Peer-review started: June 17, 2016
First decision: July 18, 2016
Revised: August 21, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: January 18, 2017
To investigate the microvascular (skeletal muscle tissue oxygenation; SmO2) response to transfusion in patients undergoing elective complex spine surgery.
After IRB approval and written informed consent, 20 patients aged 18 to 85 years of age undergoing > 3 level anterior and posterior spine fusion surgery were enrolled in the study. Patients were followed throughout the operative procedure, and for 12 h postoperatively. In addition to standard American Society of Anesthesiologists monitors, invasive measurements including central venous pressure, continual analysis of stroke volume (SV), cardiac output (CO), cardiac index (CI), and stroke volume variability (SVV) was performed. To measure skeletal muscle oxygen saturation (SmO2) during the study period, a non-invasive adhesive skin sensor based on Near Infrared Spectroscopy was placed over the deltoid muscle for continuous recording of optical spectra. All administration of fluids and blood products followed standard procedures at the Hospital for Special Surgery, without deviation from usual standards of care at the discretion of the Attending Anesthesiologist based on individual patient comorbidities, hemodynamic status, and laboratory data. Time stamps were collected for administration of colloids and blood products, to allow for analysis of SmO2 immediately before, during, and after administration of these fluids, and to allow for analysis of hemodynamic data around the same time points. Hemodynamic and oxygenation variables were collected continuously throughout the surgery, including heart rate, blood pressure, mean arterial pressure, SV, CO, CI, SVV, and SmO2. Bivariate analyses were conducted to examine the potential associations between the outcome of interest, SmO2, and each hemodynamic parameter measured using Pearson’s correlation coefficient, both for the overall cohort and within-patients individually. The association between receipt of packed red blood cells and SmO2 was performed by running an interrupted time series model, with SmO2 as our outcome, controlling for the amount of time spent in surgery before and after receipt of PRBC and for the inherent correlation between observations. Our model was fit using PROC AUTOREG in SAS version 9.2. All other analyses were also conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC, United States).
Pearson correlation coefficients varied widely between SmO2 and each hemodynamic parameter examined. The strongest positive correlations existed between ScvO2 (P = 0.41) and SV (P = 0.31) and SmO2; the strongest negative correlations were seen between albumin (P = -0.43) and cell saver (P = -0.37) and SmO2. Correlations for other laboratory parameters studied were weak and only based on a few observations. In the final model we found a small, but significant increase in SmO2 at the time of PRBC administration by 1.29 units (P = 0.0002). SmO2 values did not change over time prior to PRBC administration (P = 0.6658) but following PRBC administration, SmO2 values declined significantly by 0.015 units (P < 0.0001).
Intra-operative measurement of SmO2 during large volume, yet controlled hemorrhage, does not show a statistically significant correlation with either invasive hemodynamic, or laboratory parameters in patients undergoing elective complex spine surgery.
Core tip: Tissue oxygenation determined by Near Infrared Spectroscopy has been used to assess the adequacy of end-organ perfusion in models of trauma and sepsis and has been shown to correlate with stroke volume in models of hemorrhagic shock. We sought to investigate muscle tissue oxygenation (SmO2) during transfusion in patients undergoing complex spine surgery, and to study the association of SmO2 with invasive hemodynamic parameters in the clinical setting. In our study, we were unable to demonstrate a statistically significant correlation between SmO2 and either invasive hemodynamic, or laboratory parameters in patients undergoing elective complex spine surgery.
- Citation: Walz JM, Stundner O, Girardi FP, Barton BA, Koll-Desrosiers AR, Heard SO, Memtsoudis SG. Microvascular response to transfusion in elective spine surgery. World J Orthop 2017; 8(1): 49-56
- URL: https://www.wjgnet.com/2218-5836/full/v8/i1/49.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i1.49
Over 400000 elective spine fusion surgeries are performed annually in the United States[1]. Blood loss during complex spine surgery can be significant, and these patients frequently undergo either homologous or autologous blood transfusion, with the aim of preserving patient hemodynamics and adequate end-organ perfusion. While the transfusion of blood products is clearly indicated in situations of severe anemia associated with hemodynamic instability, the individual threshold at which a patient should undergo transfusion is less clear. Due to the properties of stored blood, unloading of oxygen may be impaired[2-4], and transfusion may thus not achieve the desired effect of optimizing oxygen supply to the tissues. Furthermore, liberal blood transfusions in patients undergoing elective orthopedic surgery are not associated with improved outcomes even in patients at high risk for cardiac complications, and may cause adverse side effects such as an increase in surgical side infections, pulmonary complications, and increased length of hospital stay in general patient populations undergoing non-cardiac surgery[5,6]. In addition, blood transfusions can cause a significant economic burden on the healthcare system if not clearly indicated[7].
Tissue oxygenation determined non-invasively by Near Infrared Spectroscopy (NIRS) has been suggested as one possible modality to determine the adequacy of end-organ perfusion in models of trauma and sepsis[8,9], and has been shown to correlate well with stroke volume in states of acute, untreated hypovolemia in models of hemorrhagic shock outside of the clinical arena[10]. We sought to investigate the microvascular (skeletal muscle tissue oxygenation; SmO2) response to transfusion in patients undergoing elective complex spine surgery, and to study the association of muscle tissue oxygenation with invasive hemodynamic parameters obtained by pulse contour analysis in the clinical setting, thereby providing guidance as to when to transfuse a patient.
After obtaining approval from the Institutional Review Board (Hospital for Special Surgery, New York, NY), potential participants were identified by review of the surgical schedule and approached on the day of surgery in the preoperative holding area. Twenty patients aged 18 to 85 years of age undergoing > 3 level anterior and posterior spine fusion surgery were enrolled. Exclusion criteria included minors, mentally disabled patients, pregnant women, employees, and prisoners. In addition, patients with skin lesions at the sensor placement site, and a history of allergies to skin adhesives were excluded from the study. Patients enrolled in the study were followed throughout the operative procedure, and for 12 h postoperatively.
After informed consent was obtained, patients were taken to the operating room where general anesthesia was induced in standard fashion. In addition to standard American Society of Anesthesiologists (ASA) monitors, patients received an invasive arterial blood pressure catheter (Edwards Lifesciences, Irvine, CA) in the radial artery position, as well as a multi-lumen central venous catheter (Arrow International, Reading, PA) for administration of fluids, blood products, blood sampling, and measurement of central venous pressure. The arterial pressure transducer was connected to a pulse contour analysis module (FloTrac, Edwards Vigileo®, Edwards Lifesiences, Irvine, CA) for continual analysis of stroke volume (SV), cardiac output (CO), cardiac index (CI), and stroke volume variability (SVV). To measure skeletal muscle oxygen saturation (SmO2) during the study period, a non-invasive adhesive skin sensor based on NIRS (CareGuide, Reflectance Medical, Westborough, MA) was placed over the deltoid muscle. After a 5-min period to obtain a stable baseline signal, continuous recording of optical spectra was performed throughout the operative procedure. Measurements were interrupted during prone positioning of the patient (anterior-posterior procedures), and during patient transport.
Central venous and mean arterial pressures were recorded with every routine lab draw during the procedure, and no less than every two hours intra-operatively. Lactate, hematocrit, base excess, arterial blood gases and central venous oxygen saturation were determined from blood samples that were drawn as part of routine care, and no less than every two hours intra-operatively.
No standardized protocol for administration of crystalloids, colloids, and blood products was used for the purpose of this study. Administration of fluids and blood products was performed at the discretion of the Attending Anesthesiologist based on individual patient comorbidities, hemodynamic status, and laboratory data. Crystalloid solutions were administered in the form of Lactated Ringer’s. The colloid administered during the study period was human albumin 5% in 250 mL aliquots. Blood products transfused were either autologous blood from cell saver (Hemonetics, Braintree, MA) in 125 mL aliquots, or allogenic packed red blood cells (PRBC) from the blood bank. No specific transfusion triggers were used, and the healthcare team was blinded to the collection of NIRS spectra. All administration of fluids and blood products followed standard procedures at the Hospital for Special Surgery, without deviation from usual standards of care. Time stamps were collected for administration of colloids and blood products, to allow for analysis of SmO2 immediately before, during, and after administration of these fluids, and to allow for analysis of hemodynamic data around the same time points. In general, PRBC were infused using pressure infusion bags (Vital Signs Inc, Totowa, NJ), whilst blood processed with the cell saver system was infused as a free flowing infusion.
Data on stroke volume, SmO2, transfusion, and blood gas were collected on a total of 20 patients. Several variables were collected on a continuously throughout the surgery and included heart rate, blood pressure, mean arterial pressure, SV, CO, CI, SVV and SmO2. These variables were identified at each time point with a time recording out to seconds. Patient data from the four sources was merged by a unique patient ID and timed at which the measurement occurred rounded to the nearest minute. Bivariate analyses were conducted to examine the potential associations between the outcome of interest, SmO2, and each hemodynamic parameter measured using Pearson’s correlation coefficient, both for the overall cohort and within-patients individually.
To examine the association between receipt of packed red blood cells and SmO2, we created a dataset that included 11 patients who had received PRBC with documented administration times. After conducting an exploratory data analysis on SmO2 using graphical techniques and percentile ranges, we excluded SmO2 values ≤ 30 as well as values that jumped by > 10% from one time point to the next, unless the jump in values was typical for that specific patient. Due to the few occurrences of multiple PRBC administration during surgery in our data, we only focused on the first PRBC event.
A variable, “timecount”, was created to standardize the time units, where the first minute of each patient surgery in our data was equal to one and subsequently increased by one unit for every minute until the end of the surgery. A second variable was created to flag the time at which PRBC was administered, where the variable was equal to 0 until the time at which PRBC were administered and 1 at every time point thereafter. Finally, an additional time variable, “postcount”, was created to count the time after PRBC was administered, where postcount = 1 referred to the minute at which PRBC was given and increased by one unit per minute of time remaining in surgery for each subsequent measurement. These flagging variables allowed us to run an interrupted time series model, with SmO2 as our outcome, controlling for the amount of time spent in surgery before and after receipt of PRBC and for the inherent correlation between observations. Our model was fit using PROC AUTOREG in SAS version 9.2. All other analyses were also conducted in SAS version 9.2 (SAS Institute Inc., Cary, NC, United States).
Summative patient demographics are presented in Table 1. Pearson correlation coefficients varied widely between SmO2 and each hemodynamic parameter examined. When examined for the overall cohort, where each unit of time served as an observation, the maximum number of observations was 8490 among the 20 participants. Several parameters, including central venous oxygen saturation ScvO2, lactate, venous blood oxygen tension (PvO2), arterial pH (pH-A), hematocrit, PRBC, cell saver, and albumin had less than 100 observations among all participants. The strongest positive correlations existed between ScvO2 (P = 0.41) and SV (P = 0.31) and SmO2; the strongest negative correlations were seen between albumin (P = -0.43) and cell saver (P = -0.37) and SmO2. Correlations for other laboratory parameters studied were weak and only based on a few observations (Table 2). When correlations were examined within individual patients, values varied widely; for example, correlations for SV varied from 0.40590 to -0.66903 for 18 patients with recorded SV values (data not shown).
| Age (yr) | Height (cm) | Weight (kg) | BMI | Sex (m/f) | |
| Mean (SD) | 59.80 (10.96) | 165.99 (9.83) | 75.84 (15.75) | 27.58 (5.45) | 5/15 |
| Variable | ρ(correlation) | No. of observations1 |
| ScvO2 | 0.40704 | 92 |
| SV | 0.30967 | 8490 |
| PvO2 | 0.20475 | 92 |
| HCT | 0.19402 | 89 |
| CO | 0.06498 | 8490 |
| Lactate | 0.0609 | 61 |
| CI | 0.05839 | 8490 |
| pH A | 0.0578 | 92 |
| SVV | -0.10652 | 8490 |
| RBC | -0.25085 | 18 |
| Cell saver | -0.37471 | 37 |
| Albumin | -0.42714 | 29 |
Average SmO2 for the 11 patients included in the final modeling was 57.63 (± SD 7.68), with a range of mean values from 47.30-61.92. The total number of observations analyzed was 7677, representing 7677 time counts, or minutes, of surgery over all patients included. Individual patient time counts ranged from 110 to 1609 (Table 3).
| Patient | No. of observations (time counts) | SmO2 statistics | |||
| Mean | Std Dev | Minimum | Maximum | ||
| 1 | 1609 | 47.30205 | 4.231185 | 39.24457 | 59.81775 |
| 2 | 261 | 54.48111 | 4.559080 | 41.36314 | 60.91571 |
| 3 | 110 | 58.08155 | 3.474810 | 49.92571 | 63.56974 |
| 4 | 1061 | 59.45269 | 5.814339 | 42.39521 | 67.48903 |
| 5 | 333 | 59.86487 | 6.765487 | 43.87553 | 70.40168 |
| 6 | 284 | 60.44015 | 2.490064 | 53.33939 | 68.04648 |
| 7 | 486 | 60.78656 | 13.90398 | 30.77515 | 75.79442 |
| 8 | 1440 | 60.95438 | 4.090378 | 48.86891 | 73.39652 |
| 9 | 420 | 61.17271 | 2.276567 | 54.89989 | 65.64407 |
| 10 | 1470 | 61.18899 | 2.880967 | 52.65419 | 69.78379 |
| 11 | 203 | 61.95770 | 3.067270 | 47.69575 | 71.06789 |
| Total | 7677 | 57.63074 | 7.677996 | 30.77515 | 75.79442 |
The final model showed a small, but significant increase in SmO2 at the time of PRBC administration by 1.29 units (P = 0.0002). SmO2 values did not change over time prior to PRBC administration (P = 0.6658) but following PRBC administration, SmO2 values declined significantly by 0.015 units (P < 0.0001).
The key finding of our study is that when compared to experimental[11], and clinical settings of uncontrolled hemorrhagic shock[8], intra-operative measurement of SmO2 during large volume, yet controlled hemorrhage does not show a statistically significant correlation with either invasive hemodynamic, or laboratory parameters in patients undergoing elective complex spine surgery.
The non-invasive assessment of tissue perfusion has garnered increasing interest in acute care medicine, based on the fact that traditional hemodynamic and oxygenation parameters such as blood pressure, central venous pressure, pulse oximetry, and central venous oxygen saturation are not necessarily reflective of the actual amount of blood loss, or the degree of shock a patient is experiencing. Furthermore, monitoring the macrocirculation with standard blood pressure, and heart rate monitors may not provide the clinician with relevant information about the adequacy of end-organ perfusion in certain disease states.
The ability of SmO2 values derived by NIRS to track the adequacy of fluid resuscitation has been demonstrated in a model of swine hemorrhagic shock[12], and a NIRS-derived variable of tissue oxygenation (StO2) has been used to guide closed-loop fluid resuscitation in animal models[13]. SmO2 has also been shown to have a strong correlation with stroke volume in a human model of acute central hypovolemia[14]. Recently, Bohula May and coworkers were able to demonstrate correlation of SmO2 with invasively-measured SvO2, and cardiac index (CI) in patients hospitalized with heart failure and cardiogenic shock[15].
In disease states such as septic shock and traumatic hemorrhage, low tissue oxygen saturation determined by modalities such as NIRS, and persistent alterations in microcirculatory blood flow determined with OPS-imaging have been shown to correlate with severity of illness, and to be associated with organ failure, and death[9,16-18].
While we were able to demonstrate a positive correlation between SmO2 and SV, no statistical significance was found in our study, despite significant blood loss experienced by some of the patients. It is important to note however that data generated in models of acute, un-resuscitated shock may not be comparable to the situation found during elective surgery, where patients undergo continuous administration of crystalloid, colloids, and blood products. The same is likely true for patients in cardiogenic shock on vasopressor therapy, where accumulation of significant oxygen debt is not uncommon, and is more likely to negatively affect tissue oxygenation.
No definitive transfusion triggers exist to guide clinicians during the intraoperative period, particularly during surgical procedures associated with substantial blood loss such as complex spine surgery. There is compelling retrospective data suggesting that intraoperative transfusion of blood in patients with anemia undergoing non-cardiac surgery, including orthopedic surgery is associated with an increase in morbidity and mortality[19]. The situation is different for post-operative patients, including those admitted to an intensive care unit with severe sepsis, as well as patients with active gastrointestinal hemorrhage outside the operating room. Several prospective, randomized controlled trials have shown that restrictive transfusion strategies are either superior, or non-inferior to liberal transfusion strategies with respect to outcomes such as in-hospital and 90-d mortality, infection, cardiac ischemia, and in-hospital acute myocardial infarction[5,6,20,21].
The most recent clinical practice guideline for perioperative blood management by ASA recommends that monitoring perfusion and oxygenation of vital organs should be continuous, and may include cerebral oximetry, and NIRS, in addition to standard hemodynamic monitors[22]. While conceptually attractive due to its non-invasive nature, and the ability to reliably monitor the microcirculation in a variety of tissue beds, data on the ability of NIRS to provide clinically relevant information in the intraoperative period during elective surgery is sparse, and is mostly restricted to the monitoring of cerebral oxygenation.
The microvascular response to red blood cell transfusion has been studied in patients with severe sepsis and trauma, however to our knowledge, no such data has been collected in patients undergoing elective orthopedic surgery associated with significant blood loss to date. Sakr and coworkers analyzed the microvascular response to transfusion in patients with severe sepsis. The authors were unable to demonstrate an overall effect of transfusion on sublingual microvascular perfusion as assessed with OPS-imaging. However, baseline microvascular blood flow predicted the microvascular response to transfusion. Those patients who were shown to have reduced microvascular blood flow at baseline demonstrated improved perfusion with transfusion whereas those with normal perfusion suffered a decrease in microvascular blood flow after transfusion[23]. The same pattern has been demonstrated in trauma patients using Sidestream Dark Field imaging, with some decline in microvascular blood flow in response to transfusion of stored RBC in patients who had normal sublingual perfusion patterns at baseline[24]. More recent investigations using NIRS-based technology to analyze the microvascular response to transfusion in trauma patients suggest that increasing age of transfused RBC results in decreased StO2 levels. This effect was demonstrated both in critically injured, as well as stable, but anemic patients[25,26]. These effects are likely attributable to “storage defects” of red blood cells, which decrease post-transfusion RBC survival. Changes that have been reported in the literature include depletion of adenosine triphosphate, and 2,3 diphosphoglycerate (2,3 DPG), a decrease in pH, release of potassium, reduced nitric oxide, increased cell volume, and reduced RBC deformability[27]. Further experimental evidence in support of a negative impact on physiologic properties of stored RBC comes from a recent analysis of the impact of exchange transfusion in a rat model using intravital microscopy among other techniques. Yalcin and coworkers were able to demonstrate that exchange transfusion with stored RBC’s produced microcirculatory vasoconstriction resulting in decreased blood flow and oxygen delivery that was not found in anemia alone, or transfusion with fresh red blood cells. In addition, the authors showed that stored RBC’s have a shorter circulating lifetime, and appear to be removed from circulating blood due to their impaired elastic, and hydrodynamic behavior[28].
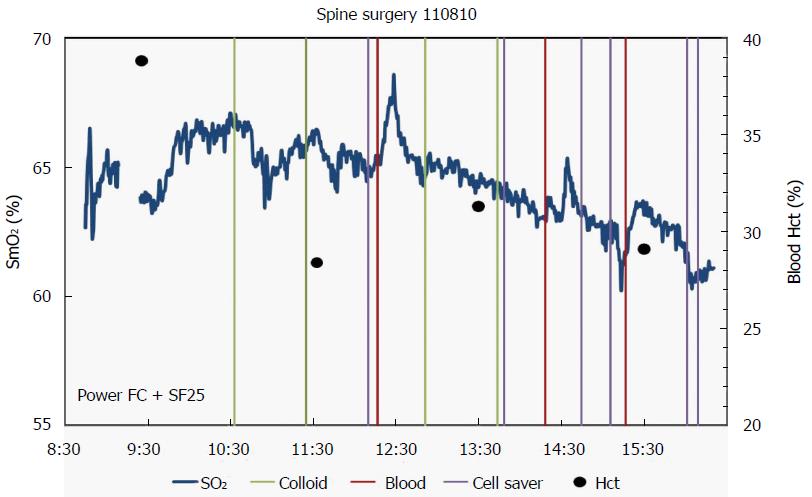
In conclusion, while we detected a short-lived increase in SmO2 in response to transfusion of PRBC, we were unable to detect a sustained, and relevant change in SmO2 signal in a patient population subjected to significant intra-operative blood loss. The reasons for the short-lived increase (Figure 1) remain speculative, but might be explained by the aforementioned changes found in stored RBC’s.
The limitations of our study are significant, and include the small number of subjects enrolled, and the fact that there were no specific treatment algorithms or outcomes studied in this proof-of-concept design. In addition, the age of transfused PRBC was not documented in our study protocol, which may limit the interpretability of our results. We were able however to evaluate a promising, non-invasive technology based on near-infrared spectroscopy in a “real-world” clinical setting, combined with a complex statistical analysis of continual oxygenation data. The results of this prospective, observational pilot study may provide a framework for future studies looking at specific patient outcomes associated with a hemodynamic management strategy incorporating real-time, microvascular blood flow data based on NIRS.
The assessment of the adequacy of end-organ perfusion in states of shock from sepsis or hemorrhage remain a challenge, as global hemodynamic measurements such as blood pressure, and cardiac filling pressures may not be reflective of perturbations of microcirculatory blood flow, and hence inadequate oxygen supply to critical end organs. Furthermore, standard physiologic parameters may not be sensitive to the early changes associated with hypovolemia from hemorrhage or anemia resulting in undetected tissue hypoxemia. Various technologies have been tested in both, exercise physiology laboratories as well as the clinical arena in an attempt to provide clinicians with more complete information regarding the state of the (micro) circulation, and oxygen supply to critical end organs. At the same time, individualized transfusion triggers based on objective data remain elusive, and there is ongoing research to determine rational, and safe transfusion patterns for hemodynamic impairment in states of shock from both, hemorrhagic, and non-hemorrhagic causes.
One of the non-invasive technologies, which have shown promise in experimental settings both in the laboratory, as well as the clinical arena, is based on Near Infrared Spectroscopy (NIRS). The technology allows for accurate assessment of skeletal muscle oxygen saturation (SmO2), which has been demonstrated to be a very early indicator of central hypovolemia in humans in a model of lower body negative pressure. It also shows excellent correlation with non-invasively measured stroke volume in the same model. A decrease in peripheral muscle NIR spectra is reflective of a decrease in tissue blood volume, and increased oxygen extraction by the peripheral tissues. NIRS has shown promise as an adjunct monitoring system for patients undergoing emergency surgery for trauma, or during treatment of patients in septic shock in addition to standard physiologic monitors for hemodynamic evaluation.
This study is the first to investigate tissue oxygenation based on NIRS in orthopedic patients undergoing elective complex spine surgery with anticipated high volumes of blood loss. While restrictive transfusion strategies appear safe in high-risk patients undergoing hip surgery, intra-operative blood loss during these procedures is usually much lower compared to estimated blood loss incurred during complex spine surgery. Optimal transfusion strategies during the latter type of surgery remain elusive, and largely based on experience and local practice patterns. The authors sought to determine in this observational pilot study if NIRS derived data on microcirculatory blood flow may provide useful objective information on the microcirculatory response to transfusion, which could guide subsequent prospective randomized controlled clinical trials on rational transfusion strategies in patients undergoing complex spine surgery. They also developed a complex statistical model for the analysis of continuous NIR spectra and their correlation with invasive hemodynamic and laboratory parameters, which can serve as a template for future trials in this area.
As they were unable to detect a sustained, and relevant change in SmO2 signal in a patient population subjected to significant intra-operative blood loss, the role of NIRS during elective surgery associated with large volume blood loss will require further investigation.
Light emitted in the near infrared spectrum from 700 to 100 nm near infrared spectroscopy can penetrate deep in to the muscle, and be reflected back to a sensor bundle providing information on the absorption spectra of hemoglobin, and de-oxyhemoglobin. This spectral information allows for the calculation of skeletal muscle tissue oxygenation with great accuracy. The sensor used in this clinical study also allows for correction of fat thickness and skin pigmentation, thus further increasing the accuracy of the spectral information derived from the tissues.
This manuscript is a well-written report of an original study, with good analysis and methodology, informative tables, and clear results.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alimehmeti R, Elgafy H, Kahveci R, Lakhdar F S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 695] [Cited by in F6Publishing: 734] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 2. | Benesch RE, Benesch R. The reaction between diphosphoglycerate and hemoglobin. Fed Proc. 1970;29:1101-1104. [PubMed] [Cited in This Article: ] |
| 3. | Sladen RN. The oxyhemoglobin dissociation curve. Int Anesthesiol Clin. 1981;19:39-70. [PubMed] [Cited in This Article: ] |
| 4. | Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA. 1993;269:3024-3029. [PubMed] [Cited in This Article: ] |
| 5. | Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114:283-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 380] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 6. | Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 937] [Cited by in F6Publishing: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 7. | Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 495] [Cited by in F6Publishing: 528] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 8. | Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Beilman GJ. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma. 2007;62:44-54; discussion 54-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 10. | Soller BR, Yang Y, Soyemi OO, Ryan KL, Rickards CA, Walz JM, Heard SO, Convertino VA. Noninvasively determined muscle oxygen saturation is an early indicator of central hypovolemia in humans. J Appl Physiol (1985). 2008;104:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Soller BR, Ryan KL, Rickards CA, Cooke WH, Yang Y, Soyemi OO, Crookes BA, Heard SO, Convertino VA. Oxygen saturation determined from deep muscle, not thenar tissue, is an early indicator of central hypovolemia in humans. Crit Care Med. 2008;36:176-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Soller BR, Cingo N, Puyana JC, Khan T, Hsi C, Kim H, Favreau J, Heard SO. Simultaneous measurement of hepatic tissue pH, venous oxygen saturation and hemoglobin by near infrared spectroscopy. Shock. 2001;15:106-111. [PubMed] [Cited in This Article: ] |
| 13. | Chaisson NF, Kirschner RA, Deyo DJ, Lopez JA, Prough DS, Kramer GC. Near-infrared spectroscopy-guided closed-loop resuscitation of hemorrhage. J Trauma. 2003;54:S183-S192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 14. | Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol (1985). 2004;96:1249-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | May EB, Soller B, O’Brien M, Kidd S, Berg D, O’Malley R, Morrow D, Wiviott S. 173: Non-Invasive Measure of Tissue Perfusion, SMO2, Compared with Standard Invasive Assessements of Shock. Crit Care Med. 2014;42:A1402. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825-1831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 806] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 17. | De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1005] [Cited by in F6Publishing: 936] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 19. | Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3699] [Cited by in F6Publishing: 3245] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 20. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1186] [Cited by in F6Publishing: 978] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 21. | Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 578] [Cited by in F6Publishing: 545] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 22. | American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122:241-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 23. | Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, Creteur J, Gullo A, Vincent JL, De Backer D. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35:1639-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, Angotti JM, Magnotti LJ, Kerby JD, Rue LW, Barnum SR, Patel RP. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, Magnotti LJ, Kerby JD, Rue LW, Angotti JM, Garrett CA, Hendrick LE, Croce MA. The deleterious effect of red blood cell storage on microvascular response to transfusion. J Trauma Acute Care Surg. 2013;75:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063-17068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 469] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 28. | Yalcin O, Ortiz D, Tsai AG, Johnson PC, Cabrales P. Microhemodynamic aberrations created by transfusion of stored blood. Transfusion. 2014;54:1015-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |