Res. 1984;16:43-53. [PubMed] [Cited in This Article: ] |
| 8. | Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1-19. [PubMed] [Cited in This Article: ] |
| 9. | Op de Beeck K, Schacht J, Van Camp G. Apoptosis in acquired and genetic hearing impairment: the programmed death of the hair cell. Hear Res. 2011;281:18-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Hu BH, Cai Q, Manohar S, Jiang H, Ding D, Coling DE, Zheng G, Salvi R. Differential expression of apoptosis-related genes in the cochlea of noise-exposed rats. Neuroscience. 2009;161:915-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229-236. [PubMed] [Cited in This Article: ] |
| 12. | Shi X, Nuttall AL. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 2003;967:1-10. [PubMed] [Cited in This Article: ] |
| 13. | Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504-508. [PubMed] [Cited in This Article: ] |
| 14. | Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201-209. [PubMed] [Cited in This Article: ] |
| 15. | Yamashita D, Miller JM, Jiang HY, Minami SB, Schacht J. AIF and EndoG in noise-induced hearing loss. Neuroreport. 2004;15:2719-2722. [PubMed] [Cited in This Article: ] |
| 16. | Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71:654-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Wakabayashi K, Fujioka M, Kanzaki S, Okano HJ, Shibata S, Yamashita D, Masuda M, Mihara M, Ohsugi Y, Ogawa K. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci Res. 2010;66:345-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Keithley EM, Wang X, Barkdull GC. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otol Neurotol. 2008;29:854-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Thorne PR, Nuttall AL, Scheibe F, Miller JM. Sound-induced artifact in cochlear blood flow measurements using the laser Doppler flowmeter. Hear Res. 1987;31:229-234. [PubMed] [Cited in This Article: ] |
| 20. | Seidman MD, Quirk WS, Shirwany NA. Mechanisms of alterations in the microcirculation of the cochlea. Ann N Y Acad Sci. 1999;884:226-232. [PubMed] [Cited in This Article: ] |
| 21. | Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163-173. [PubMed] [Cited in This Article: ] |
| 22. | Jaumann M, Dettling J, Gubelt M, Zimmermann U, Gerling A, Paquet-Durand F, Feil S, Wolpert S, Franz C, Varakina K. cGMP-Prkg1 signaling and Pde5 inhibition shelter cochlear hair cells and hearing function. Nat Med. 2012;18:252-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci USA. 1998;95:7127-7132. [PubMed] [Cited in This Article: ] |
| 24. | Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2166] [Cited by in F6Publishing: 2144] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 25. | Phan PA, Tadros SF, Kim Y, Birnbaumer L, Housley GD. Developmental regulation of TRPC3 ion channel expression in the mouse cochlea. Histochem Cell Biol. 2010;133:437-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tadros SF, Kim Y, Phan PA, Birnbaumer L, Housley GD. TRPC3 ion channel subunit immunolocalization in the cochlea. Histochem Cell Biol. 2010;133:137-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wong AC, Birnbaumer L, Housley GD. Canonical transient receptor potential channel subtype 3-mediated hair cell Ca(2+) entry regulates sound transduction and auditory neurotransmission. Eur J Neurosci. 2013;37:1478-1486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J Neurosci. 2001;21:5066-5078. [PubMed] [Cited in This Article: ] |
| 29. | Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693-18696. [PubMed] [Cited in This Article: ] |
| 30. | Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. N Engl J Med. 2005;352:1557-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249-254. [PubMed] [Cited in This Article: ] |
| 32. | Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res. 2007;227:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109-2114. [PubMed] [Cited in This Article: ] |
| 34. | Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077-14085. [PubMed] [Cited in This Article: ] |
| 35. | Maison SF, Casanova E, Holstein GR, Bettler B, Liberman MC. Loss of GABAB receptors in cochlear neurons: threshold elevation suggests modulation of outer hair cell function by type II afferent fibers. J Assoc Res Otolaryngol. 2009;10:50-63. [PubMed] [Cited in This Article: ] |
| 36. | Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128-141. [PubMed] [Cited in This Article: ] |
| 37. | Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Järlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Muñoz DJ. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7:55-61. [PubMed] [Cited in This Article: ] |
| 38. | Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Järlebark L, Burton LD, Setz VC, Cannell MB, Soeller C. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377-8388. [PubMed] [Cited in This Article: ] |
| 39. | Housley GD, Morton-Jones R, Vlajkovic SM, Telang RS, Paramananthasivam V, Tadros SF, Wong AC, Froud KE, Cederholm JM, Sivakumaran Y. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc Natl Acad Sci USA. 2013;110:7494-7499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Yan D, Zhu Y, Walsh T, Xie D, Yuan H, Sirmaci A, Fujikawa T, Wong AC, Loh TL, Du L. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc Natl Acad Sci USA. 2013;110:2228-2233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hear Res. 2002;165:96-102. [PubMed] [Cited in This Article: ] |
| 42. | Canlon B, Meltser I, Johansson P, Tahera Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear Res. 2007;226:61-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Basappa J, Graham CE, Turcan S, Vetter DE. The cochlea as an independent neuroendocrine organ: expression and possible roles of a local hypothalamic-pituitary-adrenal axis-equivalent signaling system. Hear Res. 2012;288:3-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Kumagami H, Terakado M, Takahashi H. Distribution of glucocorticoid receptors and 11β-hydroxysteroid dehydrogenase isoforms in the human inner ear. Otol Neurotol. 2013;34:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Terakado M, Kumagami H, Takahashi H. Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. Hear Res. 2011;280:148-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocorticoid receptors in the murine inner ear. Ann Otol Rhinol Laryngol. 2002;111:1133-1138. [PubMed] [Cited in This Article: ] |
| 47. | Piccirillo JF. Steroids for idiopathic sudden sensorineural hearing loss: some questions answered, others remain. JAMA. 2011;305:2114-2115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Wei BP, Mubiru S, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2006;CD003998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297-328. [PubMed] [Cited in This Article: ] |
| 50. | Tahera Y, Meltser I, Johansson P, Bian Z, Stierna P, Hansson AC, Canlon B. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. J Neurosci Res. 2006;83:1066-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Graham CE, Vetter DE. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci. 2011;31:1267-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Graham CE, Basappa J, Vetter DE. A corticotropin-releasing factor system expressed in the cochlea modulates hearing sensitivity and protects against noise-induced hearing loss. Neurobiol Dis. 2010;38:246-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Holme RH, Steel KP. Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol. 2004;5:66-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999;4:237-246. [PubMed] [Cited in This Article: ] |
| 55. | Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243-254. [PubMed] [Cited in This Article: ] |
| 56. | Fairfield DA, Lomax MI, Dootz GA, Chen S, Galecki AT, Benjamin IJ, Dolan DF, Altschuler RA. Heat shock factor 1-deficient mice exhibit decreased recovery of hearing following noise overstimulation. J Neurosci Res. 2005;81:589-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Sugahara K, Inouye S, Izu H, Katoh Y, Katsuki K, Takemoto T, Shimogori H, Yamashita H, Nakai A. Heat shock transcription factor HSF1 is required for survival of sensory hair cells against acoustic overexposure. Hear Res. 2003;182:88-96. [PubMed] [Cited in This Article: ] |
| 58. | Dixon MJ, Gazzard J, Chaudhry SS, Sampson N, Schulte BA, Steel KP. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet. 1999;8:1579-1584. [PubMed] [Cited in This Article: ] |
| 59. | Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106-1111. [PubMed] [Cited in This Article: ] |
| 60. | Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4795] [Cited by in F6Publishing: 3964] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 61. | Rabinowitz PM, Pierce Wise J, Hur Mobo B, Antonucci PG, Powell C, Slade M. Antioxidant status and hearing function in noise-exposed workers. Hear Res. 2002;173:164-171. [PubMed] [Cited in This Article: ] |
| 62. | Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, Vitale DF, La Manna P, Saulino C, Marcelli V. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 2004;50:2012-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Carlsson PI, Van Laer L, Borg E, Bondeson ML, Thys M, Fransen E, Van Camp G. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res. 2005;202:87-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Konings A, Van Laer L, Pawelczyk M, Carlsson PI, Bondeson ML, Rajkowska E, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet. 2007;16:1872-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Konings A, Van Laer L, Wiktorek-Smagur A, Rajkowska E, Pawelczyk M, Carlsson PI, Bondeson ML, Dudarewicz A, Vandevelde A, Fransen E. Candidate gene association study for noise-induced hearing loss in two independent noise-exposed populations. Ann Hum Genet. 2009;73:215-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Sotomayor M, Weihofen WA, Gaudet R, Corey DP. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature. 2012;492:128-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Donaudy F, Snoeckx R, Pfister M, Zenner HP, Blin N, Di Stazio M, Ferrara A, Lanzara C, Ficarella R, Declau F. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4). Am J Hum Genet. 2004;74:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1-9. [PubMed] [Cited in This Article: ] |
| 69. | Van Laer L, Carlsson PI, Ottschytsch N, Bondeson ML, Konings A, Vandevelde A, Dieltjens N, Fransen E, Snyders D, Borg E. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mutat. 2006;27:786-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 70. | Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A, Carlsson PI, Borg E, Van Camp G, Sliwinska-Kowalska M. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Genet. 2009;73:411-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115-2123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 72. | Järlebark LE, Housley GD, Thorne PR. Immunohistochemical localization of adenosine 5’-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. J Comp Neurol. 2000;421:289-301. [PubMed] [Cited in This Article: ] |
| 73. | Wang JC, Raybould NP, Luo L, Ryan AF, Cannell MB, Thorne PR, Housley GD. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport. 2003;14:817-823. [PubMed] [Cited in This Article: ] |
| 74. | Telang RS, Paramananthasivam V, Vlajkovic SM, Munoz DJ, Housley GD, Thorne PR. Reduced P2x(2) receptor-mediated regulation of endocochlear potential in the ageing mouse cochlea. Purinergic Signal. 2010;6:263-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Thorne PR, Munoz DJ, Nikolic P, Mander L, Jagger DJ, Greenwood D, Vlajkovic S, Housley GD. Potential role of purinergic signalling in cochlear pathology. Audiol Neurootol. 2002;7:180-184. [PubMed] [Cited in This Article: ] |
| 76. | Thorne PR, Muñoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea pig. J Assoc Res Otolaryngol. 2004;5:58-65. [PubMed] [Cited in This Article: ] |
| 77. | Fritzsch B, Pirvola U, Ylikoski J. Making and breaking the innervation of the ear: neurotrophic support during ear development and its clinical implications. Cell Tissue Res. 1999;295:369-382. [PubMed] [Cited in This Article: ] |
| 78. | Zhai SQ, Guo W, Hu YY, Yu N, Chen Q, Wang JZ, Fan M, Yang WY. Protective effects of brain-derived neurotrophic factor on the noise-damaged cochlear spiral ganglion. J Laryngol Otol. 2011;125:449-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, Dahl HH, Rose SD, Behlke MA, Smith RJ. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Okano T, Nakagawa T, Kita T, Endo T, Ito J. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol Ther. 2006;14:866-871. [PubMed] [Cited in This Article: ] |
| 82. | Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22-43. [PubMed] [Cited in This Article: ] |
| 83. | Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056-13065. [PubMed] [Cited in This Article: ] |
| 86. | Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271-276. [PubMed] [Cited in This Article: ] |
| 87. | Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 584] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 88. | Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 89. | Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699-6710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 90. | Jeon SJ, Fujioka M, Kim SC, Edge AS. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J Neurosci. 2011;31:8351-8358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 91. | Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 92. | Shi F, Edge AS. Prospects for replacement of auditory neurons by stem cells. Hear Res. 2013;297:106-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265-273. [PubMed] [Cited in This Article: ] |
| 94. | Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596-8607. [PubMed] [Cited in This Article: ] |
| 95. | Zine A, van de Water TR. The MAPK/JNK signalling pathway offers potential therapeutic targets for the prevention of acquired deafness. Curr Drug Targets CNS Neurol Disord. 2004;3:325-332. [PubMed] [Cited in This Article: ] |
| 96. | Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43-50. [PubMed] [Cited in This Article: ] |
| 97. | Wang J, Ding D, Shulman A, Stracher A, Salvi RJ. Leupeptin protects sensory hair cells from acoustic trauma. Neuroreport. 1999;10:811-816. [PubMed] [Cited in This Article: ] |
| 98. | Minami SB, Yamashita D, Schacht J, Miller JM. Calcineurin activation contributes to noise-induced hearing loss. J Neurosci Res. 2004;78:383-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Uemaetomari I, Tabuchi K, Hoshino T, Hara A. Protective effect of calcineurin inhibitors on acoustic injury of the cochlea. Hear Res. 2005;209:86-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591-3604. [PubMed] [Cited in This Article: ] |
| 101. | McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol. 1999;413:101-112. [PubMed] [Cited in This Article: ] |
| 102. | Kopke R, Bielefeld E, Liu J, Zheng J, Jackson R, Henderson D, Coleman JK. Prevention of impulse noise-induced hearing loss with antioxidants. Acta Otolaryngol. 2005;125:235-243. [PubMed] [Cited in This Article: ] |
| 103. | Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs. 2011;16:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 105. | Coleman JK, Kopke RD, Liu J, Ge X, Harper EA, Jones GE, Cater TL, Jackson RL. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hear Res. 2007;226:104-113. [PubMed] [Cited in This Article: ] |
| 106. | Bielefeld EC, Kopke RD, Jackson RL, Coleman JK, Liu J, Henderson D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 2007;127:914-919. [PubMed] [Cited in This Article: ] |
| 107. | Kopke RD, Jackson RL, Coleman JK, Liu J, Bielefeld EC, Balough BJ. NAC for noise: from the bench top to the clinic. Hear Res. 2007;226:114-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, Ma MC, Guo YL. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 109. | Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 110. | Clifford RE, Coleman JK, Balough BJ, Liu J, Kopke RD, Jackson RL. Low-dose D-methionine and N-acetyl-L-cysteine for protection from permanent noise-induced hearing loss in chinchillas. Otolaryngol Head Neck Surg. 2011;145:999-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Campbell K, Claussen A, Meech R, Verhulst S, Fox D, Hughes L. D-methionine (D-met) significantly rescues noise-induced hearing loss: timing studies. Hear Res. 2011;282:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Samson J, Wiktorek-Smagur A, Politanski P, Rajkowska E, Pawlaczyk-Luszczynska M, Dudarewicz A, Sha SH, Schacht J, Sliwinska-Kowalska M. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by D-methionine. Neuroscience. 2008;152:146-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear Res. 2007;226:44-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181:100-108. [PubMed] [Cited in This Article: ] |
| 115. | Yamasoba T, Pourbakht A, Sakamoto T, Suzuki M. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neurosci Lett. 2005;380:234-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004;114:333-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Wong AC, Velamoor S, Skelton MR, Thorne PR, Vlajkovic SM. Expression and distribution of creatine transporter and creatine kinase (brain isoform) in developing and mature rat cochlear tissues. Histochem Cell Biol. 2012;137:599-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Christie DL. Functional insights into the creatine transporter. Subcell Biochem. 2007;46:99-118. [PubMed] [Cited in This Article: ] |
| 119. | van de Kamp JM, Mancini GM, Pouwels PJ, Betsalel OT, van Dooren SJ, de Koning I, Steenweg ME, Jakobs C, van der Knaap MS, Salomons GS. Clinical features and X-inactivation in females heterozygous for creatine transporter defect. Clin Genet. 2011;79:264-272. [PubMed] [Cited in This Article: ] |
| 120. | Anselm IA, Alkuraya FS, Salomons GS, Jakobs C, Fulton AB, Mazumdar M, Rivkin M, Frye R, Poussaint TY, Marsden D. X-linked creatine transporter defect: a report on two unrelated boys with a severe clinical phenotype. J Inherit Metab Dis. 2006;29:214-219. [PubMed] [Cited in This Article: ] |
| 121. | Salomons GS, van Dooren SJ, Verhoeven NM, Marsden D, Schwartz C, Cecil KM, DeGrauw TJ, Jakobs C. X-linked creatine transporter defect: an overview. J Inherit Metab Dis. 2003;26:309-318. [PubMed] [Cited in This Article: ] |
| 122. | Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371-386. [PubMed] [Cited in This Article: ] |
| 123. | Minami SB, Yamashita D, Ogawa K, Schacht J, Miller JM. Creatine and tempol attenuate noise-induced hearing loss. Brain Res. 2007;1148:83-89. [PubMed] [Cited in This Article: ] |
| 124. | Béard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010;115:297-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 125. | Adhihetty PJ, Beal MF. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med. 2008;10:275-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 126. | Psillas G, Pavlidis P, Karvelis I, Kekes G, Vital V, Constantinidis J. Potential efficacy of early treatment of acute acoustic trauma with steroids and piracetam after gunshot noise. Eur Arch Otorhinolaryngol. 2008;265:1465-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Vlajkovic SM, Lee KH, Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR. Adenosine amine congener mitigates noise-induced cochlear injury. Purinergic Signal. 2010;6:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 128. | Von Lubitz DK, Beenhakker M, Lin RC, Carter MF, Paul IA, Bischofberger N, Jacobson KA. Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur J Pharmacol. 1996;302:43-48. [PubMed] [Cited in This Article: ] |
| 129. | Blum D, Gall D, Galas MC, d’Alcantara P, Bantubungi K, Schiffmann SN. The adenosine A1 receptor agonist adenosine amine congener exerts a neuroprotective effect against the development of striatal lesions and motor impairments in the 3-nitropropionic acid model of neurotoxicity. J Neurosci. 2002;22:9122-9133. [PubMed] [Cited in This Article: ] |
| 130. | Von Lubitz DK, Lin RC, Bischofberger N, Beenhakker M, Boyd M, Lipartowska R, Jacobson KA. Protection against ischemic damage by adenosine amine congener, a potent and selective adenosine A1 receptor agonist. Eur J Pharmacol. 1999;369:313-317. [PubMed] [Cited in This Article: ] |
| 131. | Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear Res. 1997;111:143-152. [PubMed] [Cited in This Article: ] |
| 132. | Vlajkovic SM, Abi S, Wang CJ, Housley GD, Thorne PR. Differential distribution of adenosine receptors in rat cochlea. Cell Tissue Res. 2007;328:461-471. [PubMed] [Cited in This Article: ] |
| 133. | Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21-32. [PubMed] [Cited in This Article: ] |
| 134. | Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997;113:198-206. [PubMed] [Cited in This Article: ] |
| 135. | Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR, Vlajkovic SM. Post exposure administration of A(1) adenosine receptor agonists attenuates noise-induced hearing loss. Hear Res. 2010;260:81-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247-264. [PubMed] [Cited in This Article: ] |
| 137. | Fetoni AR, Mancuso C, Eramo SL, Ralli M, Piacentini R, Barone E, Paludetti G, Troiani D. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575-1588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 138. | Yamashita D, Jiang HY, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |