INTRODUCTION
Crohn’s disease (CD) begins in childhood, below 20 years of age, in 20% of cases and may involve characteristically any part of the gastrointestinal (GI) tract. The Paris classification has recently revised the Montreal classification, that had several weakness points relating children’s classification[1]. Important modifications developed regarding CD include classifying age at diagnosis as A1a (0 to < 10 years), A1b (10 to < 17 years), A2 (17 to 40 years), and A3 (> 40 years), distinguishing disease above the distal ileum as L4a (proximal to ligament of Treitz) and L4b (ligament of Treitz to above distal ileum), allowing both stricturing and penetrating disease to be classified in the same patient (B2B3), and denoting, at any time, the presence of growth failure in the patient as G1 versus G0 (never growth failure)[2].
Although the exact frequency of the small bowel (SB) CD is unknown, most gastroenterologists believe that its prevalence has been underestimated and that it may have an increased incidence among children and young adolescents[2]. A recent study performed in pediatric patients with magnetic resonance (MR) enterography[3], according to previous studies[4], confirmed that the involvement of the terminal ileum is common to more than three times compared to that of the jejunum and the rest of the ileum. Moreover, the involvement of the jejunum alone is uncommon and it is more difficult to investigate because of its tendency to stay non distended[3].
The clinical importance of the SB CD phenotype is the impact that a diffuse SB disease is expected to have on a child’s growth and development. Moreover, patients with SB CD are more likely to experience complications, including intestinal obstruction and less commonly fistulization[4,5].
Thus, objective evaluation of the SB is essential in differentiating CD from other enteropathies and in directing the management of the patients with inflammatory bowel disease (IBD)[6,7]. The morphological evaluation of the SB, useful in the diagnosis and management of CD, has long been made only with conventional radiology. In the last decade there has been a progressive improvement of cross-sectional imaging [ultrasonography (US), computed tomography (CT), and magnetic resonance (MR)] that has significantly changed the way to diagnose and treat the patients[8,9]. Indeed, their accuracy in detecting mucosal alterations and transmural and perienteric inflammations, has led to a new disease staging, a detection of asymptomatic disease and a better assessment of response to therapy[10]. For these reasons modern cross-sectional imaging have replaced the traditional fluoroscopy-based for visualization of the SB. In the “Porto criteria” small-bowel follow-through (SBFT) was the recommended imaging modality in children[11]. However, concerns about the proven increased risk of high radiation exposure in pediatric patients mandates the use of alternative techniques when possible[12-14]. In the European Crohn’s and Colitis Organization (ECCO) guidelines[14] it is stated that MR and CT enterography or enteroclysis are the imaging modalities with the highest diagnostic accuracy. Moreover in the pediatric section of the ECCO guidelines[15,16] dynamic contrast-enhanced MRI is considered the best imaging to show most of the CD’s lesions without exposure to ionizing radiation. In the same way the Appropriateness Criteria of the American College of Radiology[17] point out that, in the pediatric patients, MR enterography may have sensitivity and specificity similar to CT enterography and avoids radiation risks. Ultimately the same accuracy, the choice of examination depends on several variables, such as institutional preferences and resources (US, CT, or MR scan), age and compliance of the patient, the eventually acute presentation, and finally radiologist expertise.
In this article we discuss all the methods commonly used for imaging the small bowel in paediatric patients with Crohn’s disease analyzing the advantages and disadvantages of each modality, with particular emphasis on MR imaging.
BARIUM STUDIES
Traditionally in children with suspected CD has been studied with barium studies, enteroclysis (SBE) or SB follow-through (SBFT), considered to be the gold standard examination for the SB[18,19]. SBE was considered the best exam for SB disease, however children poorly tolerate the required insertion of a naso-jejunal tube. SBFT has long been considered as the most common, non-invasive, inexpensive and easily accessible radiological method[14], but, currently, it has only a secondary role in small bowel imaging. US and MR enterography are methods of choice for imaging SB diseases in pediatric populations.
Early mucosal changes, such as aphthous ulceration, can be detected by SBFT. This technique can also assess bowel motility that help to differentiate strictures from mural thickening and allows a functional evaluation of the pathological segment studying the SB transit time[14-16].
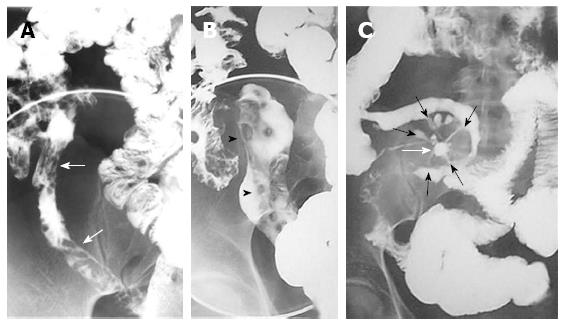
Although SBE and SBFT can effectively depict the presence of mucosal abnormalities effectively, including fissures, cobblestone mucosa, pseudo-polyps, and skip lesions, they are imprecise for the diagnosis of transmural and extramural disease[14,20,21], except in the overt forms (Figure 1).
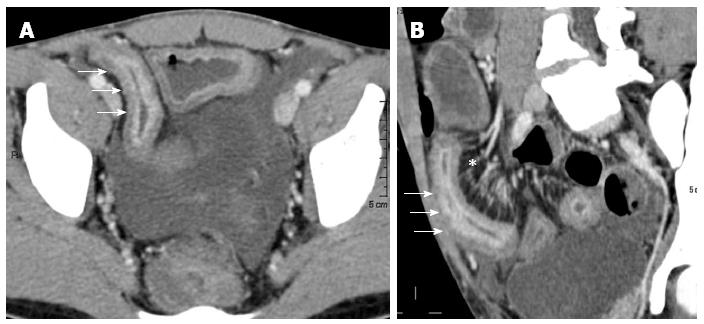
Figure 1 Barium studies in patients with Crohn’s disease.
Double-contrast barium enema examination (A and B) demonstrate longitudinal (arrows) and perpendicular (arrowheads) ulcerations in the terminal ileum. Small-bowel follow-through (C) demonstrates an abscess cavity (white arrow) with fistulae connecting the cavity to the adjacent small bowel (black arrows).
A retrospective analysis of 164 children revealed a diagnostic sensitivity of only 45% for SB radiography compared with ileo-colonoscopy[22]. Moreover, SBFT is not accurate for the detection of active CD in the SB[20-23]. In fact, it can directly examine the mucosa demonstrating early active mucosal disease such as aphthous and linear ulcers, but it does not allow to evaluate the small bowel wall and the mesentery, except with indirect signs. Moreover, superimposed bowel loops or non-palpable bowel loops deep in the pelvis can hide active disease or its complications[20].
Concerns regarding the risks of radiation exposure in the pediatric population has increased with the spread use of these imaging studies. Children especially are at risk because they are inherently more radiosensitive and because they have more remaining years of life during which a radiation-induced cancer could develop[24].
A major disadvantage of barium studies, especially in children, is the radiation exposure, particularly if fluoroscopy time is not kept to a minimum[13]. Gaca et al[13] studied a total of 176 children with CD who underwent averaging 1.2 SBFTs. On average SBFT took 5.1 min with 3.3 abdominal radiographs. The effective doses (mSv) for a 5-min fluoroscopy were 0.15 for the central abdomen, 0.35 for the right lower quadrant, and 0.56 for the pelvis, yielding an average effective dose for SBFT (5-min fluoroscopy, 3.3 abdominal radiographs) of 1.8-2.2 mSv. Although 5 min of fluoroscopy time for an SBFT might seem excessive, this was calculated based on the average of 30 examinations performed by five experienced pediatric radiologists[13]. In the young population of CD patients, the ionizing radiation required for SBE limits the use of this technique for the follow-up of the disease. Moreover, SBFT and SBE examinations can often result in incomplete studies. In fact they cause more patient discomfort compared with CT[25] and MRI[26], barium contrast can be poorly tolerated by children especially in severe and advanced disease and abdominal pain can limit compression preventing the adequate visualization of overlapping loops.
ULTRASONOGRAPHY
US has the distinct advantage of being widely available, inexpensive, non-invasive, radiation-free and relatively easy to perform[7,27]. Over the past few years, improvements in US equipment such as high-frequency transducers (7-12 MHz), combined with oral and intravenous (CE-US) contrast agents[28,29], have overcome some of the obstacles in bowel US that existed in the past, thus raising a great enthusiasm for its use in IBD children.
US can be considered a valuable tool in the preliminary diagnostic process of paediatric patients with suspected IBD, prior to further invasive tests[30,31].
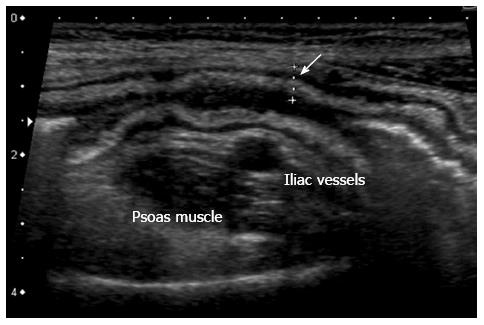
Inflamed bowel can show both mural and extramural pathological changes. Bowel wall thickness is the most important US sign of IBD (Figure 2), with different thickness values used as a threshold for a positive diagnosis in the various reports (from 1.5 mm to 3 mm in the terminal ileum and < 2 mm in the colon)[27,32-35].
Figure 2 Thickening of the bowel wall, 5 mm, (arrow) with wall layers preserved.
The hyperechoic band corresponds to thickened submucosa.
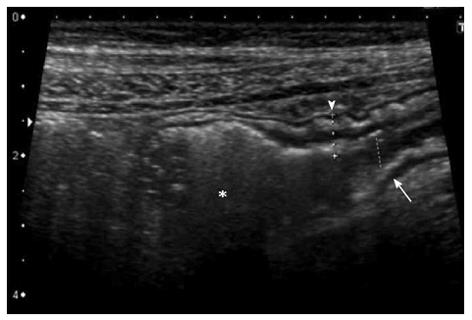
The other US signs are altered echogenicity, loss of the normally visible stratification (Figure 3), increased Colour-Doppler signal denoting hyperaemia (Figure 4) and relative decrease or lack in peristalsis signifying some degree of stiffness[31]. Extra-mural findings include changes involving the surrounding mesentery, that appears thickened and hyperechoic and generally shows enlarged mesenteric lymph-nodes (Figure 4)[30,36].
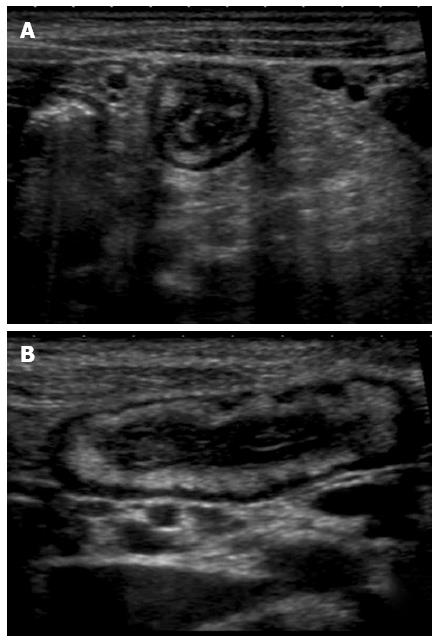
Figure 3 Transversal (A) and longitudinal (B) section of a thickened ileal loop due to Crohn’s disease.
The “target” sign, corresponding to remarkable bowel wall thickness, is visible as a strong echogenic centre surrounded by a hypoechoic border (A). The adjacent mesentery is thickened and hyperechoic, due to the transmural nature of inflammation in Crohn’s disease (A and B).
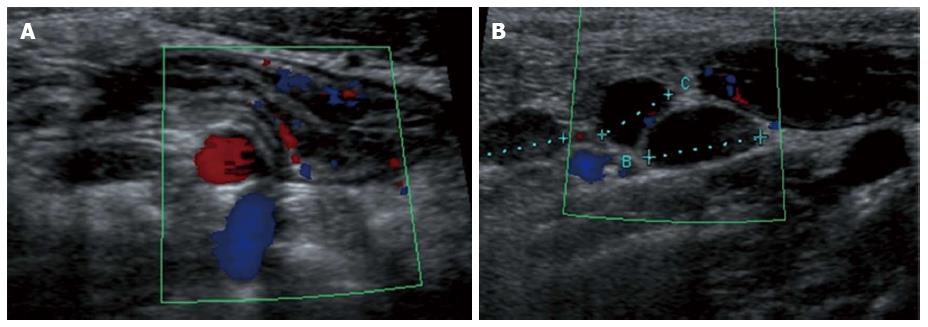
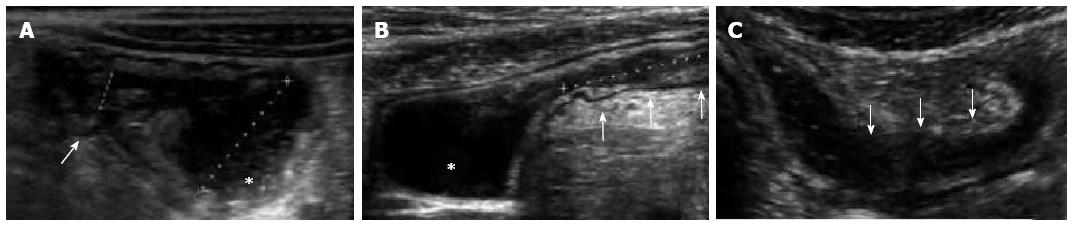
Figure 4 Longitudinal view of the terminal ileum in a 13-year-old boy with active Crohn’s disease.
The bowel wall is thickened and shows increase in Color Doppler signals denoting inflammatory hyperemia (A). Note in (B) the fibrofatty proliferation of the surrounding mesentery which appears hyperechoic and the enlarged mesenteric lymph-nodes.
Bowel US and ileocolonoscopy with histology have demonstrated an overall sensitivity of 74 and 88% and a specificity of 78 and 93%, respectively, in the detection of SB CD lesions[35,37]. The sensitivity of US in the detection of SB lesions is greater for those of the terminal ileum (approximately 90%-95%) than for those of the proximal SB (75%)[38]. US is useful for the follow-up of patients with CD[39,40]. and in early identifying intra-abdominal complications, such as abscesses, fistulae and strictures[41-44]. The reported sensitivity of US in detecting CD strictures approximately is 74%-80%[41]. US can also aid differentiate between fibrotic and inflammatory strictures. An increased Colour-Doppler signal in the stenosis is suggestive of activity, while poor vascularity of the bowel wall, an adjacent loops’ retraction and a pre-stenotic bowel segment distension are suggestive of fibrotic stenosis (Figure 5)[31].
Figure 5 Longitudinal section of a distal ileal loop showing stiffness, mural thickening (arrowhead), lumen narrowing (arrow) and mild dilatation of the pre-stenotic segment (asterisk), suggesting a fibrotic nature of the stenosis.
Some challenges about US still remain: US strongly relies on the operator’s experience and skill more than other imaging modalities, and it requires an high resolution equipment. Moreover, even in expert hands, US may result in false positive findings[34]: thickening of the intestinal wall is not specific for CD, also being present in infectious, neoplastic and other inflammatory conditions. US may also provide false negative results[38,42], for example in obese patients or when CD is characterized by only superficial lesions, like isolated aphthous ulcers and mucosal erosions, or in presence of intestinal gas that make hardly visible the bowel wall, particularly the proximal ones.
Small Intestine Contrast US (SICUS). Some studies in adults have demonstrated that the so called SICUS, enables to overcome limits of standard US. Dissociation of intestinal overlapping loops and visualization of the entire SB, from the Treitz angle to the ileo-cecal valve, are obtained by distending the SB with an oral anechoic non-absorbable contrast solution (iso-osmolar polyethylene glycol)[36]. Briefly, US examination in performed after the ingestion of the oral contrast solution, that fill in the loops, and the administration of the intravenous (iv) contrast. Unfortunately the procedure is time consuming, requiring in some cases more than 2 hours. Pallotta et al[28] studied 148 patients, 57 with a known diagnosis of CD, showing SICUS to be more sensitive and specific (94% and 98%, respectively) than conventional US (57% and 100% respectively) in assessing SB lesions. The use of an oral contrast agent can significantly improve US sensitivity, approximately of 90% (Figure 6) and of over 75% for a single and multiple SB stenosis, respectively[42].
Figure 6 The oral anechoic contrast solution, (polyethylene glycol), allows a better definition of lumen narrowing (arrow) and pre-stenotic dilatation (asterisk) as well as a more accurate measurement of the length of the stenosis.
Small intestine contrast ultrasonography also provides a dynamic assessment of the stenotic bowel loop: poor distensibility and presence of pre-stenotic dilatation suggest fibrosis, whereas normal distensibility and preserved peristalsis are features of inflammatory stenosis.
SICUS can make a dynamic evaluation of the affected segment differentiating between inflammatory and fibrotic stenosis. In fact, it can assess lumen stenosis and dilatation of the prestenotic segment, mural thickness with bowel wall stratification pattern and peristalsis of the affected segment differentiating between. SICUS is a very promising technique in children with CD, but its use in pediatric patients has still to be investigated.
CROSS SECTIONAL IMAGING
Good distension of SB loops during the CT and MRI examination is crucial for the correct evaluation of bowel wall abnormalities since collapsed SB loops are difficult to evaluate for bowel wall thickening or hyper-enhancement. Distension of the SB can be achieved by fluid administration after naso-jejunal intubation (CT/MR enteroclysis)[45,46] or per Os (CT/MR enterography)[47,48]. Although better distension of the loops is obtained with the MR/CT enteroclysis, there are various studies that show comparable accuracy between the two techniques[20,45,47,48]. The placement of the nasojejunal tube, necessary for the enteroclysis, is invasive, requires the use of ionizing radiations, which also entails the need for coordination between MR/CT suites and fluoroscopy units, and it can be very anxiety provoking resulting in poor patient tolerance. Moreover, the increased time and costs compared to enterography, make CT/MR enterography the preferred imaging method, respect to CT/MR enteroclysis, in pediatric patients.
An important limitation of CT/MR enterography is the need to drink an important amount of fluid in a short time, particularly uncomfortable in young patients. The assessment of mucosal abnormalities requires a correct distension of the loops obtained only with a proper timing of the study both in scanning or fluid ingestion. The collapsed bowel loops may mimic wall thickening and hyper-enhancement, leading to false-positive results.
The younger patients and the parents have to be motivated and aware of the importance of performing an adequate SB distension, which is fundamental for obtaining an optimal MR examination. The presence of a parent in the MR room is reassuring for younger children.
Most children diagnosed with IBD are of school age or adolescence. In a study from uniform data collected from a cohort of pediatric patients with IBD (1370 children), the mean age at diagnosis was 10.3 years with 47.7% diagnosed at 6 to 12 years of age and 36.9% at 13 to 17 years of age[49]. In our experience, children of this age are generally able to undergo the exam without sedation.
In the event a child refuses to drink the contrast agent, we will offer placement of a temporary nasogastric tube for contrast agent delivery. The enteric tube will be removed right after all of the contrast material is given and before the MR examination begins.
Patients under the age of 6 years, who require anesthesia do not undergo MR enterography and are usually imaged by using high-resolution bowel US or MR enteroclysis under anesthesia. The positioning of the tube takes place after the anesthesia. Children between 6 mo and 6 years of age are usually sedated with the intravenous injection of midazolam (Versed; Hoffman-La Roche, Basel, Switzerland) (0.05 mg per kilogram of body weight) or pentobarbital (Nembutal; Ovation Pharmaceuticals, Deerfield, Ill) (5 mg per kilogram of body weight). The sequences are performed in free breathing, making them as short as possible (Figure 7).
Figure 7 Magnetic resonance enteroclysis under anesthesia in one-year old male with Crohn’s disease at colonoscopy.
Coronal thick-slab HASTE image (A) shows good opacification of proximal and distal small bowel. The coronal T2-weighted (T2-w) image (B) and coronal fat-suppressed (FS) three-dimensional (3D) T1-weighted (T1-w) breath-hold gradient-echo (GE) image (C) are performed in free breathing.
Computed Tomography
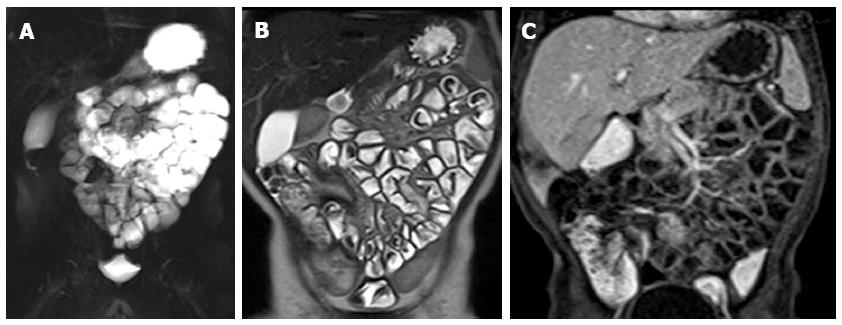
The relatively high radiation exposure is a limitation to the use of multi-detector CT (MDCT) enterography in children, and, in fact, most data regarding this method come from studies in adults[50]. The only recent pediatric IBD population’s study[51] concluded that MDCT can be used as an alternative to barium studies for the evaluation of the SB and that most of the children prefer CT rather than barium studies. Several studies on adult population showed that is an excellent non-invasive tool for diagnosing CD, and for the follow-up of the disease during therapy[19,20,52-54]. CT enterography can establish disease extension and activity on the basis of wall thickness and increased iv contrast enhancement. In recent studies bowel wall thickness is considered pathological when exceeds 3 mm[55,56] (Figure 8). A sign of active disease is an increased bowel wall enhancement after administration of iv contrast medium[57,58]. The post-contrast wall pattern depends on the different enhancement of the mucosa and/or serosa and the submucosa, usually hypodense for the presence of edema, and can be seen as mural stratification or target sign (Figure 9), with two or three different layers of density respectively. In chronic CD, the affected segments may present a non-enhancement pattern after contrast medium, with the loss of mural stratification, suggestive of fibrosis. Another typical extramural lesion in CD is the comb sign due to an increased vascularity of the mesentery seen in the images as tortuous dilated vessels associated with a wide spacing of the vasa recta (Figure 8).
Figure 8 Transverse (A) and coronal (B) computed tomography show bowel wall thickening and mucosal hyper-enhancement with pseudo polyps (white arrows) as well as mesenteric lymph nodes, that are irregular in size and shape (black arrowhead) and increased mesenteric vascularity (asterisk).
These features may be detected in case of acute exacerbation on a background of longstanding disease.
Figure 9 Transverse (A) and MRP sagittal (B) computed tomography show stratified enhancement of terminal ileum (arrows), and hyperemic mesentery with the “comb sign” (asterisk).
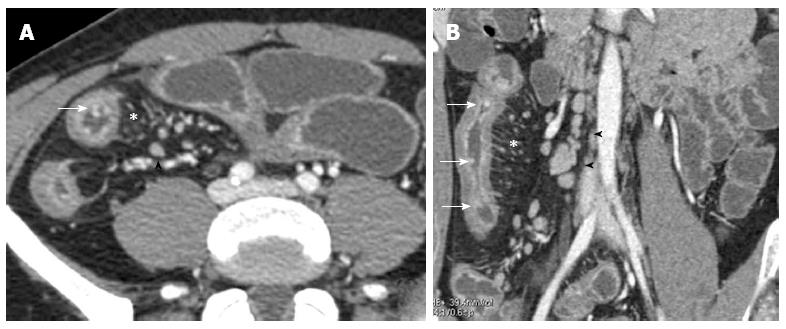
The most reliable criterion to define a stricture is a localized, persistent narrowing, whose functional effects may be judged from pre-stenotic dilatation[59]. CT, together with MRI, is the most accurate technique to detect the presence of extraluminal complications, such as abscesses, fistulae (Figure 10) and inflammatory conglomerates in CD[20,46,53,54,60-62].
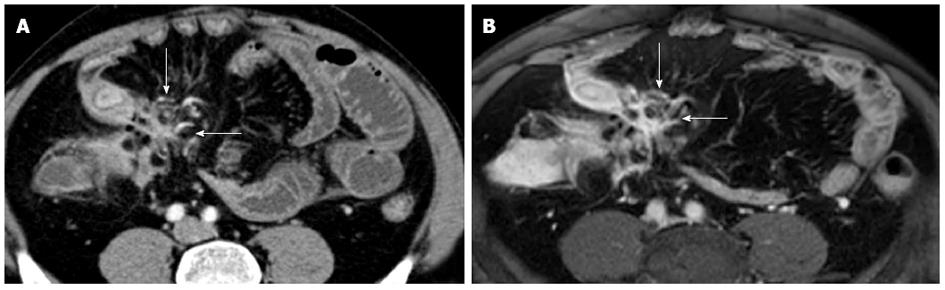
Figure 10 Transverse computed tomography (A) and post contrast T1-fat sat magnetic resonance imaging (B) images show a complex network between closely adherent small bowel loops appearing as a stellate configuration (arrows) due to entero-enteric fistulas.
CT has a high accuracy in the imaging of CD but it is limited by the use of ionizing radiations especially in children particularly in these type of chronic diseases that require a close follow up[13,63].
The radiation dose can be significantly reduce by the use of last generation MDCT scan with specific pediatric protocols[64,65] which include the introduction of noise to simulate low-dose exams[66].
Still, in pediatric patients MR must be preferred to MDCT, since it does not use ionizing radiation to which children are more vulnerable than adults for their longer life expectancy. Moreover, despite new formulations and improved safety, iodinated contrast media for CT are not without risk and the risks must be balanced against the possible benefits. However, in the hospitals without MR scan, or where it is difficult to schedule an emergent MRI, or in emergency situations, such as high-grade SB occlusion, MDCT remains the best technique in pediatric patients, too. In fact CT has greater availability and it is less time-consuming than MR (20-30 min for MR, respect to 10 s for MDCT).
Magnetic Resonance
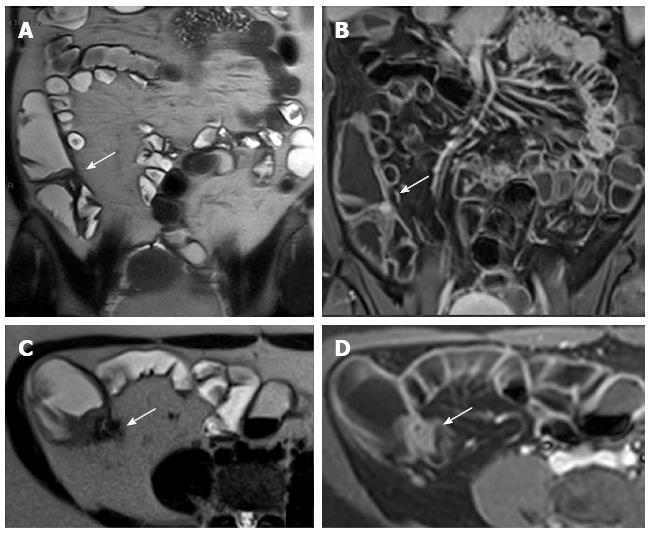
The main advantages of MRI are, in addition to the lack of ionizing radiations, a superior soft tissue contrast with a better assessment of trans and extramural disease, its noninvasiveness and the multiplanar capability. Additionally, some MRI sequences (diffusion, perfusion, motility) can provide functional and quantitative information of the bowel wall (diffusion, perfusion, motility) that CT cannot obtain. Especially, diffusion-weighted sequence does not significantly increase the time of the examination and may provide helpful clues for the identification of areas of active inflammation and of abscesses (Figure 11) without iv contrast agent. Moreover, the use of cine MRI in patients suffering from CD proves the association of motility changes of the SB wall and extraluminal alterations, which can help in the differential diagnosis between fibrotic and inflammatory stenosis[67].
Figure 11 Eighteen-years-old female with active Crohn’s disease and abscess.
Transverse T2-w (A), DWI (B), transverse (C) and coronal (D) post-contrast FS-T1-w image (C) show inflamed segments of the terminal ileum (arrowhead) with pericecal fluid-collection around ticked and hype-vascular walls (arrow), according to abscess. A small air bubble in the antideclive portion of the fluid collection is visible in the transverse T2-w (A) and transverse post-contrast FS-T1-w image (C).
In relation to imaging features, CD may present as active inflammation (without strictures or fistulas), penetrating lesions, or fibrostenotic disease[68]. Patients may present characteristics of more than one disease subtypes.
Active disease. Various MR imaging findings have been proposed as correlating with CD activity. Increased wall thickening and mural contrast enhancement (CE) on MRI were found to be sensitive and specific for active CD in pediatric population[69]. Laghi et al[69] found a strong correlation between a semi-quantitative score (reflecting bowel-wall CE and thickening) and Pediatric Crohn’s Disease Activity Index (PCDAI) in CD patients. In a recent study on pediatric population, Alexopoulou et al[70] showed that the MR percentage of CE (%CE) of the bowel wall do not correlate with PCDAI values. Other studies have reported similar results in the past[71,72], while a correlation with C-reactive protein (CRP) was already demonstrated in pediatric[70] and adult[71] population. In children, clinical evaluation of disease activity may be even more subjective due to incomplete cooperation, and this explains the observed lack of correlation between PCDAI and %CE values, while in contrast, %CE values were correlated with CRP, which is a more objective marker of inflammation[70].
The wall thickening, its high mural signal intensity on T2-weighted fat-saturated (FS-T2-w) images, and the presence of mural stratification on post-contrast T1-weighted fat-saturated (FS-T1-w) images reflect histologic features of acute SB inflammation in CD[69,71,72] (Figure 12). A purely quantitative approach would be desirable for MRI evaluation of active disease. However, in patients with CD, measurements of the bowel wall MR signal intensity are subjected to wide limits of both inter- and intra-reader agreement, which may substantially limit their utility when applied to the development of quantitative measures of inflammatory activity in the affected bowel segments[73,74].
Figure 12 Thirteen years old male with active Crohn’s disease.
Coronal T2-weighted image (A), and transverse fat saturation T2-weighted images (B and C), show mural thickness and increased mural signal (arrowhead in B, C, and D) in the terminal ileum due to edema. Coronal post-contrast T1-weighted fat-suppressed image (D) at the same level shows mural stratification (asterisk).
Acute inflammation can also present with the comb sign (Figure 13), due to an increased vascularity of the mesentery, ulcers (Figure 14) and enlarged and high enhancing lymph-nodes[74-77].
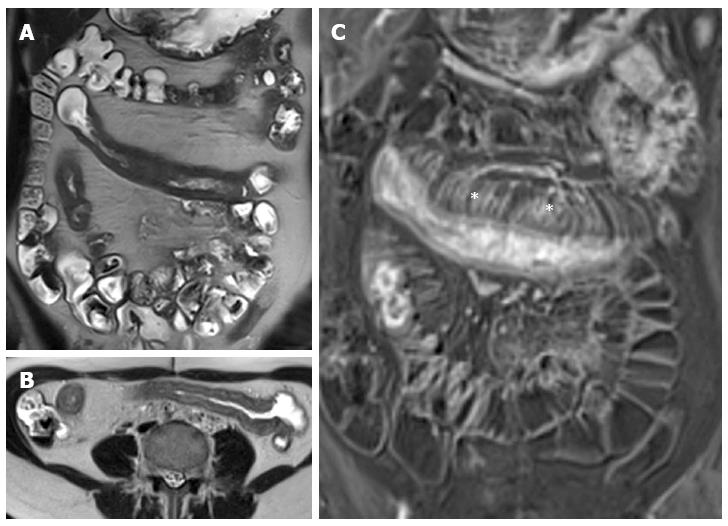
Figure 13 Thirteen years old female with active Crohn’s disease.
Coronal (A), transverse (B) T2-weighted images show thickened, inflamed segments of ileum and fat proliferating in the mesentery. The thin layer of high signal on T2 in b represents edema. The mass of the fat will displace adjacent small bowel. Coronal post-contrast T1-weighted fat-suppressed image (C) shows increased vascularity (asterisks), named “comb sign”, adjacent to a hyper-enhancing thickened segment of ileum.
Figure 14 Coronal (A), transverse (B) T2-weighted images show thickened, inflamed segments of the terminal ileum with deep ulcers seen as high-contrast protrusions within bowel wall (arrows).
A proper luminal distension is essential to assess ulcers on MRI, especially if superficial[74]. In a systematic review of seven studies[52], MRI showed an accuracy of 91%, 62% and 62% in correctly staging a frank, mild and in remission disease, respectively. MRI more often overstaged than understaged disease activity in CD, but in most of these patients radiological staging and disease staging by the reference standard differed one grade. However, this review has the limit of including old studies without considering the new state-of-the-art techniques.
Actually radiologist use different protocols and features for grading CD, as recently showed by Ziech et al[78]. The authors show that the most frequently used MR protocols include T2-w (79%) and CE FS-T1-w (83%) sequences and that the features most frequently seen as important for grading are the presence of bowel wall thickening (79% of radiologists), abscesses (75%) and CE (75%) and stratification (46%) at T1-w images.
Currently, the most important applications of MR care the confirmation of the disease and the follow-up of patients with an already established diagnosis of CD, both by monitoring the response to medical treatment by assessing disease activity (Figure 15) and by early identifying of abscesses, fistulae and strictures.
Figure 15 Twelve years old female with active disease and follow-up.
Transverse T2-weighted image (A) shows mural thickening (arrows) and increased mural signal (arrow) in the terminal ileum and coronal T1-weighted image (B) shows mural stratification (arrow), increased mesenteric vascularity adjacent to the inflamed bowel loop (the comb sign), and enlargement and hyper-enhancement of lymph-nodes (asterisk). In the same patient and at the same level, six months later after therapy, transverse T2-weighted image (C) shows the loss of increased mural signal (arrow) and coronal T1-weighted image (D) shows homogeneous enhancement without mural stratification (arrow), reduction of increased mesenteric vascularity adjacent to the inflamed bowel loop, and disappearance of lymp-nodes (asterisk).
Penetrating disease
Transmural inflammation can result from ulcers that deeply penetrate the bowel wall forming serpiginous tracts and fistulas.
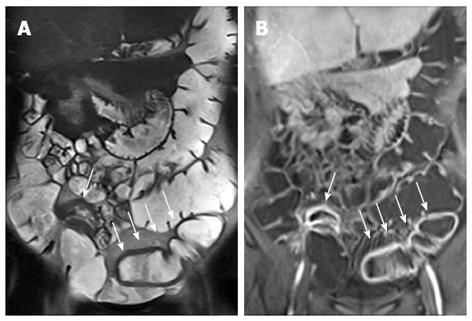
MR enterography is accurate in identifying extraluminal complications of CD. In young adults MR enterography showed a diagnostic value similar to MDCT enterography at least for acute complications of CD, such as fistulas and abscesses[79]. The abscesses can be treated by percutaneous interventions. Whereas penetrating disease may benefit from antibiotics or biologic therapies, while the use of steroids is usually avoided. Because of the exquisite sensitivity to detect fluid as well as its superior soft tissue contrast, MR easily depicts entero-entero (Figure 16), entero-vesicular, entero-cutaneous, perianal fistulaes and abscesses (Figure 17). MR imaging may also detect small volumes of gas within an abscess (Figure 11). MR enterography can assess fistulizations, sinus tracts, and abscesses, especially with the use of post-contrast FS-T1-w images (Figure 10) because of their avidly enhancing walls[80]. Entero-enteric fistulas often form a complex network between closely adherent SB loops that may appear as a stellate configuration on CE MR images.
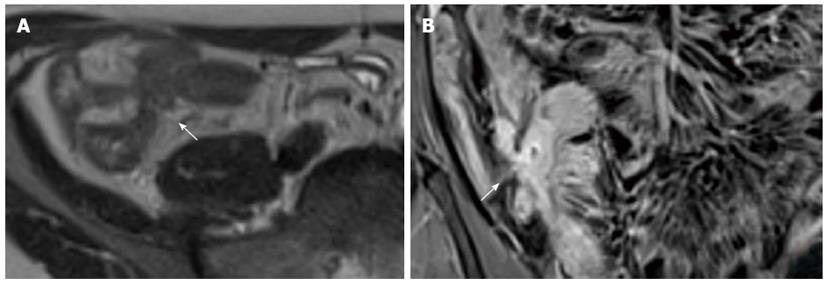
Figure 16 Transverse T2-w image (A) and coronal post-contrast FS-T1-w image (B) show cluster of bowel loops (arrow) interconnected by fistulas and adhesions.
Figure 17 Coronal (A) and transverse (B and C) CE FS-T1-w 3D gradient-echo image show a small abscess close to the terminal ileum (arrows).
Mural stratification and “comb sign” of the right colon flessure (black arrow), cecum (curved arrow), and appendix (white arrow) and homogeneous avid enhance of the terminal ileum (arrowhead). Enlargement and hyper-enhancement lymp-nodes (asterisk) are also visible.
Fibrostenosing disease
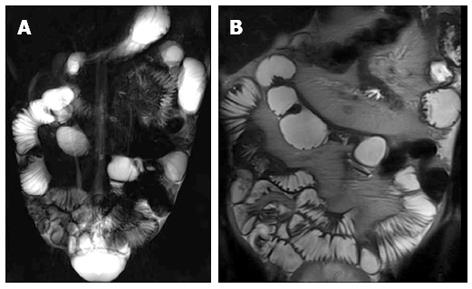
Over the time, chronic inflammation of the bowel wall may evolve in mural fibrosis that can lead to intestinal occlusion if it causes strictures. The identification of fibrotic stenosis is fundamental for they are not responsive to medical therapy and need surgical resection. In general MR enterography has a good accuracy in assessing SB strictures that are considered significant if the dilatation of the upstream bowel exceeds 3 cm[77] (Figure 18). When there is mural fibrosis with permanent strictures, the thickened bowel wall of the pathological segment does not show a hyperintensity on T2-w images or a stratified post-contrast pattern on T1-w images typical of acute inflammation[74]. These items may be useful to distinguish between transient strictures supported by acute inflammation or fibrostenosing disease (Figure 19). Cine MRI sequences, allowing the evaluation of bowel motility, can further help this differential diagnosis. Inflammation along the mesenteric border often result in pseudosacculations along the antimesenteric border and can be thought of as the MR equivalent of the mesenteric border linear ulcer seen at SBFT examination (Figure 20). In general, the affected segments are characterized by increased rigidity, loss of distensibility and diminished peristalsis.
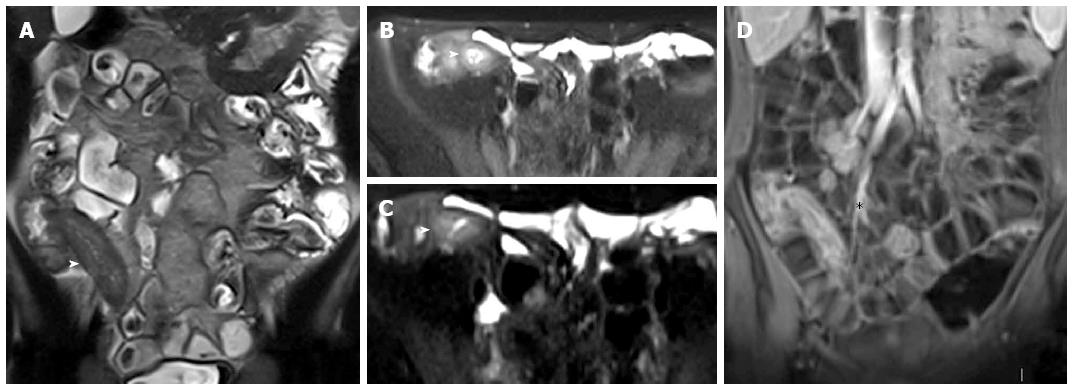
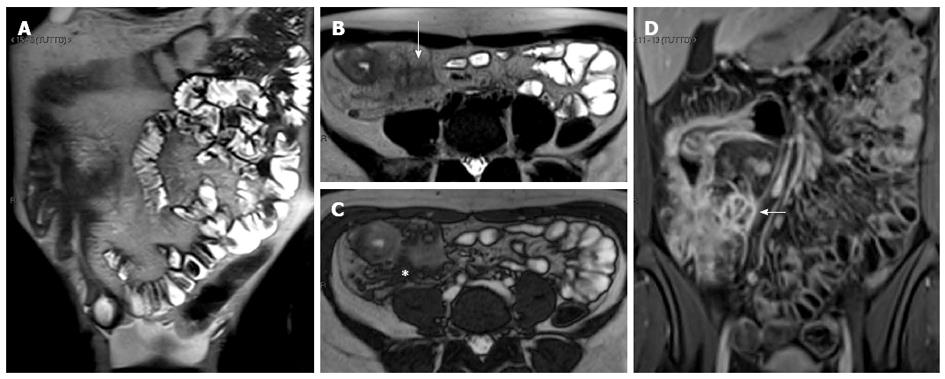
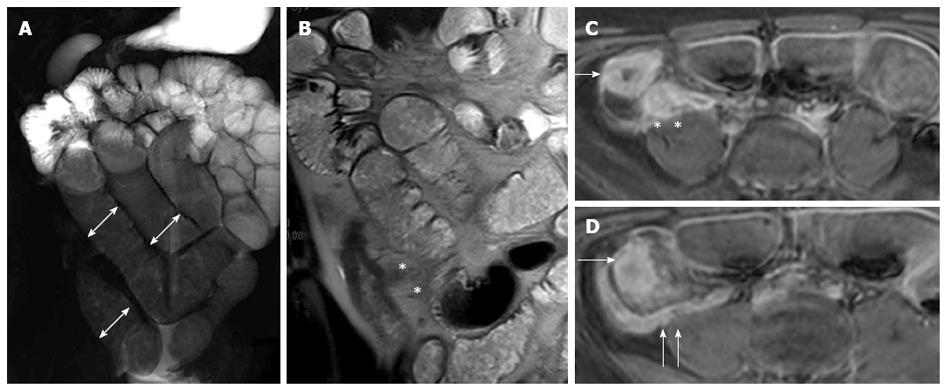
Figure 18 Fifteen years old male with small bowel obstruction caused by fibrotic stricture.
Thick-slab T2-w sequence (A) shows bowel dilatation greater than 3 cm (double arrows), according to functionally significant stricture. Coronal T2-w sequence (B) shows mural thickening of the terminal ileum without increased wall signal (asterisks). Transverse (C and D) CE FS-T1-w 3D GE show a homogeneous avidly enhancing of the cecum (arrow in C and D), terminal ileum (asterisks in C) and appendix (arrows in D). These findings can be finding in the small bowel obstruction due to fibrotic stricture.
Figure 19 Eighteen-years-old female with long standing Crohn’s disease.
Coronal (A), transverse (B) T2-w images and coronal (C), transverse (D) post-contrast FS-T1-w images show thickening and hyper-enhancement of the ileocecal valve causing stricture.
Figure 20 Coronal thick-slab HASTE (A) and coronal T2-weighted (B) images show pseudosacculations produced by asymmetric thickening of the ileal mesenteric border.
Suspected CD: In young patients with suspected CD, MR enterography is a valid method to diagnose or exclude the disease. Particularly, it can be used as the first radiological modality in pediatric patients in which the results of endoscopy examinations are normal but a high suspicion of CD is still present[81]. However, there is much debate about the best modality to use to examine the SB, because wireless endoscopic examinations, like radiological studies, have their advantages, such as the non-invasiveness and the high diagnostic accuracy in evaluating the small intestine, especially in patients who cannot undergo MR, whose bowel loops are not optimally distended or who are uncooperative, and disadvantages, such as capsule retention due to ileal strictures and delayed capsule transit due to inflammatory lesions[82,83]. If a bowel obstruction is suspected, MR enterography is preferred to capsule endoscopy for the risk of capsule retention[84]. Moreover, MR enterography can help the diagnosis of terminal ileitis in symptomatic patients when endoscopy is unsuccessful.
Additional MRI findings: Extra-intestinal lesions are detected with MRI in 24%-58% of patients[84-86]. Herfarth et al[86] performed MR enteroclysis in 710 patients with a suspicion or a diagnosis of IBD finding extra-intestinal lesions in 57% of patients of which the 12% of great clinical importance. The colonic features of CD at MR enterography can reliably be diagnosed only in severe inflammation (Figure 21).
Figure 21 Colonic distention is optimized with a rectal enema.
Coronal T2-w (A) and post-contrast FS-T1-w (B) images show bowel wall thickening, and mural stratification of terminal ileum (arrow) and sigma (arrows) walls.
The detection of colonic mild disease can be very difficult even if the colon is well distended with the help of a rectal enema (MR colonography); moreover the main weaknesses of MR colonography is the impossibility of obtaining tissue sampling. However, Rimola et al[87] demonstrated good correlations between the presence and the severity of CD lesions depicted with endoscopy and MR Colonography.
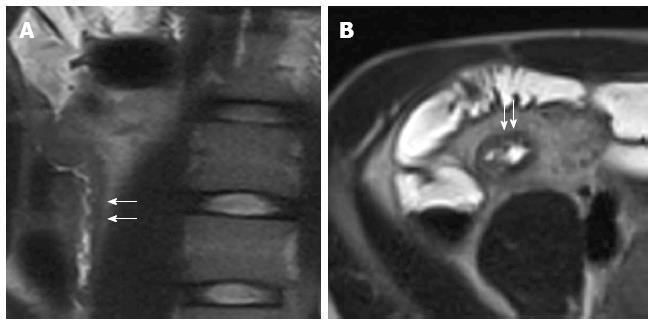
MR is an effective imaging technique for the evaluation of patients with perianal CD. During MR enterography, it is possible to perform specific pelvic sequences, such as FS-T2-w, to evaluate perianal CD, increasing only few minutes of exam time (Figure 22).
Figure 22 Twelve years old male with terminal ileum Crohn’s disease and perianal fistula.
Transverse T2-w (A) and post-contrast FS-T1-w (B) shows terminal ileum submucosa edema (black arrow) and mural stratification (arrows), respectively. Transverse FS-T2-w (C) image shows perianal fistula (arrowhead) in the same patient.
MR has an accuracy of 76%-100% compared to examination under anesthesia for fistulae and may provide additional information[88]. This technique is an important tool because it can accurately reveal the location and extent of disease, including a clinically undetected fistula or abscess, and it can guide surgery[89].
3T MRI: There has been little experience of the use of 3 Tesla (T) MR imaging in children with CD, but it seems to have the advantage of increasing the signal-to-noise ratio (SNR) of approximately 1.7-1.8 times compared to 1.5T MR. This increases the spatial and/or temporal resolution, improving the detection of early, superficial or subtle abnormalities. However, the 3T advantages are potentially offset by other issues such as greater chemical shift, susceptibility artifact, B1 inhomogeneity and specific absorption rate[90,91].