Published online Feb 6, 2016. doi: 10.4292/wjgpt.v7.i1.156
Peer-review started: August 11, 2015
First decision: September 11, 2015
Revised: October 6, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: February 6, 2016
AIM: To study the effectiveness of melatonin vs placebo in children with functional dyspepsia (FD).
METHODS: The study was conducted as a double blind, randomized, placebo controlled crossover trial. Subjects were aged 8-17 years and diagnosed with FD based on Rome III criteria. All subjects had failed to respond to 4 wk of acid suppression. Subjects receive a continuous two weeks of placebo and a continuous two weeks of melatonin in an order blinded to the participant and the study team. A Global Clinical Score was obtained to assess changes in abdominal pain. Pain was self-reported to be worse (grade 1), no change (grade 2), moderate improvement (grade 3), good (grade 4; minimal pain and not interfering with daily activities), or excellent (grade 5; no pain), respectively. A positive clinical response was defined as a grade 3 or greater response. Subjects wore an actigraph to assess sleep during a one week baseline period and during each treatment period. Subjects’ sleep latency and total sleep time were recorded throughout the duration of the study.
RESULTS: Fourteen subjects were enrolled and 12 completed the study. One withdrew prior to starting both melatonin and placebo and the other before starting melatonin. A positive clinical response (grade 3-5) was achieved in 42% of subjects on melatonin vs 50% of subjects on placebo (NS). Effect size was calculated and revealed a Cohen’s D of 0.343 which demonstrates a medium effect favoring placebo. A grade 4 or grade 5 response was seen in 4 patients on melatonin and 5 patients on placebo. Baseline sleep parameters were in the healthy range with the longest sleep latency being just over 20 min (mean 7.46 ± 8.53 min) and the shortest sleep duration just over 7 h (mean 10.09 ± 2.72 h). The mean latency did not differ between periods of treatment with melatonin as compared to placebo (4.48 ± 6.45 min vs 3.58 ± 4.24 min; NS). The mean sleep duration did not differ between periods of treatment with melatonin as compared to placebo (9.90 ± 3.53 h vs 9.41 ± 2.70 h; NS).
CONCLUSION: Melatonin does not appear to have efficacy in relieving pain in unselected pediatric FD. Future studies should consider FD subtypes, pathophysiologic mechanisms, and baseline sleep disturbances.
Core tip: Medical therapy is limited in children with functional dyspepsia. This creates a challenging clinical dilemma with regards to managing their symptoms. Melatonin has been shown to have a positive effect on pain in adults with functional dyspepsia or irritable bowel syndrome, independent of its effects on sleep. To date, there have been no studies to evaluate the effect of melatonin on abdominal pain in children. In the current study, melatonin did not result in improvement in abdominal pain or sleep parameters in children with functional dyspepsia.
- Citation: Zybach K, Friesen CA, Schurman JV. Therapeutic effect of melatonin on pediatric functional dyspepsia: A pilot study. World J Gastrointest Pharmacol Ther 2016; 7(1): 156-161
- URL: https://www.wjgnet.com/2150-5349/full/v7/i1/156.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i1.156
Pediatric functional dyspepsia (FD) is defined as persistent or recurrent pain or discomfort centered in the upper abdomen (above the umbilicus) that is unrelated to a change in stool frequency or form and not exclusively relieved by defecation[1]. It is a common diagnosis in children with population prevalence estimates of 3.5% to 27%[1,2]. A number of biologic contributors to the generation of FD have been identified and include inflammation, electromechanical dysfunction (e.g., altered gastric emptying) and visceral hyperalgesia[3,4].
Melatonin, a hormone produced, in part, by the pineal gland, has a well-known role in regulation of sleep-wake cycles through its sleep promoting effects. Melatonin is commonly used to treat sleep disturbances related to sleep-onset latency in children and has been shown to be effective at a dose of 5 mg[5]. Parent-reported sleep disturbances occur in nearly half of children with FD with the most common problems being related to sleep onset and maintenance[6]. These sleep disturbances are positively associated with functional disability in FD, with this association mediated through physical symptoms, including pain[6]. Thus, melatonin may be a useful treatment in this population to decrease pain indirectly through improvement in sleep.
Less widely recognized is melatonin’s production in parts of the body other than the pineal gland, such as in the digestive system and in immune cells including mast cells[7]. The total amount of melatonin in the digestive tract exceeds that of the pineal gland and blood. Melatonin is produced at high levels in the gastrointestinal mucosa in response to food. It exhibits a large number of biologic effects which may be relevant to FD including both excitatory and inhibitory effects on the enteric nervous system, anti-inflammatory properties, and both anxiolytic and antidepressant effects[8-10]. At pharmacologic doses, melatonin delays gastric emptying, in part acting as a partial 5-HT antagonist[10]. Thus, melatonin may also have a direct impact on visceral sensitivity.
One study of melatonin in adult patients with irritable bowel syndrome (IBS), another functional gastrointestinal disorder which shares common pathophysiologic mechanisms with FD, provides support for this secondary pathway of action[9]. In this study, two weeks of treatment with melatonin resulted in decreased mean abdominal pain scores and an increased mean rectal pain threshold without influencing sleep, anxiety, or depression scores[9]. To date, melatonin efficacy has been demonstrated in reducing pain in adults with FD in a single study[8]. Specifically, twelve weeks of melatonin (5 mg taken at bedtime) resulted in complete resolution of pain in 56.6% of patients as compared to only 6.7% of the patients who received placebo[8]. However, the specific mechanism of action was not entirely clear, given limited information provided related to methods of measurement on key variables. Although significant differences for complete resolution of pain did not emerge until the second month of treatment, participants receiving placebo took a significantly larger number of “sham” pain pills, nearly doubling those take by the melatonin group in the first month of treatment. This would suggest that they may have had a partial response during the first month that was not captured by the primary measurement strategy. Taken together, these studies suggest that treatment with melatonin may have a beneficial effect on pain in children with FD within a relatively short window of time.
Of note, there have been only a few placebo controlled trials of medications in children specifically with FD. This holds true for melatonin, as well, which has not yet been studied in children with abdominal pain in general or with FD specifically. This paucity of studies reflects the difficulty with conducting such trials in children, as well as concerns regarding the ethics of witholding treatment for prolonged periods (during the placebo phase) in a vulnerable population. These issues might be addressed, in part, by small n trials which create the potential for establishing preliminary efficacy of an intervention and establishing benefit and feasibility of completing a similar trial with a larger sample size[11]. The length of treatment can be based on the suspected mechanism of action to minimize study duration and the associated potential for unnecessary delay in treatment modification if/when a treatment is found to be ineffective. In a small n preliminary trial for FD, a cross-over design is important to not only control for the large number of biopsychosocial factors which may affect treatment response but also to evaluate the effects of treatment withdrawal, an important aspect of small n trials.
The aim of the current study was to assess the effectiveness of melatonin vs placebo in children with FD. The study was designed as a preliminary small n cross-over trial to establish an anticipated effect size from which a larger, adequately powered study could be based if results appeared promising. The specific study duration was selected based on previous work in adults with IBS and FD suggesting the beneficial impact of melatonin may be seen in as little as 2-4 wk. Thus, a 1-mo cross-over trial was deemed appropriate at this stage both from a scientific and ethical perspective.
The study was conducted as a single site, double blind, randomized, placebo-controlled crossover trial. The study involved a one week baseline sleep data collection period followed by four weeks of sleep data collection and medication administration. The four weeks of medication administration involved a continuous two weeks of placebo and a continuous two weeks of melatonin in an order blinded to the participant and the study team. The study protocol was approved by the Children’s Mercy Kansas City Institutional Review Board. Informed parental consent and participant assent were obtained prior to completion of any study procedures.
Patients ages 8-17 seen in the pediatric gastroenterology clinic with a diagnosis of FD defined by Rome III criteria and persistent pain despite a minimum of 4 wk of acid suppression were included. Patients were excluded if they received opiates, tramadol, gabapentin or benzodiazepines in the 4 wk prior to enrollment.
Melatonin and placebo were compounded by the Children’s Mercy Hospital Investigational Drug Services (CMH IDS) Pharmacy in liquid form. Melatonin bottles contained 70 mg of melatonin (5 mg/dose × 14 d). Bottles were labeled A or B and d 8-21 or d 22-35 with dosing instructions of “take 20 mL at bedtime per measurement on dropper”.
At enrollment, each participant received both treatment bottles and each participant was given a sleep diary with instructions and an actigraph watch with instruction on its usage. On D 1-7, in order to obtain baseline sleep parameters, participants were instructed to wear the actigraph and complete the sleep diary (sleep latency and sleep duration each day). On D 8-21, participants were instructed to wear the actigraph, complete the sleep diary and take the medication labeled “D 8-21”. On D 22, a follow up phone call was made and a standardized history was utilized to assign a Global Clinical Score (see Measures). On D 22-35, participants were instructed to wear the actigraph, complete the sleep diary and take the medication labeled “D 22-35”. Between day 36-40, participants returned to clinic and turned in the sleep diary and actigraph. A standardized history was utilized to obtain a second Global Clinical Score.
Clinical response: A Global Clinical Score was determined by interview to measure changes in abdominal pain as follows: (1) Grade 1: Worse - clinical deterioration with increasing pain intensity and/or frequency; (2) Grade 2: No change - no increase or decrease in pain intensity or frequency; (3) Grade 3: Moderate improvement - partial clinical response with definite improvement in pain, but not meeting criteria for Grade 4 response; (4) Grade 4: Good - nearly complete relief of symptoms with minimal residual pain and not interfering with daily activities; and (5) Grade 5: Excellent - complete relief of pain. A positive clinical response was defined as a response grade of 3 or greater, with subjects dichotomized into “responders” and “non-responders” following each 2-wk medication trial.
Sleep parameters: Time in bed and out of bed were taken from the sleep diaries and uploaded to the actigraph data. Sleep latency and duration were calculated from actigraphy data. The ActiGraph brand accelerometry monitor watches and its accompanying software were used for this study. The accelerometer measures movement several times per second. Scoring programs show activity levels for specified time periods and determine when an individual is asleep or awake. This methodology has been validated in studies comparing actigraphy to polysomnography (the gold standard for evaluating sleep)[12,13].
SPSS version 18 was utilized. The response rate on melatonin was compared to placebo using Fischer’s exact test. The mean sleep latency and sleep duration, respectively, on melatonin vs placebo were compared using Wilcoxon Signed Rank Test.
Fourteen subjects were enrolled and 12 completed the study. One withdrew prior to starting both melatonin and placebo. The other withdrew after completing placebo and before starting melatonin (this participant did not respond to placebo). The two participants who withdrew were not included in analysis. Fifty-eight percent of the participants were female and 83% were Caucasian. Patients ranged in age from 11 to 16 years old with a mean age of 13.8 ± 1.6 years. Pain was the predominant symptom in each patient and associated symptoms included nausea in 67%, bloating in 42%, early satiety in 33%, and vomiting in 25%. All participants met criteria for FD, while three participants also met criteria for IBS.
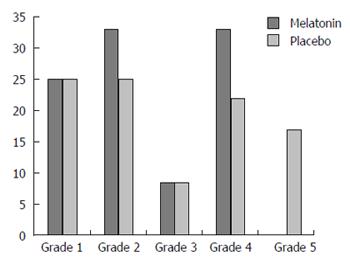
A positive clinical response (Grade 3-5) was achieved in 42% of subjects on melatonin vs 50% of subjects on placebo (NS). Effect size was calculated and revealed a Cohen’s D of 0.343 which demonstrates a medium effect favoring placebo. A summary of the post treatment pain relief grades are shown in Figure 1. Individual global clinical scores for melatonin and placebo are shown in Table 1. Of the three patients who also met criteria for IBS, two responded to placebo and not melatonin. The third responded to both.
Baseline sleep parameters were in the healthy range with the longest sleep latency being just over 20 min (mean 7.46 ± 8.53 min) and the shortest sleep duration just over 7 h (mean 10.09 ± 2.72 h). The mean latency did not differ between periods of treatment with melatonin as compared to placebo (4.48 ± 6.45 min vs 3.58 ± 4.24 min; NS). The mean sleep duration did not differ between periods of treatment with melatonin as compared to placebo (9.90 ± 3.53 h vs 9.41 ± 2.70 h; NS).
Just one participant had improved sleep (50% reduction in sleep latency and 20% increase in total sleep time), and this was while taking melatonin. However, this participant was classified as a non-responder, with a clinical grade of 2 following the melatonin treatment phase. Therefore, improved sleep could not be correlated to improved pain in this case.
In contrast to the previous study of FD in adults, melatonin had no significant impact on pain in children with FD in the current study[8]. In fact, the study was discontinued following interim analyses which indicated no beneficial response of melatonin and, in fact, appeared to favor placebo. The 50% placebo response rate in the current study was similar to what has been reported in other pediatric abdominal pain trials where placebo response has generally been 32%-58%[14]. These discordant results may be a function of different pathophysiologic processes being more or less important at different ages or may be a result of differences in study design which target different pathophysiologic factors.
One potential differential factor relates to FD subtyping across the lifespan. In adults, there are two recognized FD subtypes, epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS)[2]. The pediatric FD criteria do not contain these subtypes. EPS is defined by pain or burning confined to the epigastrium whereas PDS is defined by the presence of early satiety and/or postprandial bloating[2]. The participants in the one adult FD study previously noted consisted of patients with symptoms of EPS. EPS is rare in pediatric FD and, in fact, none of the subjects in our study had pain localized only to the epigastrium[15]. Most of the participants in the current study would have met adult criteria for PDS. This may be important in that it has been suggested that adults with EPS may have altered melatonin production[16]. Thus, differences in response between the two studies may indicate different mechanisms of symptom generation for EPS as compared to PDS.
A second factor that may have led to different results is duration of treatment. As noted previously, we chose the treatment duration for the current study based on initial findings in adults with IBS and FD that suggested a treatment response of melatonin could be detected in as little as 2-4 wk[8,9]. In the previous adult FD study, although a partial response arguably was seen within the first month of treatment, significant differences in pain resolution did not emerge between the melatonin and placebo groups until the second month of treatment[8]. This overall delay in treatment response suggests that the observed benefits of melatonin treatment may have been due to improvement in some factor other than visceral hypersensitivity, possibly mediated through improvement in sleep over time. In short, treatment duration in our study may simply not have been long enough to test an indirect effect of melatonin on pain through improvement in sleep.
Third, and related to the above, is the relative composition of samples. The adult study contained patients with sleep disturbances and these disturbances frequently improved or resolved with melatonin. However, the current study utilized an unselected group of children and adolescents presenting for evaluation of FD who all ultimately demonstrated sleep parameters in the normal range at baseline and which did not change significantly on melatonin. The composition of our sample is both a strength and limitation for our study. On the one hand, there was limited ability to test the impact of melatonin on pain via a sleep improvement pathway. On the other hand, having a group of participants without discernible sleep problems at baseline allowed us to more clearly assess the effects of melatonin independent from sleep, i.e., via a visceral hypersensitivity pathway.
In conclusion, the short-term (i.e., 2 wk) use of melatonin does not appear to have efficacy in relieving pain in an unselected pediatric FD population. The strengths of our study are the cross-over design and that we were able to study melatonin in a group of patients without sleep disturbances so we were able to assess efficacy independent from effects on sleep. In contrast, we studied a relatively small population and did not address whether melatonin might be effective over a longer duration of treatment and/or in patients with comorbid sleep issues. Future studies involving melatonin may take into consideration FD subtypes and whether melatonin has effects on specific potential pathophysiologic factors including the presence of inflammation, electromechanical dysfunction, or the presence of baseline sleep disturbances.
Pediatric functional dyspepsia (FD) is defined as persistent or recurrent pain or discomfort centered in the upper abdomen (above the umbilicus) that is unrelated to a change in stool frequency or form and not exclusively relieved by defecation.
Melatonin and placebo were compounded by the Children’s Mercy Hospital Investigational Drug Services Pharmacy in liquid form.
The mean sleep latency and sleep duration, respectively, on melatonin vs placebo were compared using Wilcoxon Signed Rank Test.
Subjects wore an actigraph to assess sleep during a one week baseline period and during each treatment period. Subjects’ sleep latency and total sleep time were recorded throughout the duration of the study.
The study was conducted as a double blind, randomized, placebo controlled crossover trial.
This is an original study looking for a new therapeutic indication for melatonin in children with FD.
P- Reviewer: Chiarioni G, Dumitrascu DL, Rodella LF S- Editor: Qiu S L- Editor: A E- Editor: Wang CH
| 1. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [PubMed] [Cited in This Article: ] |
| 2. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [PubMed] [Cited in This Article: ] |
| 3. | Friesen CA, Schurman JV, Colombo JM, Abdel-Rahman SM. Eosinophils and mast cells as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther. 2013;4:86-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Rosen JM, Cocjin JT, Schurman JV, Colombo JM, Friesen CA. Visceral hypersensitivity and electromechanical dysfunction as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther. 2014;5:122-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33:1605-1614. [PubMed] [Cited in This Article: ] |
| 6. | Schurman JV, Friesen CA, Dai H, Danda CE, Hyman PE, Cocjin JT. Sleep problems and functional disability in children with functional gastrointestinal disorders: an examination of the potential mediating effects of physical and emotional symptoms. BMC Gastroenterol. 2012;12:142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res. 2010;62:282-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Klupińska G, Poplawski T, Drzewoski J, Harasiuk A, Reiter RJ, Blasiak J, Chojnacki J. Therapeutic effect of melatonin in patients with functional dyspepsia. J Clin Gastroenterol. 2007;41:270-274. [PubMed] [Cited in This Article: ] |
| 9. | Song GH, Leng PH, Gwee KA, Moochhala SM, Ho KY. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402-1407. [PubMed] [Cited in This Article: ] |
| 10. | Mozaffari S, Rahimi R, Abdollahi M. Implications of melatonin therapy in irritable bowel syndrome: a systematic review. Curr Pharm Des. 2010;16:3646-3655. [PubMed] [Cited in This Article: ] |
| 11. | Cushing CC, Walters RW, Hoffman L. Aggregated N-of-1 randomized controlled trials: modern data analytics applied to a clinically valid method of intervention effectiveness. J Pediatr Psychol. 2014;39:138-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Cellini N, Buman MP, McDevitt EA, Ricker AA, Mednick SC. Direct comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiol Int. 2013;30:691-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201-207. [PubMed] [Cited in This Article: ] |
| 14. | Friesen CA, Schurman JV, Abdel-Rahman SM. Present state and future challenges in pediatric abdominal pain therapeutics research: Looking beyond the forest. World J Gastrointest Pharmacol Ther. 2015;6:96-104. [PubMed] [Cited in This Article: ] |
| 15. | Schurman JV, Singh M, Singh V, Neilan N, Friesen CA. Symptoms and subtypes in pediatric functional dyspepsia: relation to mucosal inflammation and psychological functioning. J Pediatr Gastroenterol Nutr. 2010;51:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Chojnacki C, Poplawski T, Blasiak J, Chojnacki J, Klupinska G. Does melatonin homeostasis play a role in continuous epigastric pain syndrome? Int J Mol Sci. 2013;14:12550-12562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |