Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.342
Peer-review started: October 5, 2015
First decision: December 28, 2015
Revised: January 15, 2016
Accepted: January 28, 2016
Article in press: January 31, 2016
Published online: April 28, 2016
Processing time: 204 Days and 21.4 Hours
Uterine cervical cancer still remains an important socioeconomic issue because it largely affects women of reproductive age. Prognosis is highly depended on extent of the disease at diagnosis and, therefore, accurate staging is crucial for optimal management. Cervical cancer is clinically staged, according to International Federation of Gynecology and Obstetrics guidelines, but, currently, there is increased use of cross sectional imaging modalities [computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT)] for the study of important prognostic factors like tumor size, parametrial invasion, endocervical extension, pelvic side wall or adjacent/distal organs involvement and lymph node status. Imaging indications also include cervical cancer follow-up, evaluation of tumor response to treatment and selection of suitable candidates for less radical surgeries like radical trachelectomy for fertility preservation. The preferred imaging method for local cervical cancer evaluation is MRI; CT is equally effective for evaluation of extrauterine spread of the disease. PET-CT shows high diagnostic performance for the detection of tumor relapse and metastatic lymph nodes. The aim of this review is to familiarize radiologists with the MRI appearance of cervical carcinoma and to discuss the indications of cross sectional imaging during the course of the disease in patients with cervical carcinoma.
Core tip: Although cervical cancer staging is based on clinical assessment, there is a wide use of cross sectional imaging [magnetic resonance imaging, computed tomography (CT), positron emission tomography-CT] in the pre- and post-treatment work up of these patients. Imaging may provide important information for the discrimination between operable and advanced cervical cancer, the evaluation of tumor response to therapy and the detection of recurrent disease. The aim of this study is to summarize current literature data regarding the use of imaging in cervical carcinoma evaluation and to familiarize radiologists with the available imaging techniques and the corresponding imaging features of cervical tumors.
- Citation: Bourgioti C, Chatoupis K, Moulopoulos LA. Current imaging strategies for the evaluation of uterine cervical cancer. World J Radiol 2016; 8(4): 342-354
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/342.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.342
Worldwide, uterine cervical carcinoma is the second most common gynecologic malignancy. The incidence of invasive cervical cancer is higher in low-income countries due to lack of screening programs. In developed countries, the incidence of invasive cervical cancer dropped after implementation of the Papanicolaou smear test[1]; indeed, in the United States, cervix uteri cancer represents only 0.8% of all new cancer cases. It affects mostly women of reproductive age; 14.0% of patients diagnosed with cervical cancer are between 20 and 34 and 25.9% between 35 and 44 years of age[2].
Invasive cervical cancer remains, however, a fatal disease with no significant improvement of survival rates for patients with advanced disease. It is estimated that 275000 die annually from cervical cancer. In the United States, 4100 women with invasive cervical cancer were expected to die from the disease in 2015, according to Surveillance, Epidemiology and End Results data[2].
Squamous cell carcinoma (SCC) accounts for 85% of all cervical cancers. Non squamous histologies (adenocarcinoma, adenosquamous, undifferentiated) are less common (15%) and are usually associated with poorer prognosis. The main risk factor for developing cervical cancer is infection by the human papilloma virus (HPV), especially subtypes 16 (mostly associated with SCC) and 18 (mostly associated with adenocarcinoma). Other predisposing factors include low socioeconomic background, early sexual life, multiple partners, immunosuppression and smoking[3].
Prognosis of invasive cervical carcinoma is strongly associated with the stage of the disease at the time of diagnosis. Approximately 46.4% of patients with invasive cervical cancer are diagnosed at an early (local) stage; 5-year survival for these patients remains high (up to 91.5%). When there is regional lymph node or distant metastases, the 5-year survival drops to 57.4% and 16.5%, respectively[2].
A systematic literature search was conducted using MEDLINE (PubMed) Library; applied key words included “cervical cancer”, “FIGO guidelines”, “uterine cervical cancer staging”, “MRI”, “CT”, “PET-CT”. Search period extended from 1988-2015. Original research articles (prospective or retrospective), observational studies and review articles with or without meta-analysis data were reviewed. A total of 62 studies were selected with consensus by all authors. Eligibility criteria included adequate study population (> 20 patients), use of clear diagnostic evidence and reliable statistical data.
Early cervical cancer diagnosis remains a clinical issue and it is based on results derived from the Pap smear test, colposcopy and diagnostic biopsies. Despite significant improvements in the field of imaging technology, imaging alone is not adequate for the diagnosis of cervical cancer since it cannot discriminate between invasive cancer and precursor (Cervical Intraepithelial Neoplasm) lesions or other non-neoplastic pathological processes, like cervicitis; therefore, biopsy of suspicious lesions on colposcopy, is currently the gold standard method for cervical cancer diagnosis.
Most of the published literature regarding cervical cancer imaging refers to the evaluation of patients with macroscopically visible tumors [International Federation of Gynecology and Obstetrics (FIGO) ≥ IB]. Inherently, microscopic cervical cancer (stage ≤ IA) cannot be reliably detected by any of the available imaging modalities; therefore the use of imaging is not suggested as a routine neither for the diagnosis nor for the staging of these patients. However, due to technical advances, like the use of endovaginal coils and the wide implementation of magnetic resonance imaging (MRI) functional techniques [dynamic contrast enhanced (DCE) or diffusion weighted imaging (DWI) sequences] to conventional MRI protocols, even small (≤ 1 cm) cervical tumors may be detected[4]; small tumors may be seen on MRI, as foci of enhancement on early arterial DCE images or as foci of restricted diffusion within the cervix[5]. Positron emission tomography/computed tomography (PET/CT) may detect small FDG-avid tumors 7 mm or less[6]; PET/MRI, a new hybrid imaging method, may have the potential to improve imaging performance rate for detection of small cervical cancers, but more data is needed to establish clinical implementation.
Cervical cancer is the only gynecological cancer still clinically staged. The FIGO staging system provides health care professionals with a common language and can be easily applied to countries with a low socioeconomic background and high prevalence of cervical cancer. Currently, FIGO clinical classification is the most commonly used staging system for uterine cervical cancer[7] (Table 1).
| Stage | Sub-stage | Sub-stage |
| (description) | (description) | (description) |
| In situ | ||
| I | IA | IA1 |
| Tumor confined to the cervix (may extend to the uterine corpus) | Miscroscopic tumor: Depth of invasion ≤ 5 mm, horizontal spread ≤ 7 mm | Depth of invasion ≤ 3 mm, horizontal spread ≤ 7 mm |
| IA2 | ||
| Depth of invasion > 3 mm and ≤ 5 mm, horizontal spread ≤ 7 mm | ||
| IB | IB1 | |
| Macroscopically visible tumor | Lesion of maximal diameter ≤ 4 cm | |
| IB2 | ||
| Lesion of maximal diameter > 4 cm | ||
| II | IIA | IIA1 |
| Cervical carcinoma extends to the upper vagina or parametrium | Cervical cancer extents to the upper 2/3 of the vagina | Lesion of maximal diameter ≤ 4 cm |
| IIA2 | ||
| Lesion of maximal diameter > 4 cm | ||
| IIB | ||
| Tumor invades parametrial space (uni- and/or bilateral) | ||
| III | IIIA | |
| Cervical cancer invades pelvic sidewall and/or lower 1/3 vagina and/or causes hydronephrosis | Tumor invades lower 1/3 of vagina | |
| IIIB | ||
| Tumor invades pelvic wall and/or ureter causing hydronephrosis | ||
| IV | IVA | |
| Cervical cancer invades adjacent pelvic organs and/or extending distally (beyond the pelvis) | Bladder and/or rectal mucosa invasion | |
| IVB | ||
| Distant metastasis, peritoneal or paraaortic node involvement |
Early-stage cervical carcinoma (FIGO < IIB) is effectively treated with either surgery or external radiation plus brachytherapy, but for most gynecologic-oncology centers, radical hysterectomy (Wertheim type) combined with pelvic lymphadenectomy is the therapeutic option of choice; the application of neoadjuvant chemotherapy followed by debulking surgery is another acceptable treatment option for bulky, early-stage tumours (IB2, IIA2)[8].
More conservative surgeries, like radical trachelectomy (through a vaginal or abdominal approach) may be applied to young patients with early cervical cancer who wish to preserve their fertility potential. However, strict eligibility criteria, including tumor size < 4 cm, internal os integrity and absence of extrauterine spread, are required to ensure low recurrence rates in this subset of patients[9,10].
Chemoradiation is the optimal treatment for advanced cervical cancer (≥ IIB); in these patients, surgery provides no additional survival benefit, while it exposes the patients to the surgical risk and delays radiation therapy. In a significant number of patients treated with surgery, more advanced disease requiring adjuvant chemoradiation is found on final surgicopathological examination; interestingly, it has been shown that surgical treatment followed by chemoradiation, increases significantly the morbidity of these patients. Therefore, the ability to distinguish early (operable) from advanced (non-operable) cervical cancer is crucial[8,11].
The FIGO staging system has several limitations which may lead to under- or overestimation of the extent of disease at diagnosis, adversely affecting patient optimal care. Literature data report erroneous clinical staging in 17%-32% of patients with early disease (< FIGO IIB) and in up to 65% of patients with advanced disease (FIGO II-IV)[12-14]; difficulties of clinical staging include assessment of tumor growth to the endocervix and parametria.
The internal cervical os (ICO) is not accessible to clinical examination and more invasive techniques, like hysteroscopy, are required. ICO involvement is an important adverse prognostic factor because it is associated with a high incidence of nodal metastases[15]. Lack of free margins between ICO and tumor, precludes fertility-sparing surgery[16].
Another important limitation of the FIGO staging system is that nodal involvement, which is associated with poor survival in patients with cervical carcinoma, cannot be addressed. Furthermore, clinical examination cannot detect distant metastases in patients with advanced disease.
FIGO guidelines for the staging of cervical cancer include a significant number of time-consuming “old fashioned” imaging methods (barium enema, intravenous urography, lymphangiography, chest and skeletal radiographs) and invasive procedures (cystoscopy, rectosigmoidoscopy); today, in clinical practice, these are rarely used. Cross-sectional imaging (CT, MRI, PET-CT) provides more accurate evaluation of local and extrauterine cervical cancer spread and, at the same time, limits the cost of staging. Although the last revision of FIGO staging criteria recommends inclusion of these imaging techniques when possible, their use remains optional[7].
A significant number of clinicians prefer CT for the evaluation of oncologic patients, due to its wide availability[17]. Reported CT accuracy for overall cervical cancer staging, ranges from 32%-80%. CT though, cannot discriminate between normal cervical stroma and cancerous tissue; it performs poorly, therefore, in assessing local spread with lower than 58% PPV for detection of early parametrial invasion[13,18]. Recent studies with multislice CT, exhibit somewhat better results[19]. Relative to extrauterine tumor spread, most authors consider CT the imaging modality of choice, even though the ACRIN trial reported low CT sensitivity values (42%) for depicting advanced cervical disease[20,21]. Currently, staging with CT is limited to patients with clinical evidence of advanced disease or contraindications to MRI.
Transvaginal ultrasound (TVUS) is not part of the routine cervical cancer staging; the location of the cervix deep within the pelvis and the relatively small field of view may be responsible for poor TVUS performance. However, several dedicated imaging centres, report high TVUS accuracy for cervical cancer staging and assessment of parametrial involvement, comparable to those of MRI[22,23].
Hybrid imaging (PET-CT or PET-MRI) is superior to conventional cross- sectional techniques for identifying metastatic lymph nodes, with excellent diagnostic accuracy, ranging from 85%-99%[24,25]. However, its role in the initial evaluation of cervical cancer has not been established. Accuracy of PET-CT for local staging of cervical cancer is moderate (53.3%). PET-MRI may be promising for the assessment of primary tumor, with sensitivity and specificity values over 90%, but further studies with larger patient populations are needed to support clinical implementation[25].
MRI is the preferred imaging modality for evaluating local extent of cervical cancer due to its high contrast resolution which enables differentiation between cancerous and normal tissues[5,26,27]. In the following paragraphs, we will focus on the role of MRI in the pre-treatment staging of cervical cancer.
Currently, in cervical cancer patients, MRΙ is primarily used for the evaluation of tumor morphology and local extent; it accurately evaluates tumor features with a significant prognostic value, like size, endocervical growth, parametrial infiltration and pelvic side wall or adjacent organ (bladder, rectum) involvement. Reported MRI accuracy values for determining tumor stage (operable vs advanced disease) range from 75%-96%[5,21,26,28-30]. Clinical staging of early cervical cancer and assessment of important prognostic factors significantly improves when MRI information is added to the clinical data; MRI helps select surgical candidates among patients with cervical cancer, because it has a high negative predictive value (94%-100%) for parametrial involvement. Also, when findings of clinical examination are unclear or when it cannot be performed due to patient obesity or discomfort, MRI may be used for treatment planning.
Cervical cancer appears as a moderately hyperintense area on T2-weighted (T2-W) images easily separated from the normal, hypointense cervical stroma. Microinvasive or superficial disease is hard to detect; small (< 1 cm) cervical tumors, may be apparent only on early arterial DCE-MRI as enhancing foci[31,32].
Tumor size is an important prognostic factor for early cervical cancer; larger tumors are associated with a high incidence of extrauterine spread and nodal involvement. Patients with large tumors (≥ 4 cm, FIGO IB2-IIA) receive neoadjuvant chemoradiation, and should be excluded from fertility sparing surgery. Exophytic cervical tumors may be accurately examined with colposcopy, but in cases with endocervical tumor extension, clinical examination may underestimate tumor size. MRI is highly accurate in assessing tumor size, coming within 5 mm from that of the histological specimen in 70%-90% of patients[33]. Concomitant uterine pathology like cervical polyps or multiple Nabothian cysts may lead to erroneous size estimates. Sometimes it is difficult, even for radiologists with experience in pelvic imaging, to distinguish adenoma malignum, a special subtype of cervical adenocarcinoma, from multiple Nabothian cysts; deep cervical location of the cysts and the presence of a solid component favour malignancy. In large tumors, peritumoral edema due to tumor inflammation or post-biopsy changes may be mistaken for tumor. In such cases, DWI may be of value, since edema lacks molecular restriction[34].
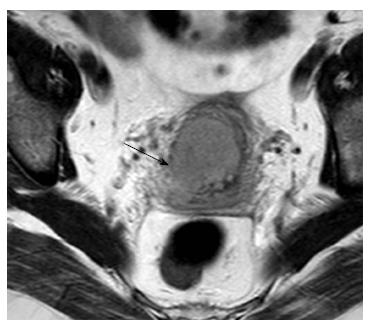
Tumor extension to the parametrial fat is better demonstrated on T2-W sequences. MRI features suggestive of parametrial involvement include cervical contour nodularity, irregular borders between tumor and parametrial tissue and presence of a soft-tissue mass within the parametrium, possibly encasing the periuterine vascular plexus (Figure 1). Stranding of the parametrial fat may be indicative of tumor extension but it is also a sign of peritumoral inflammation. A > 3 mm T2 hypointense stromal cervical rim, excludes parametrial involvement with extremely high specificity (96%-99%) and NPV (94%-100%)[31]. When there is total loss of the hypointense cervical rim without parametrial irregularity, it may be hard to identify early extension of the tumor to parametrial tissue; this leads to lower accuracy rates for detection of parametrial invasion in this subset of patients[30,35]. False positive results may occur when peritumoral post-biopsy or inflammatory changes are present. Microscopic tumor accounts for false negative results.
Parametrial invasion (FIGO IIB) precludes surgical treatment. Clinical examination exhibits low sensitivity for the detection of parametrial involvement, even when performed under anaesthesia. Reported MRI accuracy values for parametrial extension range from 88%-97%, significantly higher compared to clinical examination. When information acquired from MRI is added to clinical assessment, the predictive ability of the latter significantly increases[5,26,30,31].
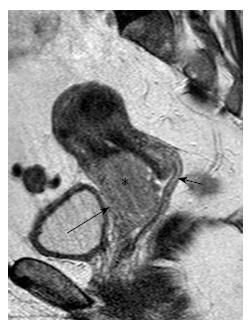
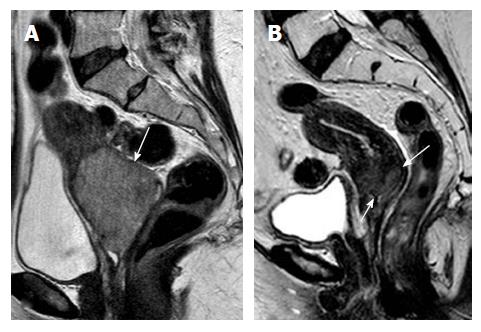
In stage IIA cervical tumors, there is invasion of the upper 2/3 of the vagina; involvement of the lower vagina classifies the tumor as stage IIIA. MRI is sensitive in evaluating the vaginal fornices; preservation of the low signal intensity of the vaginal wall on T2-W images is a reliable sign of vaginal wall integrity (Figure 2). However, MRI results may be false positive for vaginal wall invasion in large exophytic tumors, because they distend the vagina causing the vaginal wall to appear thin; in such cases, application of endovaginal gel may be of value. Overall MRI accuracy for vaginal invasion is high, ranging from 86% to 93%[31].
Usually, clinicians can reliably assess the vagina with physical examination and colposcopy; therefore, in practice, imaging is reserved for a small number of indeterminate cases.
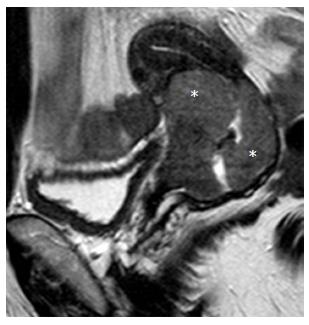
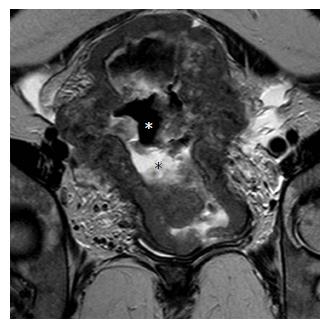
ICO involvement is another important poor prognostic factor for cervical cancer because it is associated with an increased incidence of nodal metastases[15]; ICO integrity is crucial for planning fertility sparing surgery[16,32]. The ICO is not accessible to clinical examination and hysteroscopy is costly and invasive. MRI is safe with high sensitivity (86%-91%) and specificity (94%-96%) values for evaluating tumor extension to the corpus uteri[32,36] (Figure 3).
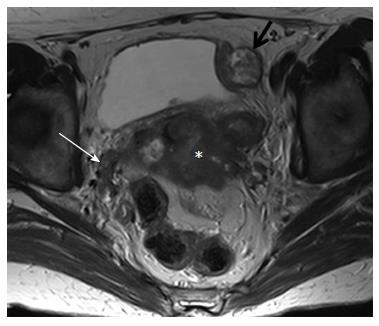
MRI provides excellent images of the normal pelvic floor anatomy; therefore, pathology may easily be detected. Pelvic wall involvement (stage IIIB) is diagnosed when tumor is seen within 3 mm from the internal obturator, levator ani or pyriformis muscles; encasement of the iliac vessels is also diagnostic of pelvic sidewall invasion (Figure 4).
The presence of hydronephrosis/hydroureter should always be investigated since it is diagnostic of stage IIIB disease; coronal T2-W images including the kidneys provide a quick, reliable and safe evaluation of urinary tract status, obviating the need for intravenous urography.
Invasion of the bladder or rectal mucosa classifies the tumor as stage IVA. Positive signs for bladder or rectal invasion include full disruption of the low signal intensity wall on T2-W images, presence of an intraluminal mass or demonstration of a vesicovaginal or rectovaginal fistula. Uniform thickening and hyperintesity of the bladder or rectal wall on T2-W images, is more suggestive of edema than tumor invasion (bullous edema sign). Preservation of the fat plane between cervical tumor, urinary bladder or rectum on MRI, excludes bladder or rectal invasion with high accuracy (100%)[31]. Therefore, the use of invasive diagnostic procedures like cystoscopy or rectosigmoidoscopy is limited to cases with indeterminate MRI findings[37].
Normal lymph nodes are seen as ovoid homogeneous structures of intermediate signal intensity on T2-W, low signal intensity on T1-W and high signal intensity on DW images; normal nodes enhance homogeneously after intravenous injection of paramagnetic contrast.
Lymph node metastasis is the most important, independent adverse prognostic factor for patients with cervical cancer. Surgical lymphadenectomy (via laparoscopy) is considered as the gold standard procedure for the diagnosis of nodal metastases, but as it is an invasive and expensive procedure, with a high risk of complications, it is not routinely performed[38]. If metastatic pelvic nodes are found at surgery, adjuvant chemoradiation is required, although tumor stage remains the same; positive paraaortic or inguinal lymph nodes classify the tumor as stage IVB.
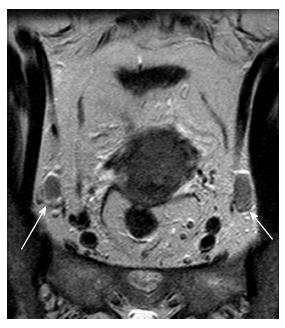
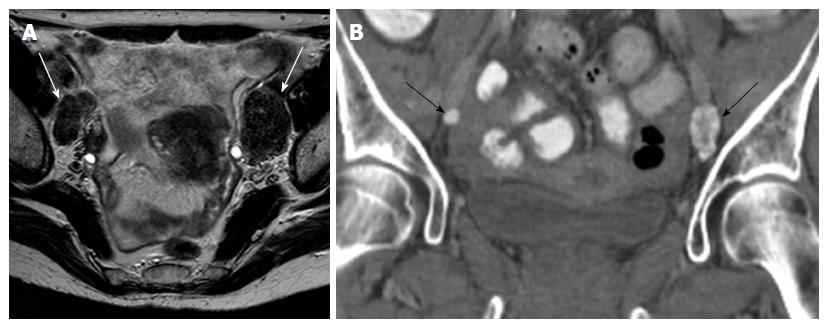
Even today, size (short axis > 1 cm) is the main cross-sectional imaging criterion for discriminating normal from metastatic nodes (Figure 5). Micrometastases cannot be reliably detected even when functional MRI techniques are applied. MRI cannot discriminate between enlarged inflammatory lymph nodes and metastatic nodes. This results in moderate sensitivity and specificity values for both CT and MRI (43%-73%) for detection of metastatic lymph nodes[5,26,27]. PET/CT has the highest diagnostic performance for detecting nodal metastases in cervical cancer patients; however, its accuracy decreases for nodal size < 5 mm[24].
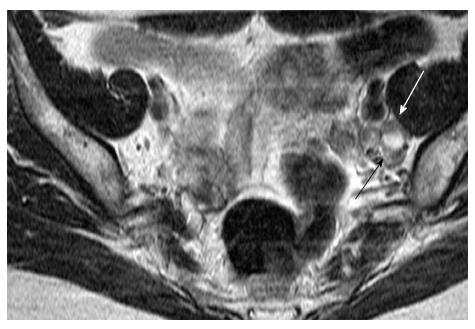
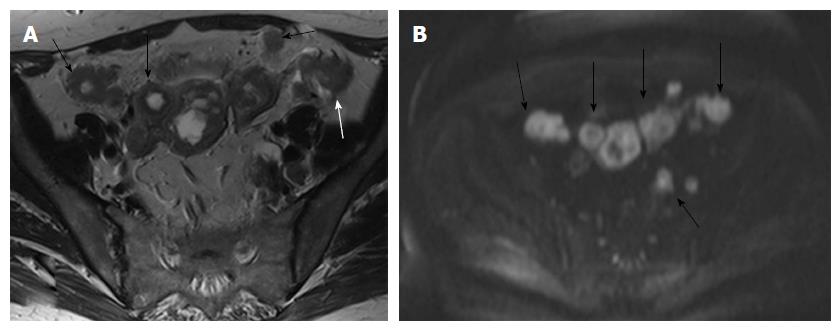
Other morphologic features suggestive of a malignant node include round shape, irregular margins or extracapsular mass; central necrosis has 100% PPV for the diagnosis of nodal metastases. Care should be taken not to mistake a necrotic lymph node for a normal ovary with follicles[27,31]. Rarely, calcifications within the metastatic nodes may be present (Figures 6 and 7).
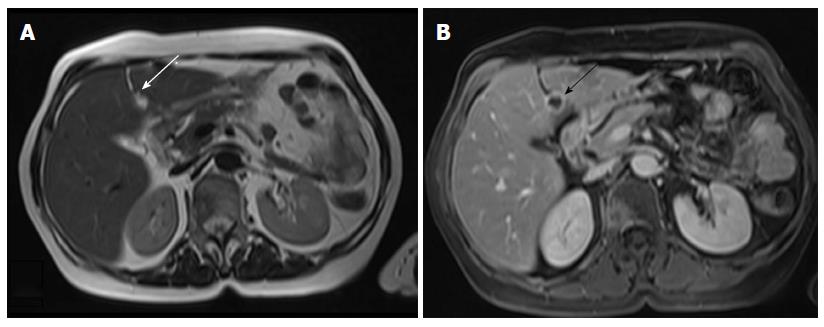
In stage IVB disease, metastases usually involve the lungs, liver, adrenals or bones. Peritoneal deposits are also considered as stage IVB disease. The abdomen may be effectively evaluated with Transabdominal Ultrasound-TAUS, CT or MRI (Figures 8 and 9).
In early cervical cancer, the incidence of lung involvement is extremely low and routine chest X-ray is usually sufficient for evaluating these patients. In the presence of advanced cervical carcinoma, chest CT should be performed. In cervical cancer of squamous cell origin, metastases may present as cavitating lung nodules[39].
Bone metastases are rare, with a reported incidence of 1.1%[40]. Plain radiographs, CT or scintigraphy are routinely used for bone lesion detection, while MRI is an excellent method for the evaluation of bone marrow.
Cervical cancer follow-up includes physical examination, vaginal inspection/colposcopy and Pap smear, every 3 mo for a total of 2 years and then less often[41]. When there are suspicious findings, vaginal biopsy is performed.
There is no consensus regarding the use of imaging for monitoring patients treated for cervical cancer. The use of cross-sectional modalities (CT, MRI, PET-CT) for routine follow-up should be rational, because of high cost and radiation exposure.
Currently, in surgically treated patients with no clinical evidence of tumor, no imaging is recommended. In some centers and mostly for patients with adverse surgico-pathological features predisposing to relapse, like large tumor volume, small cell histology, lymph-vascular space invasion, FIGO stage ≥ IIB and positive surgical margins, follow-up MRI is recommended every 6 mo for a total of 2 years; the same follow-up is recommended for patients who had fertility preserving surgery. In patients with clinical evidence of tumor recurrence, PET-CT is the preferred imaging modality because it can more accurately evaluate lymph node status and extrapelvic spread of the disease[42].
In patients with advanced cervical cancer who received conservative therapy (chemotherapy, external radiation and brachytherapy), determination of complete vs partial response of the tumor is important due to differences in management; patients with residual cancer after completion of chemoradiation, may be candidates for pelvic exenteration (Figure 10); patients with complete regression of tumor do not need further imaging, if suspicious clinical findings are not present[39,42].
In patients with advanced disease, there are several findings on MRI studies following initiation of treatment, which may help early prediction of the therapeutic outcome. MRI features good response to treatment include: Significant decrease in tumor size 2 mo after initiation of the therapy[43], increased tumor ADC values on DWI 2 wk after indicative of the start of radiotherapy[44] and changes in quantitative pharmacokinetic parameters on DCE-MRI during the course of early (after 20-25 Gy) and mid-term (after 45-50 Gy) radiotherapy treatment[45]. Some authors have proposed DWI with ADC measurements, as a cancer biomarker for tumor response to therapy[46]; however, since there is variability amongst imaging protocols and heterogeneity in ADC analysis, its use is still restricted. Complete reconstitution of cervical architecture and normal low T2 signal of cervical stroma, are the most reliable MRI features for successful response to radiation therapy, with NPV up to 97%[31].
Tumor recurrence may be local (e.g., cervix, vaginal vault after radical hysterectomy), regional (e.g., pelvic side wall, pelvic lymph nodes) or distal (e.g., lung, liver, paraaortic, inguinal and supraclavicular lymph nodes). CT detects enlarged lymph nodes or distant metastases, but its role is limited in the evaluation of local recurrence. MRI provides excellent anatomic detail of the uterus, vagina and pelvic floor muscles. Local relapse on MRI is shown as a lobulated, moderate to high signal intensity soft-tissue mass on T2-W images, with heterogeneous contrast enhancement. Distinction between tumor recurrence and post-treatment fibrosis may be difficult within the first 6 mo of treatment; lack of high signal intensity on high b value DWI, is more suggestive of treated disease. PET-CT is more reliable than CT or MRI for evaluating distant or nodal spread. Therefore, a combination of MRI and PET-CT is preferred for the diagnosis of cervical cancer relapse[42].
In dedicated centers, special preparation before pelvic MRI is recommended[47]; patients are instructed to fast for at least 6-8 h prior to the MRI to limit bowel peristalsis and/or they may receive antiperistaltic agents (e.g., 20 mg hyoscine butyl bromide or 1 mg glucagon via intramuscular or intravenous injection) just before the exam. Endovaginal sterilized ultrasound gel may be applied for better evaluation of the vaginal wall, in cases of exophytic cervical tumors. The urinary bladder should be moderately distended to avoid distortion of pelvic anatomy.
Dedicated external phased array coil should be used for the staging of cervical cancer. Endovaginal coil is more sensitive than the external array coil for detecting small tumors; however, in the presence of a large mass, its utility is limited, because of the inherent small field of view and technical difficulties[48]. The use of high field magnets (3T) may improve image resolution in the pelvis but does not provide significantly higher diagnostic accuracy to the overall staging in patients with cervical carcinoma[49].
MRI should be performed at least ten days after biopsy, to avoid false positive results due to local inflammation.
Conventional MRI study protocols for cervical cancer include T2-W sequences, in all imaging planes (axial, oblique sagittal, oblique coronal); T2-W images provide optimal contrast difference between tumor and normal cervical tissue and, also, enable detection of enlarged lymph nodes. High-resolution images of the lesser pelvis, with small field of view, are performed in axial oblique plane, perpendicular to the long axis of the cervix, for better evaluation of the parametrial tissues. T2-W images with fat suppression may be helpful for the evaluation of parametrial tissues in young patients with prominent periuterine vascular plexus, as well as for detection of fluid collections or bone marrow changes. T1-W images provide information on pelvic anatomy, lymph nodes and bone marrow.
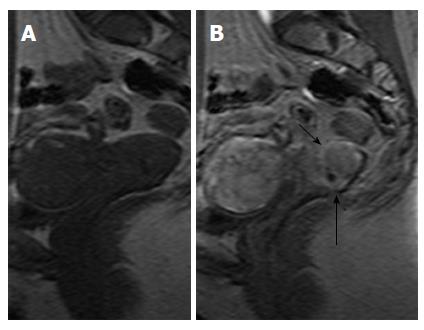
DCE-MRI is not a routine part of MRI protocols for cervical cancer staging; its role is limited to the detection of small tumors, equivocal T2-W findings, or for post-treatment assessment[47]. Some imaging centers have incorporated DCE-MRI to dedicated MRI protocols for staging cervical cancer and have supported its role in identifying endocervical extent, more accurately defining tumor borders and discriminating between cervical and endometrial carcinomas (Figure 11)[50].
DWI has been recently implemented to MRI protocols for cervical cancer staging. Cervical invasive carcinoma exhibits significantly lower ADC values than normal cervix, facilitating tumor detection and definition of extent. It may also be used to discriminate between post-biopsy changes and residual cancer; however, haemorrhage may also exhibit restricted diffusion and may, therefore, be responsible for false positive results[34,51]. DWI is excellent in identifying small, even a few mm, lymph nodes; however, so far it has not been able to discriminate between normal and malignant nodes. DWI may be especially helpful in the evaluation of pregnant patients, in whom intravenous contrast is contraindicated. Combination of DWI and T2-W images is more accurate than T2-W images alone in identifying parametrial extension or recurrent disease[52].
A dedicated MRI protocol for the pre-treatment staging of cervical cancer is presented in Table 2.
| Sequence | Plane | Technical characteristics | Utility |
| T2-W TSE (mandatory) | Axial (renal hilum-pubis) | TR/TE: 3500/90, NSA: 2, SL/G: 4.5/1, Matrix: 340 × 350, FOV: 38 | Tumor detection and morphology, evaluation of pelvic tissues, lymph node status |
| Sagittal | TR/TE: 3500/90, NSA: 3, SL/G: 3.5/1.2, Matrix: 348 × 276, FOV: 25 | ||
| Axial oblique (perpendicular to cervical axis) | TR/TE: 3900/125, NSA: 6, SL/G: 4/0.4, Matrix: 256 × 176, FOV: 18 | ||
| T1-W TSE (mandatory) | Axial (renal hilum-pubis) | TR/TE: 400/13, NSA: 1, SL/G: 6/2, Matrix: 300 × 205, FOV: 36 | Pelvic anatomy, lymph nodes, bone marrow, haemorrhage |
| DWI-EPI (mandatory) | Axial | TR/TE: 3000/68, | Tumor detection, lymph nodes, post-treatment evaluation |
| NSA: 12, SL/G: 6/1 | |||
| Matrix: 124 × 174 | |||
| FOV: 35 | |||
| DCE (optional) | Sagittal; one native, post contrast images every 17 s, total acquisition time about 3 min | TR/TE/FA: 15/4.2/45°, NSA: 2, Matrix: 228 × 75, FOV:17 | Small tumor detection, tumor borders, endocervical extent |
| T2-W TSE FS (optional) | Axial | TR/TE: 1650/70, NSA: 2, SL/G: 4.5/1, Matrix: 288 × 250, FOV: 35 | Parametrial plexus, fluid, bone marrow evaluation |
As previously mentioned, uterus-sparing operation is an alternative option for patients with early cervical cancer who wish to preserve their childbirth potential. Eligibility criteria for vaginal radical trachelectomy (VRT) and abdominal radical trachelectomy (ART) include age (< 40 years), tumor size (≤ 2 cm for VRT vs≤ 4 cm for ART), tumor to ICO distance (> 1 cm for VRT vs > 0.5 cm for ART), absence of extrauterine spread and strong desire to keep fertility potential. With ART, inclusion criteria are more flexible than with VRT, since with the abdominal approach a wide resection of parametrial tissue and upper vagina can be achieved, similar to that of abdominal radical hysterectomy[16,53].
Tumor size, ICO integrity and extrauterine spread should be accurately evaluated before fertility-sparing surgery. Tumor size is considered most significant for successful treatment; tumors > 2 cm are more often associated with recurrence (29% vs 1.6% for smaller tumors) after treatment with radical trachelectomy[54]. There is a substantial number (17%) of scheduled trachelectomies that may be abandoned during surgery because of unexpected findings of more advanced disease, like endocervical involvement or metastatic lymph nodes[55]. Preoperative MRI provides useful information on tumor size, parametrial status and lymph nodes, contributing to the more accurate selection of candidates for trachelectomy. Recently, it has been shown that patients with a ≤ 5 mm distance between tumor and ICO on MRI, fare better with radical hysterectomy than trachelectomy[56].
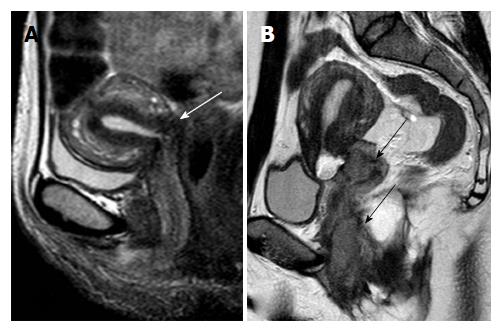
MRI may also be used for monitoring patients after trachelectomy. Typical MRI features after trachelectomy include: Utero-vaginal anastomosis, neo-vaginal fornix formation, diffuse vaginal wall edema which may persist for over a year, and vaginal wall hematomas/submucosal fluid collections, which gradually resolve on follow-up. Susceptibility artefacts may be present on MR images after trachelectomy, because of the placement of a metallic cerclage suture at the end-to-end anastomosis of the uterine body with the vaginal vault; these artefacts, usually, do not pose a problem for MRI interpretation. The most frequently reported post-trachelectomy complications include utero-vaginal stenosis, infections/abscess and lymphocysts. Hydrosalpinges may develop after ART and should be looked for, since their presence may compromise the reproductive potential of trachelectomy patients. MRI helps the detection of tumor relapse, which most commonly involves the vaginal vault or the lymph nodes (Figure 12)[32,57].
In a small number of patients with bulky uterine masses, conventional biopsy may not be able to establish a cervical or endometrial origin. In such patients, with uterine tumors of unclear histology, the use of immunohistochemistry (IHC) may provide a diagnosis; however, no single antibody is totally specific for cervical or endometrial cancer and IHC results should be always interpreted in conjunction with imaging findings[58].
Diagnosis of cervical vs endometrial carcinoma is of critical importance due to differences in staging, prognosis and therapeutic approach. Hysterectomy (simple or radical) with pelvic lymphadenectomy when tumor invades deep into the myometrium or cervical stroma, is the optimal treatment for endometrial carcinoma. Radical hysterectomy, combined with pelvic lymphadenectomy is the preferred option for early stage invasive cervical carcinoma. Advanced cervical cancer is treated with primary chemoradiation[7,59].
There is limited data on discriminant MRI features between primary cervical and endometrial carcinomas (Figure 13). Most studies[60,61] reported high accuracy (up to 88%) of tumor epicenter determination with MRI for identification of tumor origin[61]. In another study a significantly higher prevalence of certain MRI features, such as presence of an endometrial mass in patients with endometrial vs cervical cancer, was observed[62].
A recently published study, reported seven MRI features with significant discriminant predictive ability for cervical vs endometrial cancer: Tumor epicentre (uterine body or cervix), and the presence or absence of tumor hypervascularity on early arterial DCE-MRI, full depth cervical stromal invasion on T2-W images, mass within the endometrial cavity, distended endometrial cavity with secretions, deep (≥ 50%) myometrial invasion and enhancing rim at the periphery of the tumor. Based on the possible likelihood ratio values assigned to each of the above features, a 7-feature MRI scoring system was designed with the potential to help radiologists predict the histology of a bulky uterine cancer; the developed scoring system proved to be quite accurate in tumor origin prediction with high sensitivity and specificity values up to 96.6% and 100%, respectively[50].
Currently, clinical staging of cervical cancer cannot be replaced by cross- sectional imaging for various reasons including lack of availability in low-income countries, variability of imaging protocols or agreement between readers and need for dedicated imaging centres with radiologists, experts in the field. However, imaging studies provide more information than clinical examination for the evaluation of patients with cervical cancer and their results may be used by clinicians to make appropriate therapeutic decisions. MRI is the preferred imaging modality for the initial staging of uterine cervical cancer and its use may increment the clinical prognostic value. MRI is particularly helpful in evaluating patients with cervical cancer who are candidates for trachelectomy or for predicting tumor origin in patients with bulky uterine masses of indeterminate histology. In patients with clinical evidence of tumor relapse, the combination of MRI and PET/CT data may reliably evaluate both local and distant spread of the disease, optimizing the therapeutic management of these patients. Functional imaging techniques, like DWI, appear to be promising cancer biomarkers for predicting therapeutic outcome but further studies and imaging protocol standardization are needed to support clinical implementation.
P- Reviewer: Chu JP, Chow J, Storto G S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Macgregor JE, Campbell MK, Mann EM, Swanson KY. Screening for cervical intraepithelial neoplasia in north east Scotland shows fall in incidence and mortality from invasive cancer with concomitant rise in preinvasive disease. BMJ. 1994;308:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | SEER Data: Surveillance, Epidemiology and End Results. [accessed 2015 Sept 19]. Available from: http://seer.cancer.gov/statfacts/html/cervix.html. |
| 3. | HPV and Cancer. National Cancer Institute. [accessed 2015 Sept 19]. Available from: http://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet. |
| 4. | deSouza NM, Dina R, McIndoe GA, Soutter WP. Cervical cancer: value of an endovaginal coil magnetic resonance imaging technique in detecting small volume disease and assessing parametrial extension. Gynecol Oncol. 2006;102:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 6. | Mirpour S, Mhlanga JC, Logeswaran P, Russo G, Mercier G, Subramaniam RM. The role of PET/CT in the management of cervical cancer. AJR Am J Roentgenol. 2013;201:W192-W205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 513] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 8. | Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C; ESMO Guidelines Working Group. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii27-vii32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Kesic V, Rodolakis A, Denschlag D, Schneider A, Morice P, Amant F, Reed N. Fertility preserving management in gynecologic cancer patients: the need for centralization. Int J Gynecol Cancer. 2010;20:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Denschlag D, Reed NS, Rodolakis A. Fertility-sparing approaches in gynecologic cancers: a review of ESGO task force activities. Curr Oncol Rep. 2012;14:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Kesic V. Management of cervical cancer. Eur J Surg Oncol. 2006;32:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Togashi K, Morikawa K, Kataoka ML, Konishi J. Cervical cancer. J Magn Reson Imaging. 1998;8:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Subak LL, Hricak H, Powell CB, Azizi L, Stern JL. Cervical carcinoma: computed tomography and magnetic resonance imaging for preoperative staging. Obstet Gynecol. 1995;86:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 245] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Bhosale P, Peungjesada S, Devine C, Balachandran A, Iyer R. Role of magnetic resonance imaging as an adjunct to clinical staging in cervical carcinoma. J Comput Assist Tomogr. 2010;34:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Narayan K, McKenzie AF, Hicks RJ, Fisher R, Bernshaw D, Bau S. Relation between FIGO stage, primary tumor volume, and presence of lymph node metastases in cervical cancer patients referred for radiotherapy. Int J Gynecol Cancer. 2003;13:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Plante M, Gregoire J, Renaud MC, Roy M. The vaginal radical trachelectomy: an update of a series of 125 cases and 106 pregnancies. Gynecol Oncol. 2011;121:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Thomeer MG, Gerestein C, Spronk S, van Doorn HC, van der Ham E, Hunink MG. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: systematic review and meta-analysis. Eur Radiol. 2013;23:2005-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Ho CM, Chien TY, Jeng CM, Tsang YM, Shih BY, Chang SC. Staging of cervical cancer: comparison between magnetic resonance imaging, computed tomography and pelvic examination under anesthesia. J Formos Med Assoc. 1992;91:982-990. [PubMed] |
| 19. | Tsili AC, Tsangou V, Koliopoulos G, Stefos T, Argyropoulou MI. Early-stage cervical carcinoma: the role of multidetector CT in correlation with histopathological findings. J Obstet Gynaecol. 2013;33:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Hricak H, Gatsonis C, Chi DS, Amendola MA, Brandt K, Schwartz LH, Koelliker S, Siegelman ES, Brown JJ, McGhee RB, Iyer R, Vitellas KM, Snyder B, Long HJ, Fiorica JV, Mitchell DG; American College of Radiology Imaging Network 6651; Gynecologic Oncology Group 183. Role of imaging in pretreatment evaluation of early invasive cervical cancer: results of the intergroup study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. J Clin Oncol. 2005;23:9329-9337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Hricak H, Gatsonis C, Coakley FV, Snyder B, Reinhold C, Schwartz LH, Woodward PJ, Pannu HK, Amendola M, Mitchell DG. Early invasive cervical cancer: CT and MR imaging in preoperative evaluation - ACRIN/GOG comparative study of diagnostic performance and interobserver variability. Radiology. 2007;245:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Fischerova D, Cibula D, Stenhova H, Vondrichova H, Calda P, Zikan M, Freitag P, Slama J, Dundr P, Belacek J. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int J Gynecol Cancer. 2008;18:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Moloney F, Ryan D, Twomey M, Hewitt M, Barry J. Comparison of MRI and high-resolution transvaginal sonography for the local staging of cervical cancer. J Clin Ultrasound. 2016;44:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Grueneisen J, Schaarschmidt BM, Heubner M, Aktas B, Kinner S, Forsting M, Lauenstein T, Ruhlmann V, Umutlu L. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: preliminary results. Eur J Nucl Med Mol Imaging. 2015;42:1814-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics. 2012;32:1805-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Patel S, Liyanage SH, Sahdev A, Rockall AG, Reznek RH. Imaging of endometrial and cervical cancer. Insights Imaging. 2010;1:309-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Hricak H, Lacey CG, Sandles LG, Chang YC, Winkler ML, Stern JL. Invasive cervical carcinoma: comparison of MR imaging and surgical findings. Radiology. 1988;166:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Hricak H, Powell CB, Yu KK, Washington E, Subak LL, Stern JL, Cisternas MG, Arenson RL. Invasive cervical carcinoma: role of MR imaging in pretreatment work-up--cost minimization and diagnostic efficacy analysis. Radiology. 1996;198:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Bourgioti C, Chatoupis K, Rodolakis A, Antoniou A, Tzavara C, Koutoulidis V, Moulopoulos LA. Incremental prognostic value of MRI in the staging of early cervical cancer: a prospective study and review of the literature. Clin Imaging. 2016;40:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Sala E, Wakely S, Senior E, Lomas D. MRI of malignant neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol. 2007;188:1577-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Bourgioti C, Koutoulidis V, Chatoupis K, Rodolakis A, Koureas A, Thomakos N, Moulopoulos LA. MRI findings before and after abdominal radical trachelectomy (ART) for cervical cancer: a prospective study and review of the literature. Clin Radiol. 2014;69:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Mitchell DG, Snyder B, Coakley F, Reinhold C, Thomas G, Amendola M, Schwartz LH, Woodward P, Pannu H, Hricak H. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol. 2006;24:5687-5694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Charles-Edwards EM, Messiou C, Morgan VA, De Silva SS, McWhinney NA, Katesmark M, Attygalle AD, DeSouza NM. Diffusion-weighted imaging in cervical cancer with an endovaginal technique: potential value for improving tumor detection in stage Ia and Ib1 disease. Radiology. 2008;249:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Jena A, Oberoi R, Rawal S, Das SK, Pandey KK. Parametrial invasion in carcinoma of cervix: role of MRI measured tumour volume. Br J Radiol. 2005;78:1075-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Peppercorn PD, Jeyarajah AR, Woolas R, Shepherd JH, Oram DH, Jacobs IJ, Armstrong P, Lowe D, Reznek RH. Role of MR imaging in the selection of patients with early cervical carcinoma for fertility-preserving surgery: initial experience. Radiology. 1999;212:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Amendola MA, Hricak H, Mitchell DG, Snyder B, Chi DS, Long HJ, Fiorica JV, Gatsonis C. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: results of intergroup protocol ACRIN 6651/GOG 183. J Clin Oncol. 2005;23:7454-7459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Plante M, Renaud MC, Têtu B, Harel F, Roy M. Laparoscopic sentinel node mapping in early-stage cervical cancer. Gynecol Oncol. 2003;91:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, Rao GG, Small W, Varia MA, Wolfson AH. ACR Appropriateness Criteria® on Advanced Cervical Cancer Expert Panel on Radiation Oncology-Gynecology. Int J Radiat Oncol Biol Phys. 2011;81:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Thanapprapasr D, Nartthanarung A, Likittanasombut P, Na Ayudhya NI, Charakorn C, Udomsubpayakul U, Subhadarbandhu T, Wilailak S. Bone metastasis in cervical cancer patients over a 10-year period. Int J Gynecol Cancer. 2010;20:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Greer BE, Koh WJ, Abu-Rustum N, Bookman MA, Bristow RE, Campos S, Cho KR, Copeland L, Eifel P, Huh WK, Jaggernauth W, Kapp DS, Kavanagh J, Lipscomb GH, Lurain JR, Morgan M, Morgan RJ, Powell CB, Remmenga SW, Reynolds RK, Secord AA, Small W, Teng N; National Comprehensive Cancer Network. Cervical cancer. J Natl Compr Canc Netw. 2008;6:14-36. [PubMed] |
| 42. | Testa AC, Di Legge A, De Blasis I, Moruzzi MC, Bonatti M, Collarino A, Rufini V, Manfredi R. Imaging techniques for the evaluation of cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2014;28:741-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Flueckiger F, Ebner F, Poschauko H, Tamussino K, Einspieler R, Ranner G. Cervical cancer: serial MR imaging before and after primary radiation therapy--a 2-year follow-up study. Radiology. 1992;184:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol. 2005;15:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Mayr NA, Wang JZ, Zhang D, Grecula JC, Lo SS, Jaroura D, Montebello J, Zhang H, Li K, Lu L. Longitudinal changes in tumor perfusion pattern during the radiation therapy course and its clinical impact in cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1545] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 47. | Balleyguier C, Sala E, Da Cunha T, Bergman A, Brkljacic B, Danza F, Forstner R, Hamm B, Kubik-Huch R, Lopez C. Staging of uterine cervical cancer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 48. | Downey K, Attygalle AD, Morgan VA, Giles SL, MacDonald A, Davis M, Ind TE, Shepherd JH, deSouza NM. Comparison of optimised endovaginal vs external array coil T2-weighted and diffusion-weighted imaging techniques for detecting suspected early stage (IA/IB1) uterine cervical cancer. Eur Radiol. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Hori M, Kim T, Murakami T, Imaoka I, Onishi H, Tomoda K, Tsutsui T, Enomoto T, Kimura T, Nakamura H. Uterine cervical carcinoma: preoperative staging with 3.0-T MR imaging--comparison with 1.5-T MR imaging. Radiology. 2009;251:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Bourgioti C, Chatoupis K, Panourgias E, Tzavara C, Sarris K, Rodolakis A, Moulopoulos LA. Endometrial vs. cervical cancer: development and pilot testing of a magnetic resonance imaging (MRI) scoring system for predicting tumor origin of uterine carcinomas of indeterminate histology. Abdom Imaging. 2015;40:2529-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Nougaret S, Tirumani SH, Addley H, Pandey H, Sala E, Reinhold C. Pearls and pitfalls in MRI of gynecologic malignancy with diffusion-weighted technique. AJR Am J Roentgenol. 2013;200:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Lucas R, Lopes Dias J, Cunha TM. Added value of diffusion-weighted MRI in detection of cervical cancer recurrence: comparison with morphologic and dynamic contrast-enhanced MRI sequences. Diagn Interv Radiol. 2015;21:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Pareja R, Rendón GJ, Sanz-Lomana CM, Monzón O, Ramirez PT. Surgical, oncological, and obstetrical outcomes after abdominal radical trachelectomy - a systematic literature review. Gynecol Oncol. 2013;131:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Marchiole P, Benchaib M, Buenerd A, Lazlo E, Dargent D, Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH). Gynecol Oncol. 2007;106:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 55. | Plante M. Evolution in fertility-preserving options for early-stage cervical cancer: radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. Int J Gynecol Cancer. 2013;23:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 56. | Lakhman Y, Akin O, Park KJ, Sarasohn DM, Zheng J, Goldman DA, Sohn MJ, Moskowitz CS, Sonoda Y, Hricak H. Stage IB1 cervical cancer: role of preoperative MR imaging in selection of patients for fertility-sparing radical trachelectomy. Radiology. 2013;269:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Sahdev A, Jones J, Shepherd JH, Reznek RH. MR imaging appearances of the female pelvis after trachelectomy. Radiographics. 2005;25:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | McCluggage WG, Sumathi VP, McBride HA, Patterson A. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol. 2002;21:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C; ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi33-vi38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 60. | Vargas HA, Akin O, Zheng J, Moskowitz C, Soslow R, Abu-Rustum N, Barakat RR, Hricak H. The value of MR imaging when the site of uterine cancer origin is uncertain. Radiology. 2011;258:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | He H, Bhosale P, Wei W, Ramalingam P, Iyer R. MRI is highly specific in determining primary cervical versus endometrial cancer when biopsy results are inconclusive. Clin Radiol. 2013;68:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Haider MA, Patlas M, Jhaveri K, Chapman W, Fyles A, Rosen B. Adenocarcinoma involving the uterine cervix: magnetic resonance imaging findings in tumours of endometrial, compared with cervical, origin. Can Assoc Radiol J. 2006;57:43-48. [PubMed] |