Published online Jun 28, 2014. doi: 10.4329/wjr.v6.i6.388
Revised: April 14, 2014
Accepted: May 8, 2014
Published online: June 28, 2014
Seizures are one of the most common pediatric neurologic disorders. Many complications secondary to seizures have been described in the literature including head trauma, fractures, drowning and burns. However, to the best of our knowledge, rupture of the myotendinous insertion of the temporalis muscle on the mandible secondary to a seizure has never been described in the literature. We report the case of a unilateral temporalis muscle rupture in a 16-year-old boy who developed unilateral facial swelling following new onset tonic-clonic seizures. We emphasize on the computed tomography and magnetic resonance imaging findings in this case report. Two mechanisms have been proposed to explain such an injury. The favored mechanism in our patient is a pull on the temporalis myotendinous insertion on the mandible following vigorous and brisk deviation of the head and neck during seizure. Radiologists should be familiar with this type of injury following seizures in order to prevent misdiagnosis and subsequently mistreatment.
Core tip: We report the unique case of a unilateral temporalis muscle rupture following new onset tonic-clonic seizures in a 16-year-old boy. The favored mechanism in our patient is a pull on the temporalis myotendinous insertion on the mandible following vigorous and brisk deviation of the head and neck during seizure. Although this is a rare entity, it is important to be familiar with such type of injury in a patient who develops unilateral facial swelling and pain following tonic-clonic seizures in order to prevent misdiagnosis and mistreatment.
- Citation: Naffaa LN, Tandon YK, Rubin M. Myotendinous rupture of temporalis muscle: A rare injury following seizure. World J Radiol 2014; 6(6): 388-391
- URL: https://www.wjgnet.com/1949-8470/full/v6/i6/388.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i6.388
Seizures are one of the most common pediatric neurologic disorders. It is estimated that 4% to 10% of children will have at least one seizure in the first 16 years of life[1]. Many complications have been described in the literature secondary to seizures, such as head trauma, fractures, drowning and burns[2-4]. However, to the best of our knowledge, this is the first reported case of myotendinous rupture of the temporalis muscle after seizures. We report the computed tomography (CT) and magnetic resonance imaging (MRI) findings of rupture of the myotendinous insertion of the right temporalis muscle on the coronoid process of the mandible following generalized tonic-clonic seizures. Two mechanisms have been proposed to explain such an injury: significant pull on the myotendinous insertion following brisk and forceful deviation of the head and neck toward the contolateral side during seizures or direct fall on the face. In our patient, the first mechanism is favored as there was no witnessed fall nor signs of facial trauma on physical exam.
Patient is a 16-year-old male with a past medical history of Attention Deficit Hyperactivity Disorder (ADHD) who was admitted for new onset seizures. Approximately 12 h prior to his presentation to our institution, he had a sudden witnessed episode of violent bilateral shaking of his arms and legs that lasted approximately 2 min associated with foaming at the mouth. He was taken to an outside hospital emergency department (ED) where a basic metabolic panel including glucose and calcium levels was normal. His urine toxicology was negative and urine analysis was also within normal limits. Head CT was performed and was reportedly normal. Just prior to discharge from the ED, his mother noticed a small bump that had developed over his right temple.
He was discharged home after he returned to his neurological baseline. At home, the patient continued to have increasing facial swelling that was spreading down to his cheek. Patient also began to complain of pain and noted difficulty opening his mouth secondary to pain. At home, a few hours after returning from the ED, the patient’s mother again witnessed him having another seizure that lasted approximately 2 min where he was noted to have bilateral arm and leg shaking with foaming at the mouth. He was taken back to the outside hospital ED. He returned to his neurological baseline and was transferred to our institution for further management.
Shortly after arrival to our institution, he had another seizure witnessed by nursing staff where he had full body stiffening followed by bilateral jerking of his arms and legs with his eyes and head deviated to the left. This episode lasted 1.5 min. The patient’s mother and the providing physicians noted that the right sided facial swelling continued to worsen and the patient continued to experience worsening right sided facial pain. On physical exam, there was diffuse swelling over the right temporal region extending all the way down to reach the right ear and right jaw. This region was extremely tender to palpation. There were no external signs of trauma such as bruising or laceration. Laboratory tests including CBC demonstrates a high WBC of 17.8 (Normal range: 4.5-13.0 10E9/L), elevated CPK of 762(Normal range: 24-195 U/L), normal blood amylase, negative blood culture at 24 h and normal coagulation profile. The initial differential diagnosis for the facial swelling included a dental abscess, parotitis, or non specific myositis. Patient was empirically started on Clindamycin to treat any potential underlying infectious etiology.
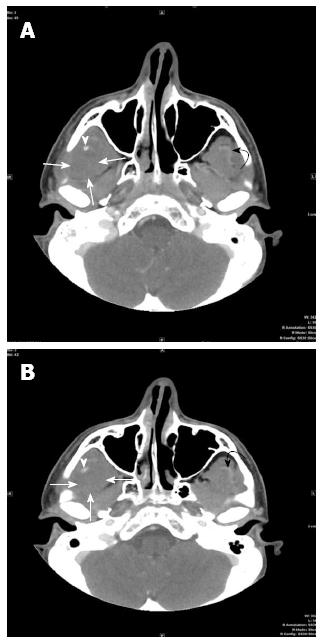
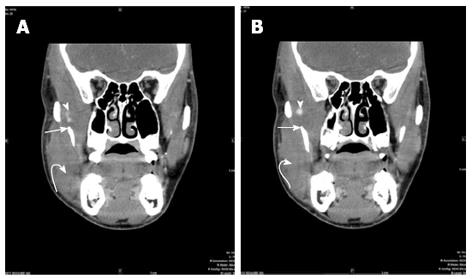
A CT scan of the face with intravenous contrast was performed in our institution for further evaluation using the following technique: GE Medical Systems, Pitch:0.562:1, Pixel spacing: 0.361 mm/0.361 mm, Kvp 120, mA 129, rotate time: 0.8, ST 2.5 mm, FOV 18 cm, Tilt 0, W:342, L:56, Contrast: 98 cc of Isovue 300. The CT images (Figures 1 and 2) demonstrate swelling and diminished attenuation of right temporalis muscle most prominent near its myotendinous portion as it passes medial to the zygomatic bone just prior to its insertion to the coronoid process and anterior ramus of the right mandible. A small high density is identified just prior to its insertion to the mandible which may represent hemorrhage. The CT findings were in favor of a non specific myositis, possibly infectious in nature. The subtle hypoattenuation near the insertion to the mandible was felt to represent a possible phlegmon without a mature abscess seen.
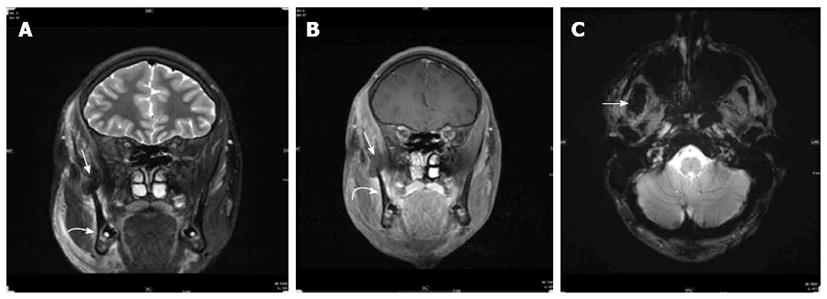
An MRI of the face prior and following intravenous contrast administration was performed the next day for better characterization. The following pulse sequences were obtained on a 3 Tesla magnet( Siemens/Skyra): T2 coronal SPIR(repetition time/echo time = 6580/91, Number of excitations (NEX) = 2, matrix = 320 × 240, slice thickness = 2.5 mm), T2 axial fat sat (6070/98, NEX = 2, 4 mm, 256 × 256, 2 mm), T1 coronal (600/8.4, NEX =1, 256 × 205, 2.5 mm), T2 axial GRE ( 627/19.9, NEX = 1, 320 × 240, 4 mm), T1 axial fat sat pre and post intravenous administration of 5.7 mL of Gadavist (661/8.4, NEX = 1, 256 × 205, 4 mm), T1 coronal fat sat post contrast (899/8.4, NEX = 1, 256 × 205, 2.5 mm). The MRI findings (Figure 3) are consistent with rupture of the right temporalis myotendinous junction with a small hematoma formation at its insertion onto the coronoid process of the mandible. There was swelling and mild inflammatory changes in the entire right temporalis muscle over the temporal calvarium. Swelling and mild inflammatory changes were also seen in the right masseter muscle especially at its attachment to the anterior ramus and angle of the mandible consistent with sprain.
Plastic surgery was consulted and after examining the patient decided to treat conservatively with pain medication and physical therapy.
The temporalis muscle is a broad, fan shaped muscle that fills the temporal fossa, superior to the zygomatic arch. The muscle originates from the temporal fossa and the deep surface of the temporal fascia. As the muscle fibers descend, they form a tendon which passes medial to the zygomatic arch and inserts into the medial surface, apex, and anterior border of the coronoid process, and the anterior border of the mandibular ramus[5,6]. Its function is to elevate and retract the mandible[5,6].
Thorough review of the literature reveals that not much has been written about rupture of the temporalis muscle insertion. Etiologies that have been described as a potential cause for injury include direct injury to the muscle fibers caused by inappropriate intra operative dissection or retraction[7-10]. Direct trauma such as a blow to the side of the head or a motor vehicle accident can also cause injury to the temporalis muscle. Sleep bruxism is a common sleep-related motor disorder characterized by tooth grinding and clenching in which there can be strain of the temporalis muscle[11]. However, frank rupture of the myotendinous insertion of the temporalis muscle has not been reported especially in the context of seizure related injuries.
We describe the CT and MRI findings of rupture of myotendinous insertion of right temporalis muscle on the mandible in a 16-year-old male following multiple witnessed generalized tonic-clonic seizures. Two mechanisms have been proposed to explain such an injury: significant pull on the myotendinous insertion following violent jerking of his head and neck to the controlateral side during seizures or a direct fall on the face. In our patient, the first mechanism is favored as no facial bruising or laceration were identified on physical exam and no direct trauma or fall to the head or face was witnessed by parents or medical staff.
Rupture of the myotendinous junction of temporalis muscle is extremely rare. It is important to be familiar with such type of injury in a patient who developed unilateral facial swelling and pain following tonic-clonic seizures in order to prevent misdiagnosis and mistreatment.
Sixty-year-old boy who developed unilateral facial swelling and pain following new onset tonic-clonic seizures.
Diffuse swelling over the right temporal region extending all the way down to reach the right ear and right jaw which was tender to palpation.
Dental abscess, parotitis, non specific myositis, rupture of the myotendinous junction of temporalis muscle.
WBC 17.8; CPK of 762; normal blood amylase; negative blood culture at 24 h; negative urine toxicology; normal coagulation profile; normal basic metabolic panel and urine analysis.
Computed tomography finding: Swelling and diminished attenuation of right temporalis muscle most prominent near its myotendinous portion as it passes medial to the zygomatic bone just prior to its insertion to the coronoid process and anterior ramus of the right mandible. A small high density is identified just prior to its insertion to the mandible which may represent hemorrhage; Magnetic resonance findings: Rupture of the right temporalis myotendinous junction with a small hematoma formation at its insertion onto the coronoid process of the mandible. Swelling and sprain of the right masseter muscle especially at its attachment to the anterior ramus and angle of the mandible.
Conservative treatment with pain medication and physical therapy.
The literature regarding rupture of the temporalis muscle insertion is sparse. Potential etiologies described in the literature include direct injury to the muscle fibers caused by inappropriate intra operative dissection or retraction or direct trauma such as a blow to the side of the head or a motor vehicle accident.
This case reports a rare case of temporalis muscle rupture following seizure. Although this is a rare entity, it is important to be familiar with such type of injury in a patient who develops unilateral facial swelling and pain following a tonic-clonic seizure in order to prevent misdiagnosis and mistreatment.
The authors present an interesting and well done clinical note and describe the neuroimaging findings of rupture of myotendinous insertion of right temporalis muscle of the mandible in a 16-year-old male following multiple witnessed generalized tonic-clonic seizures. This is the first reported case of myotendinous rupture of the temporalis muscle after seizure. This information is interesting from a clinical point of view.
P- Reviewers: Arboix A, Gumustas OG S- Editor: Song XX L- Editor: A E- Editor: Zhang DN
| 1. | McAbee GN, Wark JE. A practical approach to uncomplicated seizures in children. Am Fam Physician. 2000;62:1109-1116. [PubMed] [Cited in This Article: ] |
| 2. | Tan M, D’Souza W. Seizure-related injuries, drowning and vehicular crashes -- a critical review of the literature. Curr Neurol Neurosci Rep. 2013;13:361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Ting YW, Kwong KL. Seizure-related injuries in newly diagnosed childhood epilepsy. Pediatr Neurol. 2010;42:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Asadi-Pooya AA, Nikseresht A, Yaghoubi E, Nei M. Physical injuries in patients with epilepsy and their associated risk factors. Seizure. 2012;21:165-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Fehrenbach MJ, Herring SW. Illustrated Anatomy of the Head and Neck and Herring. 4th ed. St. Louis: Elsevier 2012; 98. [Cited in This Article: ] |
| 6. | Jacob S. Human Anatomy: A Clinically-Orientated Approach. 1st ed. New York: Elsevier 2008; 194. [Cited in This Article: ] |
| 7. | Oikawa S, Mizuno M, Muraoka S, Kobayashi S. Retrograde dissection of the temporalis muscle preventing muscle atrophy for pterional craniotomy. Technical note. J Neurosurg. 1996;84:297-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Spetzler RF, Lee KS. Reconstruction of the temporalis muscle for the pterional craniotomy. Technical note. J Neurosurg. 1990;73:636-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kadri PA, Al-Mefty O. The anatomical basis for surgical preservation of temporal muscle. J Neurosurg. 2004;100:517-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Abdulazim A, Filis A, Sadr-Eshkevari P, Schulte F, Sandu N, Schaller B. Postcraniotomy function of the temporal muscle in skull base surgery: technical note based on a preliminary study. ScientificWorldJournal. 2012;2012:427081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |