Published online May 15, 2017. doi: 10.4291/wjgp.v8.i2.93
Peer-review started: November 5, 2016
First decision: December 29, 2016
Revised: January 27, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Processing time: 193 Days and 6 Hours
Gastrointestinal involvement in plasma cell neoplasms, either as primary localizations (extramedullary plasmacytomas) or as secondary involvement in systemic multiple myeloma, is a well-known event. Accurate histological examination is crucial in defining the diagnosis. In this report, an uncommon case of duodenal localization of myeloma with plasmablastic features is described, with emphasis on the role of clinical data and findings from ancillary immunostaining techniques to avoid misdiagnosis.
Core tip: In cases of gastrointestinal involvement by high-grade plasma cell neoplasia, the presence of large atypical cells infiltrating the lamina propria of the mucosa may lead to an erroneous diagnosis of poorly differentiated carcinoma. Clinical data and findings from ancillary immunostaining techniques are crucial to avoid misdiagnosis.
- Citation: Licci S. Duodenal localization of plasmablastic myeloma. World J Gastrointest Pathophysiol 2017; 8(2): 93-95
- URL: https://www.wjgnet.com/2150-5330/full/v8/i2/93.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i2.93
Involvement of the gastrointestinal tract by plasma cell neoplasms is a well-known event, and many cases have been described in the literature, either as primary localizations (extramedullary plasmacytomas)[1] or as secondary involvement in systemic multiple myeloma[2]. In this report, the immunomorphological findings of an uncommon case of duodenal localization of myeloma with plasmablastic features are described.
A 60-year-old woman presented with unintentional weight loss, anemia and thrombocytopenia. Serum protein electrophoresis revealed a monoclonal peak of IgG (30 g/L), and immunofixation identified λ light chains. For better hematological evaluation, a bone marrow trephine biopsy was performed. Marrow spaces showed remarkably increased cellularity (about 90%), mainly represented by immature/atypical CD138+ plasma cells with prevalent λ chain immunohistochemical expression (about 80% of the total cellularity). Accordingly, the diagnosis was plasma cell neoplasm, with morphological features consistent with “high-grade” plasma cell myeloma. The residual cellularity was composed of trilineage hematopoietic cells with reactive changes, rare CD34+ blast cells and some B (CD20+) and T (CD3+) reactive small lymphocytes, showing interstitial and micronodular distribution.
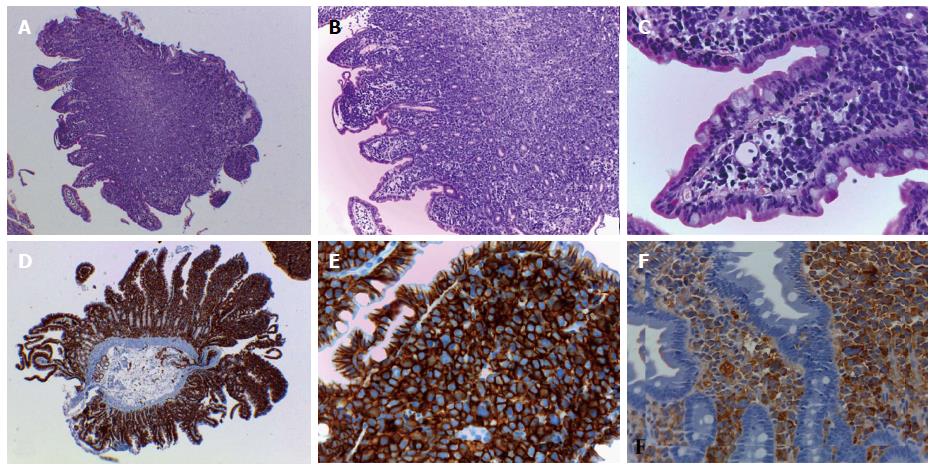
In the days following the initial presentation to clinic, the patient re-presented with an acute worsening of anemia and an episode of melena. Suspected gastrointestinal bleeding was investigated by esophagogastroduodenoscopy; the esophageal and gastric mucosa appeared normal, while the duodenal mucosa was characterized by presence of multiple micropolyps, which were biopsied for histology. Microscopic examination revealed a diffuse infiltration in the lamina propria by medium- to large-sized cells with high nuclear-cytoplasmic ratio, atypical pleomorphic nuclei and prominent nucleoli, consistent with malignant neoplasia (Figure 1A-C). Immunohistochemical study excluded infiltration by poorly differentiated carcinoma (i.e., AE1/3 cytokeratin immunostaining was negative) and revealed a diffuse and strong positivity for the CD138 plasma cell marker (Figure 1D and E). Further immunostaining analyses showed a prevalent λ chain expression (Figure 1F).
Ultimately, the diagnosis of duodenal localization of plasma cell neoplasm showing plasmablastic myeloma features was made on the basis of the cell morphology findings and consistent with the previous bone marrow trephine biopsy diagnosis.
In cases of gastrointestinal plasma cell neoplasia, histological examination can represent a diagnostic pitfall, especially when the clinical data are missing; the underlying plasma cell neoplasia can remain unknown and the tumor cells can appear immature and/or atypical. In fact, the presence of neoplastic atypical cells diffusely infiltrating the lamina propria among glandular structures may be easily misdiagnosed as a poorly differentiated carcinoma. Furthermore, even in well differentiated cases with recognizable plasma cells, the issue can be complicated by the presence of numerous Russel bodies - spherical intracytoplasmic eosinophilic immunoglobulin - containing structures, easily detectable by microscopic observation-either in association with neoplastic plasma cell proliferation, in cases of lymphoproliferative disorders displaying a certain degree of plasma cell differentiation, or in non-neoplastic, inflammatory processes characterized by a conspicuous plasma cell infiltrate, such as the so-called Russell body gastritis[3]. In such cases, plasma cells can take on the form of the signet-ring cells of poorly differentiated mucinous carcinoma of the gastrointestinal tract. Thus, careful microscopic observation must be integrated with ancillary techniques, mainly immunohistochemical staining analyses, to formulate the right diagnosis. Immunoreaction for cytokeratins is determinant for excluding a carcinoma, and demonstration of plasma cell [CD138+ and/or CD38+ and/or VS38c (plasma cell p63)+] proliferation with κ or λ light-chain restriction enables distinction between a reactive and a neoplastic plasma cell infiltrate.
For the case presented herein, the clinical data represented an important tool for making the right diagnosis, similar to a previously described case of extramedullary plasmablastic myeloma of the small bowel[4]. The patient’s experience of acute worsening of anemia and an episode of melena suggested gastrointestinal bleeding; esophagogastroduodenoscopy disclosed the duodenal mucosa lesions and the histological diagnosis were supported by the clinical-anamnestic data of a previous diagnosis of multiple myeloma. In such cases of secondary gastroenteric involvement by myeloma, therapy options include induction chemotherapy with immunomodulatory agents or proteasome inhibitors and corticosteroids. Stem cell transplantation may improve the remission rates and overall survival.
A 60-year-old woman with recent clinical history of multiple myeloma presented for acute worsening of anemia and an episode of melena.
To address suspected gastrointestinal bleeding, an esophagogastroduodenoscopy was performed and revealed multiple micropolyps of duodenal mucosa, which were biopsied for histology.
Adenomatous duodenal polyps; Primary duodenal adenocarcinoma; Secondary duodenal involvement by myeloma; Other neoplasia.
Serum protein electrophoresis monoclonal peak of IgG for the underlying disease; Severe worsening of anemia.
Bone osteolytic lesions for the underlying disease.
Secondary duodenal localization of plasmablastic myeloma.
Proteasome inhibitors and corticosteroids; Stem cell transplantation.
Because of the possible morphological overlap, a secondary gastrointestinal localization of high-grade myeloma can be mistakenly diagnosed as poorly differentiated adenocarcinoma.
The term “plasmablastic” in myeloma indicates a low degree of tumor cell differentiation, related to a more aggressive biological behavior.
Correlation of the histopathological findings with clinical and medical history is the basis of a correct diagnosis for plasmablastic myeloma with duodenal localization.
The strength of this report lies in the importance of the histopathological study, deeply influenced by the knowledge of the clinical and medical history.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mica L S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Lopes da Silva R. Extramedullary plasmacytoma of the small intestine: clinical features, diagnosis and treatment. J Dig Dis. 2012;13:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Talamo G, Cavallo F, Zangari M, Barlogie B, Lee CK, Pineda-Roman M, Kiwan E, Krishna S, Tricot G. Clinical and biological features of multiple myeloma involving the gastrointestinal system. Haematologica. 2006;91:964-967. [PubMed] |
| 3. | Licci S, Sette P, Del Nonno F, Ciarletti S, Antinori A, Morelli L. Russell body gastritis associated with Helicobacter pylori infection in an HIV-positive patient: case report and review of the literature. Z Gastroenterol. 2009;47:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Reddy R, Vohra S, Macurak R. Secondary Extramedullary Plasmablastic Myeloma of the Small Bowel. Clin Gastroenterol Hepatol. 2015;13:A23-A24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |