Published online Aug 28, 2013. doi: 10.4329/wjr.v5.i8.295
Revised: June 13, 2013
Accepted: July 17, 2013
Published online: August 28, 2013
Processing time: 124 Days and 9.9 Hours
AIM: To investigate diagnostic accuracy of high, low and mixed voltage dual energy computed tomography (DECT) for detection of prior myocardial infarction (MI).
METHODS: Twenty-four consecutive patients (88% male, mean age 65 ± 11 years old) with clinically documented prior MI (> 6 mo) were prospectively recruited to undergo late phase DECT for characterization of their MI. Computed tomography (CT) examinations were performed using a dual source CT system (64-slice Definition or 128-slice Definition FLASH, Siemens Healthcare) with initial first pass and 10 min late phase image acquisitions. Using the 17-segment model, regional systolic function was analyzed using first pass CT as normal or abnormal (hypokinetic, akinetic, dyskinetic). Regions with abnormal systolic function were identified as infarct segments. Late phase DE scans were reconstructed into: 140 kVp, 100 kVp, mixed (120 kVp) images and iodine-only datasets. Using the same 17-segment model, each dataset was evaluated for possible (grade 2) or definite (grade 3) late phase myocardial enhancement abnormalities. Logistic regression for correlated data was used to compare reconstructions in terms of the accuracy for detecting infarct segments using late myocardial hyperenhancement scores.
RESULTS: All patients reported prior history of documented myocardial infarction, with most occurring more than 5 years prior (n = 18; 75% of cohort). Fifty-five of 408 (13%) segments demonstrated abnormal wall motion and were classified as infarct. The remaining 353 segments were classified as non-infarcted segments. A total of 1692 segments were analyzed for late phase enhancement abnormalities, with 91 (5.5%) segments not interpretable due to artifact. Combined grades 2 and 3 compared to grade 3 only enhancement abnormalities demonstrated significantly higher sensitivity and similar specificity for detection of infarct segments for all reconstructions evaluated. Evaluation of different voltage acquisitions demonstrated the highest diagnostic performance for the 100 kVp reconstruction which had higher diagnostic accuracy (87%; 95%CI: 80%-90%), sensitivity (86%-93%; 95%CI: 54%-78%) and specificity (90%; 95%CI: 86%-93%) compared to the other reconstructions. For sensitivity, there were significant differences noted between 100 kVp vs 140 kVp (P < 0.0005), 100 kVp vs mixed (P < 0.0001), and 100 kVp vs iodine only (P < 0.005) using combined grade 2 and grade 3 perfusion abnormalities. For specificity, there were significant differences noted between 100 kVp vs 140 kVp (P < 0.005), and 100 kVp vs mixed (P < 0.01) using combined grades 2 and 3 perfusion abnormalities.
CONCLUSION: Low voltage acquisition CT, 100 kVp in this study, demonstrates superior diagnostic performance when compared to higher and mixed voltage acquisitions for detection of prior MI.
Core tip: Cardiac magnetic resonance (CMR) imaging is considered the gold standard non-invasive imaging technique for identification of myocardial infarction and viability. However, not all patients are eligible for CMR due to potential contraindications, especially in patients with electronic implants. Although electrocardiogram, nuclear imaging and echocardiography have been used to identify myocardial infarction, they generally have low sensitivity, particular for small scar regions. Cardiac computed tomography represents a viable alternative to CMR, and low voltage late enhancement cardiac computed tomography angiography imaging provides the best diagnostic accuracy compared to high and mixed voltage acquisitions for identification of myocardial infarct regions.
- Citation: Srichai MB, Chandarana H, Donnino R, Lim IIP, Leidecker C, Babb J, Jacobs JE. Diagnostic accuracy of cardiac computed tomography angiography for myocardial infarction. World J Radiol 2013; 5(8): 295-303
- URL: https://www.wjgnet.com/1949-8470/full/v5/i8/295.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i8.295
The identification of myocardial infarct (MI) is important in the clinical evaluation of patients. Patients may present with a variety of clinical syndromes including heart failure, asymptomatic cardiomyopathy, or sudden cardiac death, and knowledge of the underlying myocardial structure is important for directing therapy. Cardiac magnetic resonance (CMR) imaging is commonly used for evaluation of MI and viability because of its ability to depict transmural extent of myocardial fibrosis[1,2]. However, not all patients are eligible for CMR given its limited availability to specialized imaging centers, and potential contraindication in patients with electronic implants. In addition, some heart failure patients may not tolerate the somewhat prolonged examination time (30 min-1 h), or use of gadolinium based contrast agents (e.g., severe renal insufficiency including dialysis)[3] which at present are required for adequate visualization of viable and infarcted myocardium. While alternative imaging modalities such as nuclear imaging and echocardiography provide some information on MI and viability, they do not provide the high spatial resolution and tissue characterization seen with CMR[4,5].
Cardiac computed tomography angiography (CCTA) has emerged as a powerful non-invasive imaging test for coronary artery disease (CAD) evaluation. Additional diagnostic information can be obtained regarding myocardial function[6,7], valvular structure[8,9], and pericardial structure.
Early animal studies have demonstrated the general feasibility of conventional contrast-enhanced computed tomography (CT) for MI imaging[10,11], although the single-slice CT systems utilized were inadequate for cardiac imaging. The advent of multi-detector CT with improved temporal resolution capable of diagnostic coronary artery imaging stimulated investigation of the potential utility of CCTA for evaluation of MI and viability[12-17]. However, at present there is no consensus on the optimal CT imaging parameters (e.g., contrast dose, energy voltage, time phase) for MI imaging.
Mixed voltage (e.g., dual energy) imaging can enhance tissue differentiation by taking advantage of different inherent absorption characteristics exhibited by tissues when exposed to different X-ray spectra. Early CT implementation of dual energy CT (DECT) required two separate CT acquisitions at different kVp levels with subsequent image co-registration. Technological advances in scanner design have led to the development of dual source CT systems (Siemens HealthCare, Erlangen, Germany) in which two X-ray tubes and two detector arrays are mounted on the same gantry. Several studies have demonstrated the improved temporal resolution, high image quality, and improved diagnostic accuracy for detection of coronary artery disease[18-20] with use of these dual source CT systems when X-ray sources are set to the same energy voltage level. When the scanner is operated in dual-energy mode, two tubes emit different voltage level X-ray spectra that can be simultaneously registered in their corresponding detector array. At present, very limited data is available on DECT image acquisition for assessment of MI and viability[21]. The goal of this study was to evaluate diagnostic accuracy of different energy voltage reconstructions including low, high, and mixed energy voltage for detection of prior MI using DECT acquisition.
Patients referred for clinical CCTA examination for assessment of CAD or pulmonary vein and left atrial anatomy were eligible for study inclusion. Twenty-four consecutive patients (88% male, mean age 65 ± 11 years old) with clinically documented prior MI (> 6 mo) were prospectively recruited between October 2007 and March 2011 to undergo late phase DECT for characterization of their MI. Prior MI was documented either by the presence of pathological Q waves on the electrocardiogram (ECG) (11 patients), imaging evidence of a region of loss of viable myocardium that is thinned and fails to contract (22 patients), or pathological findings of a prior MI (7 patients)[22]. Our study was approved by the medical center’s Institutional Review Board and was Health Insurance Portability and Accountability Act compliant. All subjects provided written informed consent.
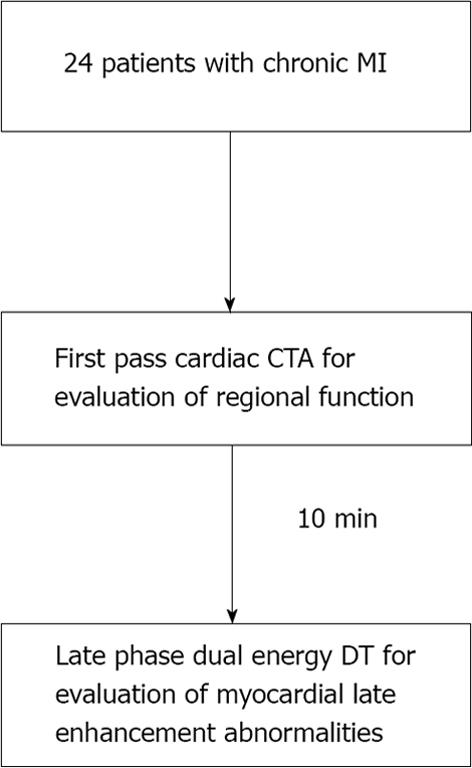
CCTA examinations were performed using a dual source CT system (64-slice Definition or 128-slice Definition FLASH, Siemens Healthcare) (Figure 1). Prior to image acquisition, 18- or 20-gauge intravenous access was obtained and ECG leads were placed. All patients referred for assessment of CAD received sublingual nitroglycerin prior to scan commencement. Eight percent of the patients received beta-blocker pre-medication to optimize coronary artery imaging. A test bolus injection method was used to calculate scan delay time, and 150 mL of 370 mg I/mL of nonionic contrast [iopamidol (Isovue 370, GE Healthcare) or iopromide (Ultravist 370, Berlex Imaging)] was intravenously administered at a rate of 5-7 mL/s (mean 6 mL/s) with 50 mL saline chaser. First pass CCTA acquisition was obtained from carina through the diaphragm, with larger coverage for patients undergoing evaluation of bypass graft patency, using thin collimation in conjunction with retrospective ECG gating using dose modulation (nominal current reduced to 25% during 0%-30% and 80%-100% phases of the cardiac cycle). A second late phase DECT scan was performed 10 min after initial contrast administration with coverage limited to only the heart. Scan parameters for first pass dual source CT scan on 64-slice CT system included gantry rotation 330 ms, tube A voltage 120 kVp, tube B voltage 120 kVp, average tube output 343 mAs per rotation (automatically adjusted for patient body habitus, range 258-438 mAs), retrospective gating with pitch automatically adjusted for patient heart rate (range 0.2-0.5), ECG-based tube current modulation, 0.6-mm collimation with z-flying focal spot technique for both detectors and 82.5 ms temporal resolution. First pass scan parameters on the 128-slice CT system were similar with the exception that gantry rotation 280 ms, average tube output 208 mAs per rotation and 75 ms temporal resolution. Scan parameters for the 7 patients who underwent late phase DECT scan on 64-slice system included gantry rotation 330 ms, tube A voltage 140 kVp, tube B voltage 100 kVp, tube A mean output 92 mAs per rotation (automatically adjusted for patient body habitus, range 65-108 mAs), tube B mean output 132 mAs per rotation (automatically adjusted for patient body habitus, range 104-145 mAs), retrospective gating with pitch automatically adjusted for patient heart rate (range 0.17-0.33), dose modulation, 1.2-mm collimation with z-flying focal spot technique for both detectors and 165 ms temporal resolution. Late phase DECT scan parameters for the 17 patients on the 128-slice system were similar with exception of gantry rotation 280 ms, tube A voltage 140 kVp + Sn filter, tube A mean output 120 mAs per rotation, range 93-149 mAs), tube B mean output 139 mAs (range 94-169 mAs) per rotation, and pitch 0.28.
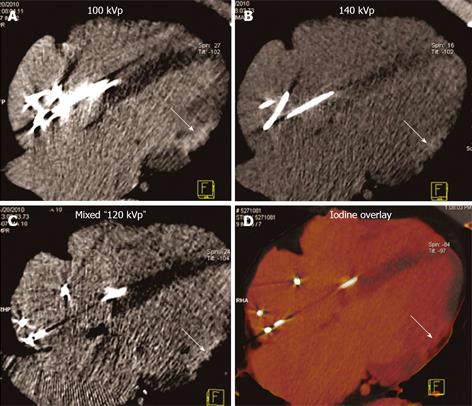
Only late phase DECT scan acquisitions were used for evaluation of myocardial enhancement abnormalities for detection of MI. DECT scans were reconstructed at the diastolic phase least affected by motion, using full gantry rotation (280 ms or 330 ms effective temporal resolution depending on scanner), 3 mm slice thickness, 1.5 mm reconstruction increment, and medium-soft reconstruction kernel (D30) into three image datasets: 140 kVp, 100 kVp, and a mixed dataset, weighted with 70% of the 140 kVp and 30% of the 100 kVp reconstructions simulating the image contrast of a single voltage 120 kVp acquisition (Figure 1). In addition, dual energy specific post-processing software was used to determine relative iodine content within the myocardium based on unique X-ray absorption characteristics of iodine at 100 and 140 kVp[23]. The resultant “iodine-only images” comprised a fourth dataset for myocardial late enhancement evaluation (Figure 2)[21,24].
First pass CCTA acquisitions were reconstructed with 0.75 mm slice thickness and 0.5 mm increments at 10% phase intervals from 0%-90% of the RR wave for evaluation of segmental left ventricular (LV) function.
Abnormal myocardial enhancement: Each DECT late phase image dataset (100, 140 kVp, mixed and iodine-only) was evaluated for myocardial late enhancement abnormalities using multiplanar reformations. Datasets were evaluated for hyperenhanced regions within the LV myocardium using the 17-segment cardiac model[25]. Datasets were evaluated in a random order in consensus by two experienced cardiac readers (8 years and 3 years) blinded to the patient’s clinical information, wall motion analysis, and reconstruction type with the exception of iodine-only datasets. The presence and severity of late myocardial hyperenhancement (i.e., “bright” regions) were evaluated as: 1 = normal, 2 = probable hyperenhancement, 3 = definite hyperenhancement, 4 = not interpretable due to artifact. Only presence or absence of late enhancement abnormalities was graded, and not transmural extent. Segments with late enhancement abnormalities limited to the subendocardium were graded as abnormal, either grade 2 or 3. Segments with step or streak (e.g., related to pacemaker wire) artifact in the late phase image were given grade 4 if the artifact limited the ability to interpret myocardial late enhancement. Datasets for 7 subjects chosen at random were individually analyzed by each cardiac reader for evaluation of interobserver variability.
Myocardial function: Multiphase first pass CCTA was evaluated in consensus by 2 experienced readers (8 years and 5 years) blinded to patient’s clinical information and late phase image analysis for presence of left ventricular wall motion abnormalities using the 17-segment model[25] and dedicated cardiac analysis software (Siemens Circulation, Siemens Healthcare). Segments were graded as normal, hypokinetic, akinetic or dyskinetic based on qualitative evaluation of wall thickening and motion (contraction pattern). Segments graded as hypokinetic, akinetic or dyskinetic with significant wall thinning that were deemed unrelated to abnormal conduction or prior cardiac surgery were considered abnormal and classified as infarct segments. When possible, corroborating information regarding myocardial infarct location including location of Q waves on ECG (n = 8), prior revascularization procedures (n = 10), echocardiography (n = 18), SPECT (n = 6), or CMR (n = 1) was collected from the patient’s electronic medical record.
Logistic regression for correlated data was used to compare reconstructions with respect to the percentage of times readers rated segments as non-evaluable for late myocardial enhancement abnormalities and in terms of the accuracy of detecting wall motion abnormalities using late myocardial hyperenhancement scores. Specifically, generalized estimating equations (GEE) based on a binary logistic regression model were used to model each of two indicator variables (one identifying segments rated as non-evaluable, the other identifying segments that were correctly diagnosed) as functions of reconstruction type, segment (as identified by the 17 segment model) and reader. The correlation structure imparted by the inclusion of multiple observations per patient was modeled by assuming data to be correlated when acquired from the same patient and independent otherwise. The κ-statistic was used to quantify interobserver agreement for segmental evaluation of myocardial hyperenhancement.
Upon initial analysis, the sample size of 24 patients was considered adequate since it provided 90% power at the two-sided 5% significance level to detect a 5 percentage point difference between reconstruction algorithms or between the two diagnostic tests based on perfusion score (test positive = definite vs test positive = possible or definite for enhancement abnormalities) in terms of accuracy for the detection of infarct segments and allowed diagnostic accuracy for each combination of algorithm and diagnostic test to be estimated with a precision no worse than 7 percentage points, where precision is the half-width of the 95%CI.
All reported P values are two-sided and statistical significance is defined as P < 0.05. SAS 9.3 (SAS Institute, Cary, NC) was used for all computations.
Baseline characteristics are shown in Table 1. All patients reported prior history of documented myocardial infarction, with most occurring more than 5 years prior (n = 18; 75% of cohort). Several patients underwent prior revascularization including coronary artery bypass grafting (n = 6) and percutaneous coronary angioplasty with or without stenting (n = 5).
| Clinical characteristics | Patients (n = 24) |
| Age (yr) | 65 ± 11 (40-86) |
| Male gender | 88% |
| Body mass index | 28.4 ± 5.3 (19.2-40.7) |
| Hypertension | 58% |
| Hyperlipidemia | 75% |
| Diabetes mellitus | 37.5% |
| Prior tobacco use | 29% |
| Positive family history | 62.5% |
| Prior MI > 5 yr ago | 75% |
| Prior PCI | 21% |
| Prior CABG | 21% |
| Infarct region | |
| Anterior | 9 |
| Lateral | 4 |
| Inferior | 5 |
| Septal | 7 |
| Apical | 8 |
| Examination characteristics | |
| Beta-blocker administration | 2 |
| Nitroglycerin administration | 15 |
| Mean heart rate | 63 ± 12 bpm |
| Mean contrast amount | 150 mL |
| DECT dose-length product (mGycm) | 808 ± 191 |
| DECT effective dose (mSv) | 11.3 ± 2.7 |
| Left ventricle | |
| End diastolic volume | 173 ± 60 mL |
| Ejection fraction | 55% ± 17% |
Fifty-five of 408 (13%) segments demonstrated abnormal wall motion and were classified as infarct. The remaining 353 segments were classified as non-infarcted segments. Infarct regions were noted in all vascular territories (Table 1). In 16 patients, myocardial infarction in CCTA assigned territories was confirmed by one or more additional imaging studies [echocardiography (n = 15), SPECT (n = 5) or CMR (n = 1). In 7 patients, there was evidence of coronary occlusion and/or prior revascularization in the CCTA assigned infarct territory including 6 patients without additional imaging studies. There were 2 patients who did not have a corroborating additional imaging study or X-ray coronary angiogram to confirm the infarct regions.
A total of 1692 segments were analyzed for late phase enhancement abnormalities. Ninety one (5.5 percent) segments were not interpretable due to artifact. Artifacts were more common with iodine-only reconstruction (7.4%) and least common with mixed reconstruction (4.2%) although this difference was not statistically significant. Interobserver variability for detection of late phase enhancement abnormalities was good (κ = 0.63 ± 0.11).
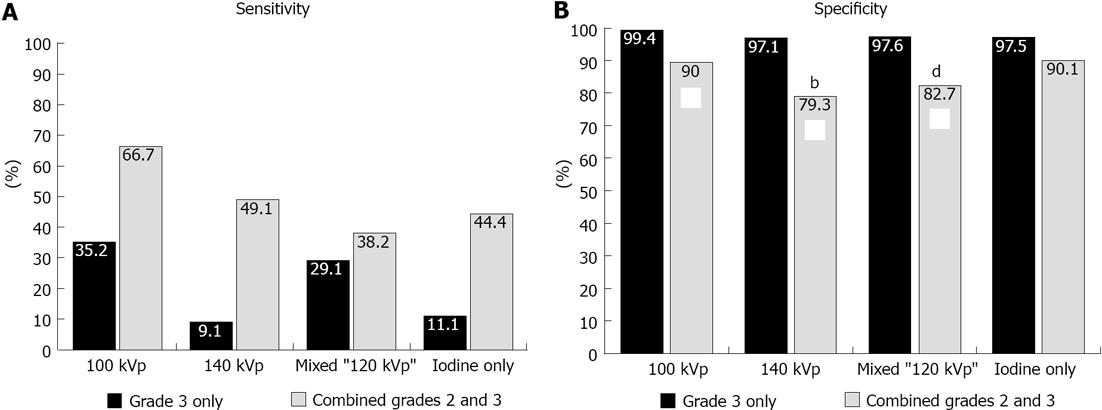
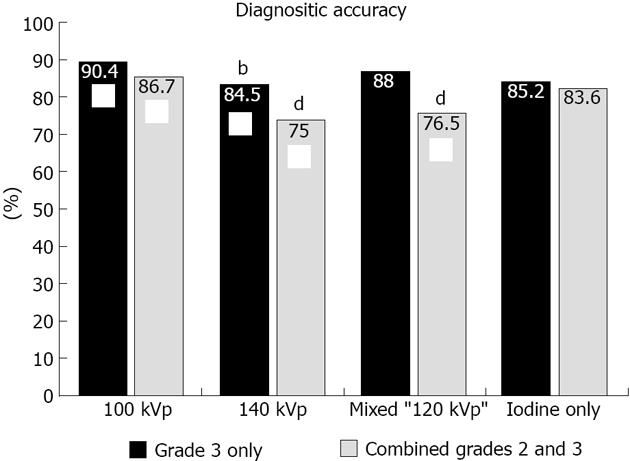
Regardless of the reconstruction algorithm used, the presence of definite (grade 3) compared to possible or definite (combined grades 2 and 3) enhancement abnormalities demonstrated similar diagnostic accuracy, although higher specificity and lower sensitivity for detection of infarct segments (Figures 3 and 4). Evaluation of different voltage acquisitions demonstrated the highest diagnostic performance for the 100 kVp reconstruction which had higher diagnostic accuracy (87%; 95%CI: 82%-90%), sensitivity (67%; 95%CI: 54%-78%) and specificity (90%; 95%CI: 86%-93%) compared to the other reconstructions (Table 2). Statistically significant differences were noted between the diagnostic accuracy for 100 kVp compared to 140 kVp (P < 0.01) using grade 3 perfusion abnormalities, and between 100 kVp compared to 140 kVp (P < 0.0001) and 100 kVp compared to mixed voltage (P < 0.0001) using combined grades 2 and 3 perfusion abnormalities. For sensitivity, there were significant differences noted between 100 kVp vs 140 kVp (P < 0.05), 100 kVp vs iodine only (P < 0.05), and 140 kVp vs mixed (P < 0.05) using grade 3 only perfusion abnormalities, and between 100 kVp vs 140 kVp (P < 0.0005), 100 kVp vs mixed (P < 0.0001), and 100 kVp vs iodine only (P < 0.005) using combined grades 2 and 3 perfusion abnormalities. For specificity, there were significant differences noted between 100 kVp vs 140 kVp (P < 0.005), and 100 kVp vs mixed (P < 0.01) using combined grades 2 and 3 perfusion abnormalities.
| Accuracy | Specificity | Sensitivity | PPV | NPV | |
| Definite | |||||
| (Grade 3) | |||||
| Hyperenhancement | |||||
| 100 kVp | 90.4% | 99.4% | 35.2% | 90.5% | 90.4% |
| 95%CI (84.3-94.2) | 95%CI (97.7-99.8) | 95%CI (19.4-55.0) | 95%CI (66.5-97.8) | 95%CI (83.6-94.5) | |
| 140 kVp | 84.5% | 97.1% | 9.1% | 33.3% | 86.6% |
| 95%CI (76.1-90.4) | 95%CI (92.5-98.8) | 95%CI (2.7-26.6) | 95%CI (9.4-70.6) | 95%CI (77.5-92.4) | |
| Mixed | 88.0% | 97.6% | 29.1% | 66.7% | 89.4% |
| 95%CI (81.1-92.6) | 95%CI (94.0-99.1) | 95%CI (14.0-50.9) | 95%CI (35.6-87.8) | 95%CI (81.5-94.2) | |
| Iodine Only | 85.2% | 97.5% | 11.1% | 42.9% | 86.8% |
| 95%CI (78.3-90.2) | 95%CI (94.2-99.0) | 95%CI (4.0-27.0) | 95%CI (14.8-76.4) | 95%CI (79.3-91.9) | |
| Definite or Possible | |||||
| 95%CI (Combined Grades 2 and 3) | |||||
| Hyperenhancement | |||||
| 100 kVp | 86.7% | 90.0% | 66.7% | 52.2% | 94.3% |
| 95%CI (82.4-90.1) | 95%CI (85.8-93.1) | 95%CI (53.6-77.6) | 95%CI (34.7-69.2) | 95%CI (89.1-97.1) | |
| 140 kVp | 75.0% | 79.3% | 49.1% | 28.1% | 90.4% |
| 95%CI (68.0-80.9) | 95%CI (71.4-85.4) | 95%CI (34.1-64.3) | 95%CI (15.6-45.2) | 95%CI (82.7-94.9) | |
| Mixed | 76.5% | 82.7% | 38.2% | 26.6% | 89.1% |
| 95%CI (69.6-82.2) | 95%CI (76.1-87.8) | 95%CI (22.7-56.5) | 95%CI (13.9-44.9) | 95%CI (80.9-94.1) | |
| Iodine Only | 83.6% | 90.1% | 44.4% | 42.9% | 90.7% |
| 95%CI (77.3-88.4) | 95%CI (83.6-94.2) | 95%CI (27.7-62.5) | 95%CI (24.6-63.3) | 95%CI (84.0-94.8) |
We evaluated the diagnostic performance of different tube energy voltage reconstructions including low, high, and mixed energies for detection of prior myocardial infarct. The use of dual energy CT enabled direct comparison of different energy voltage reconstructions including mixed voltages simulating the image contrast of a single voltage 120 kVp acquisition by combining different proportions of the acquisition energies (100 and 140 kVp), and new dual energy specific post-processing algorithms of iodine-only images, without the need for additional image acquisitions.
We found that lower voltage (100 kVp in this study) performed significantly better than higher voltage (140 kVp in this study) and mixed voltage acquisitions. Dual energy post-processing software generating iodine-only images did not perform as well as the lower voltage 100 kVp dataset. Although in certain cases iodine-only reconstructions appeared to better depict infarct regions, artifacts were more frequently observed (7.4% of segments with iodine-only reconstructions compared to 4.2% of segments with the mixed dataset) and limited interpretation of these datasets when evaluated independently. Evaluation of iodine-only reconstructions in conjunction with non-processed datasets may improve diagnostic accuracy by better distinguishing between perceived and actual artifacts within the iodine-only images.
Early animal studies have demonstrated the general feasibility of conventional CT to identify myocardial infarcts[10,11], although the single-slice systems utilized at that time were not valuable as a clinical tool for cardiac imaging. With the advent of multi-detector CT systems with improved temporal and spatial resolution, there have been several studies demonstrating the ability of cardiac CT to identify and characterize myocardial infarcts including size, transmurality and degree of collagenous scar formation[12,13,26,27]. However, there is no agreement concerning the best CT imaging protocol for imaging myocardial infarcts in patients, as current studies in the literature were performed in infarcts of varying ages (acute and prior), with different scan delay times (early and late phase), different contrast administration schemes, different scanning energy voltage (80-140 kVp), and different reconstruction algorithms[12,16,17,28,29].
To our knowledge, this is the first study that specifically evaluated the effect of different tube voltages on infarct conspicuity in a patient cohort with prior myocardial infarct. Our results compare similarly to a previous animal study evaluating low dose techniques for depiction of acute myocardial infarct regions. In that study, lower tube voltage (80 kVp compared to 120 kVp) did not affect accuracy of infarct measurement, and although image noise was higher, there was significantly improved contrast enhancement between viable and non-viable myocardium[30]. In addition, given that the radiation dose is exponentially related to tube voltage, the use of lower tube voltage should significantly reduce radiation dose exposure for the patient[31].
Although we corroborated myocardial infarct location territories with other available studies, this study was not designed to be able to specifically compare the CT techniques with these other imaging studies on a segment by segment basis. It is well known that ECG and nuclear imaging (SPECT) have limited diagnostic accuracy for detection of myocardial infarction with generally low sensitivity (22% for ECG and 67% for SPECT) although high specificity for detection of myocardial infarction when compared to CMR as the reference standard[32], particularly for small infarctions. Similarly, regional wall motion abnormalities on echocardiography are not always present in prior myocardial infarcts detected by CMR, which may be related to small scar regions, non-transmural scar, or limited sensitivity of echocardiography to detect minor wall motion abnormalities[33]. In our cohort there were 18 subjects with available ECG’s for interpretation, with 7 demonstrating no evidence for pathologic Q waves or other findings suggestive of myocardial infarction despite other correlative imaging evidence demonstrating myocardial infarction. Similarly, there were 6 echocardiographic studies that demonstrated no regional wall motion abnormalities or other findings suggestive of myocardial infarction despite other correlative evidence including coronary angiography demonstrating prior myocardial infarction. Since late phase CCTA imaging may provide information similar to late enhancement CMR, CCTA may provide additional diagnostic and prognostic information beyond that provided by ECG, echocardiography and SPECT imaging that may be important in the management of these patients. Future studies of head to head comparisons of CCTA with other imaging techniques for detection of prior myocardial infarction including safety assessments for development of contrast nephropathy and radiation exposure are warranted.
There are several limitations to our study. First, myocardial infarct segments were determined by presence of wall motion abnormality on first pass CCTA as opposed to infarct imaging with CMR or nuclear imaging techniques. Although wall motion analysis is not a perfect gold standard for depicting myocardial infarct segments, it remains the current method for identifying myocardial infarct regions by echocardiography and nuclear imaging techniques, particularly when combined with concomitant perfusion defects. In our study, corroborating evidence of infarct location was made utilizing additional imaging modalities which were available for 100% of patients in our cohort. Second, using segmental wall motion abnormality as the reference standard for infarct segments may increase false positive interpretation of small infarct regions that may not manifest as a wall motion abnormality. In the setting of prior myocardial infarction, transmural extent of infarct approaches 50% before contractile dysfunction can be systematically identified[34]. Hence while we can be fairly confident that larger, non-viable infarcts (> 50% transmural extent) were reliably assessed, the true diagnostic accuracy of CCTA for detecting smaller, non-transmural infarcts cannot be determined by our study. Finally, this study evaluated the ability to detect late phase myocardial enhancement abnormalities on a segmental basis. The transmural extent of hyperenhanced regions was not assessed nor correlated with detection of myocardial infarct segments.
In a conclusion, CCTA demonstrates significant potential for detection of prior myocardial infarct based on late phase myocardial enhancement abnormalities. Our results suggest that low voltage imaging with 100 kVp provides the best diagnostic performance with the lowest radiation dose for detection of myocardial infarct regions.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Cardiac computed tomography angiography (CCTA) has emerged as a powerful non-invasive imaging test for coronary artery disease evaluation. In addition, CCTA represents an alternative method for evaluation of myocardial scar in patients unable to undergo cardiac magnetic resonance imaging. Several different CCTA algorithms have been proposed for assessment of myocardial infarction (MI), but none have been demonstrated to be superior to another. In a small cohort of patients, authors demonstrate that lower voltage, 100 kVp in this study, provided the best diagnostic accuracy for detection of prior myocardial infarct compared to higher or mixed voltage (dual energy) acquisitions.
New applications of CCTA include myocardial scar imaging for assessment of myocardial viability. New developments in CCTA such as dual energy imaging can improve tissue differentiation by taking advantage of different inherent absorption characteristics exhibited by tissues when exposed to different X-ray spectra. Such techniques may lead to improved myocardial tissue characterization in patients with chronic ischemic heart disease.
Cardiac magnetic resonance (CMR) imaging is commonly used for evaluation of myocardial infarction and viability because of its ability to depict transmural extent of myocardial fibrosis. However, not all patients are eligible for CMR given its limited availability to specialized imaging centers, and potential contraindication in patients with electronic implants. CCTA represents a viable alternative to CMR for evaluation of myocardial infarction and viability, and low voltage late enhancement CCTA imaging provides the best diagnostic accuracy compared to high and mixed voltage acquisitions.
Late phase, low voltage (100 kVp) CCTA provides a good alternative for evaluation of myocardial scar in patients with ischemic heart disease, particularly when CMR is not available or contraindicated.
Dual energy computed tomography (CT) imaging refers to the acquisition and integration of CT datasets acquired nearly simultaneously at two different voltage settings.
This is an interesting manuscript that compares low and high voltage computed tomography acquisitions in patients with chronic myocardial infarction.
P- Reviewers Chang ST, Loffredo L, Ye YC, Zamilpa R S- Editor Wen LL L- Editor A E- Editor Liu XM
| 1. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1744] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 2. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2211] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 3. | Othersen JB, Maize JC, Woolson RF, Budisavljevic MN. Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transplant. 2007;22:3179-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Lee VS, Resnick D, Tiu SS, Sanger JJ, Nazzaro CA, Israel GM, Simonetti OP. MR imaging evaluation of myocardial viability in the setting of equivocal SPECT results with (99m)Tc sestamibi. Radiology. 2004;230:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 891] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 6. | Juergens KU, Grude M, Fallenberg EM, Opitz C, Wichter T, Heindel W, Fischbach R. Using ECG-gated multidetector CT to evaluate global left ventricular myocardial function in patients with coronary artery disease. AJR Am J Roentgenol. 2002;179:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Mahnken AH, Koos R, Katoh M, Spuentrup E, Busch P, Wildberger JE, Kühl HP, Günther RW. Sixteen-slice spiral CT versus MR imaging for the assessment of left ventricular function in acute myocardial infarction. Eur Radiol. 2005;15:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Konen E, Goitein O, Feinberg MS, Eshet Y, Raanani E, Rimon U, Di-Segni E. The role of ECG-gated MDCT in the evaluation of aortic and mitral mechanical valves: initial experience. AJR Am J Roentgenol. 2008;191:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Manghat NE, Rachapalli V, Van Lingen R, Veitch AM, Roobottom CA, Morgan-Hughes GJ. Imaging the heart valves using ECG-gated 64-detector row cardiac CT. Br J Radiol. 2008;81:275-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Higgins CB, Sovak M, Schmidt W, Siemers PT. Uptake of contrast materials by experimental acute myocardial infarctions: a preliminary report. Invest Radiol. 1978;13:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Gray WR, Buja LM, Hagler HK, Parkey RW, Willerson JT. Computed tomography for localization and sizing of experimental acute myocardial infarcts. Circulation. 1978;58:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Hoffmann U, Millea R, Enzweiler C, Ferencik M, Gulick S, Titus J, Achenbach S, Kwait D, Sosnovik D, Brady TJ. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology. 2004;231:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Buecker A, Katoh M, Krombach GA, Spuentrup E, Bruners P, Günther RW, Niendorf T, Mahnken AH. A feasibility study of contrast enhancement of acute myocardial infarction in multislice computed tomography: comparison with magnetic resonance imaging and gross morphology in pigs. Invest Radiol. 2005;40:700-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Nikolaou K, Sanz J, Poon M, Wintersperger BJ, Ohnesorge B, Rius T, Fayad ZA, Reiser MF, Becker CR. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: preliminary results. Eur Radiol. 2005;15:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Lessick J, Ghersin E, Dragu R, Litmanovich D, Mutlak D, Rispler S, Agmon Y, Engel A, Beyar R. Diagnostic accuracy of myocardial hypoenhancement on multidetector computed tomography in identifying myocardial infarction in patients admitted with acute chest pain syndrome. J Comput Assist Tomogr. 2007;31:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Lee IH, Choe YH, Lee KH, Jeon ES, Choi JH. Comparison of multidetector CT with F-18-FDG-PET and SPECT in the assessment of myocardial viability in patients with myocardial infarction: a preliminary study. Eur J Radiol. 2009;72:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Tsiflikas I, Brodoefel H, Reimann AJ, Thomas C, Ketelsen D, Schroeder S, Kopp AF, Claussen CD, Burgstahler C, Heuschmid M. Coronary CT angiography with dual source computed tomography in 170 patients. Eur J Radiol. 2010;74:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Achenbach S, Ropers D, Kuettner A, Flohr T, Ohnesorge B, Bruder H, Theessen H, Karakaya M, Daniel WG, Bautz W. Contrast-enhanced coronary artery visualization by dual-source computed tomography--initial experience. Eur J Radiol. 2006;57:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Johnson TR, Nikolaou K, Busch S, Leber AW, Becker A, Wintersperger BJ, Rist C, Knez A, Reiser MF, Becker CR. Diagnostic accuracy of dual-source computed tomography in the diagnosis of coronary artery disease. Invest Radiol. 2007;42:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Ruzsics B, Lee H, Zwerner PL, Gebregziabher M, Costello P, Schoepf UJ. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur Radiol. 2008;18:2414-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2312] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 23. | Riederer SJ, Mistretta CA. Selective iodine imaging using K-edge energies in computerized X-ray tomography. Med Phys. 1977;4:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, Fink C, Weckbach S, Lenhard M, Schmidt B. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17:1510-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1102] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 25. | Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4568] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 26. | Lardo AC, Cordeiro MA, Silva C, Amado LC, George RT, Saliaris AP, Schuleri KH, Fernandes VR, Zviman M, Nazarian S. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation. 2006;113:394-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Brodoefel H, Klumpp B, Reimann A, Fenchel M, Heuschmid M, Miller S, Schroeder S, Claussen C, Scheule AM, Kopp AF. Sixty-four-MSCT in the characterization of porcine acute and subacute myocardial infarction: determination of transmurality in comparison to magnetic resonance imaging and histopathology. Eur J Radiol. 2007;62:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Günther RW, Kühl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol. 2005;45:2042-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Nikolaou K, Knez A, Sagmeister S, Wintersperger BJ, Boekstegers P, Steinbeck G, Reiser MF, Becker CR. Assessment of myocardial infarctions using multidetector-row computed tomography. J Comput Assist Tomogr. 2004;28:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Brodoefel H, Klumpp B, Reimann A, Ohmer M, Fenchel M, Schroeder S, Miller S, Claussen C, Kopp AF, Scheule AM. Late myocardial enhancement assessed by 64-MSCT in reperfused porcine myocardial infarction: diagnostic accuracy of low-dose CT protocols in comparison with magnetic resonance imaging. Eur Radiol. 2007;17:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schömig A. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 32. | Andrade JM, Gowdak LH, Giorgi MC, de Paula FJ, Kalil-Filho R, de Lima JJ, Rochitte CE. Cardiac MRI for detection of unrecognized myocardial infarction in patients with end-stage renal disease: comparison with ECG and scintigraphy. AJR Am J Roentgenol. 2009;193:W25-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Catalano O, Moro G, Cannizzaro G, Mingrone R, Opasich C, Perotti M, Rognone F, Frascaroli M, Baldi M, Tramarin R. Scar detection by contrast-enhanced magnetic resonance imaging in chronic coronary artery disease: a comparison with nuclear imaging and echocardiography. J Cardiovasc Magn Reson. 2005;7:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Mahrholdt H, Wagner A, Parker M, Regenfus M, Fieno DS, Bonow RO, Kim RJ, Judd RM. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol. 2003;42:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |