Published online Jun 28, 2013. doi: 10.4329/wjr.v5.i6.229
Revised: April 24, 2013
Accepted: May 18, 2013
Published online: June 28, 2013
AIM: To assess the role of contrast enhanced ultrasonography in evaluation of hepatocellular carcinoma (HCC) at the first Indian tertiary liver center.
METHODS: Retrospective analysis of contrast enhanced ultrasound (CEUS) examinations over 24 mo for diagnosis, surveillance, characterization and follow up of 50 patients in the context of HCC was performed. The source and indication of referrals, change in referral rate, accuracy and usefulness of CEUS in a tertiary liver center equipped with a 64 slice dual energy computer tomography (CT) and 3 tesla magnetic resonance imaging (MRI) were studied. Sonovue (BR1, Bracco, Italy, a second generation contrast agent) was used for contrast US studies. Contrast enhanced CT/MRI or both were performed in all patients. The findings were taken as a baseline reference and correlation was done with respect to contrast US. Contrast enhanced MRI was performed using hepatocyte specific gadobenate dimeglumine (Gd-BOPTA). Iomeron (400 mg; w/v) was used for dynamic CT examinations.
RESULTS: About 20 (40%) of the examinations were referred from clinicians for characterization of a mass from previous imaging. About 15 (30%) were performed for surveillance in chronic liver disease; 5 (10%) examinations were performed for monitoring lesions after radiofrequency ablation (RFA); 3 (6%) were post trans-arterial chemo-embolization (TACE) assessments and 3 (6%) were patients with h/o iodinated contrast allergy. About 2 (4%) were performed on hemodynamically unstable patients in the intensive care with raised alpha fetoprotein and 2 (4%) patients were claustrophobic. The number of patients referred from clinicians steadily increased from 12 in the first 12 mo of the study to 38 in the last 12 mo. CEUS was able to diagnose 88% of positive cases of HCC as per reference standards. In the surveillance group, specificity was 53.3% vs 100% by CT/MRI. Post RFA and TACE specificity of lesion characterization by CEUS was 100% in single/large mass assessment, similar to CT/MRI. For non HCC lesions such as regenerative and dysplastic nodules, the specificity was 50% vs 90% by CT/MRI. The positive role of CEUS in imaging spectrum of HCC included a provisional urgent diagnosis of an incidentally detected mass. It further led to a decrease in time for further management. A confident diagnosis on CEUS was possible in cases of characterization of an indeterminate mass, in situations where the patient was unfit for CT/MRI, was allergic to iodinated contrast or had claustrophobia, etc. CEUS was also cost effective, radiation free and an easy modality for monitoring post RFA or TACE lesions.
CONCLUSION: CEUS is a valuable augmentation to the practice of ultrasonography, and an irreplaceable modality for confounding cases and interpretation of indeterminate lesions in imaging of HCC.
- Citation: Laroia ST, Bawa SS, Jain D, Mukund A, Sarin S. Contrast ultrasound in hepatocellular carcinoma at a tertiary liver center: First Indian experience. World J Radiol 2013; 5(6): 229-240
- URL: https://www.wjgnet.com/1949-8470/full/v5/i6/229.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i6.229
Ultrasound contrast media (UCM) (initially first generation and now second generation contrast media) were introduced in 1990[1,2]. Contrast enhanced ultrasound (CEUS) was subsequently introduced as a potential tool for improvised liver imaging. Since then, CEUS has brought about many significant changes to the diagnostic paradigm of hepatocellular focal lesions, particularly hepatocellular carcinoma (HCC). The guidelines for usage of these agents were formulated by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) delegates meeting at the congress at Copenhagen in March 2003[3]. Although the guidelines were meant for the use of UCM for general use, the initial emphasis was on liver lesions. Since then, US technology has come a long way with the introduction of newer second generation contrast media and real time low impedance imaging, with vast use of UCM in liver imaging[2].
The basic principle of scanning with contrast agents is that they act as blood pool agents and are completely intravascular compared to computer tomography (CT) and magnetic resonance imaging (MRI) agents which are seen to permeate into the tissue interstitium. UCM are made of small < 2 micro meter gas-filled microbubbles. Specific software modes (which can be added on to existing equipment with software compatibility) are required for CEUS. Post contrast scanning acts on the principle of filtering out the US signals from tissues and the contrast enhanced regions. The backscatter from UCM is usually non linear and is dependent on the acoustic pressure/mechanical index (MI) settings and the harmonic response from the tissues as well as mechanical oscillations of the microbubbles. First generation UCM which had room air filled microbubbles coated with galactose/palmitic acid surfactant microcrystals (Levovist-SH U 508A; Schering AG Berlin, Germany) or denatured albumin shell (Albunex) were easy to disrupt and required intermittent scanning with low frame rates or fast scanning techniques with offline review of the clips[1]. Newer second generation UCM contain perfluorocarbon gases such as perfluoropropane and sulfur hexafluoride (SonoVue, Bracco, Milan) and are encapsulated with phospholipid shells. They have longer stability within the blood pool and allow real time, low MI scanning for a span of 3-5 min with decreased background tissue interference. This low MI setting technique forms the backbone of contrast US imaging today. Flash replenishment technique (which involves small periods of high impedance acoustics) destroys microbubbles during scanning and is used to study refilling of the liver or tumor after their destruction, which helps in lesion characterization and visualization of vessel morphology in studies[4].
Contrast US can be used to assess the pelvis, prostate, kidneys, spleen, aorta and bowel[5,6]. CEUS has been extensively used to study the liver and has become one of the established modalities for successful evaluation of the liver[4,7-9]. Sonovue (Bracco, Milan, Italy) has replaced Levovist to a great extent. A new second generation contrast, which is Kupffer cell specific, is Sonazoid (Daiichi Sankyo, Tokyo, Japan). It is a lipid-stabilized suspension of perfluorobutane gas microbubble contrast agent, available only in Japan and used to study benign and malignant focal lesions in the liver [10].
The specificity and sensitivity of CEUS was compared with both CT and MRI by various study groups and found to be clinically significant. Even in multimodality centers[11-18], characterization of focal liver lesions (FLL) was found to be in the order of 90%-95% concordance with other imaging modalities[16-18]. CEUS is able to detect contrast enhancement of the smallest vessels (measuring less than 100 micrometers) in a mass, which is virtually impossible on CT or MRI[13,19]. Lesions can be labeled as benign or malignant based on hypervascularity or washout pattern of enhancement[15]. Ultrasonography has a major role in the imaging caveat of HCC. Considering the consensus of the American Association for the Study of Liver Diseases (AASLD) and Asian Pacific Association for the Study of the Liver (APASL) on surveillance of liver cancer, US is the modality of choice for routine surveillance despite the higher sensitivity and specificity of CT and MRI[18]. No obvious role of CEUS has been defined by the AASLD for either diagnosis or surveillance, probably due to the unavailability of UCM in the United States. However, APASL has recommended Sonazoid for its Kupffer cell specificity in diagnosis and problem solving role in atypical liver lesions[18].
We initiated this study, the first from the Indian subcontinent as far as we know, to assess the role of contrast US in a well equipped tertiary liver hospital, with imaging giants such as 64 slice MDCT and 3Tesla MRI in the same departmental setting, in the context of surveillance, diagnosis and interventional care of HCC. We tried to analyze the referral base, usage of CEUS and its accuracy of diagnosis by comparing results with histopathology (if required), CT/MRI and alpaa fetoprotein (AFP), as well as other clinical data so as to assess the feasibility of maintaining a robust CEUS program in conjunction with technically advanced CT and MRI programs.
The study was retrospective and hence informed consent was not obtained. It was conducted over 24 mo and retrospective analysis of 50 sequential patients who underwent CEUS of liver in the context of HCC was done. Patients referred for surveillance, characterization of an indeterminate liver mass, nodule suspicious of HCC or follow up cases of HCC were included. The clinician referral pattern over the study period was observed.
Of the 50 patients, 10 were females and 40 males, with ages ranging from 25-75 years. Furthermore, CEUS performed during that interval for surveillance of TIPS stent grafts (n = 5), evaluation of masses in other organs (n = 5) and determination of vascular patency in patients who were suspected to have hepatic venous outflow tract obstruction (n = 4) were not included in this analysis since they were not connected to the diagnosis of HCC or indeterminate masses suspected to be liver malignancies.
Dynamic, triple phase contrast enhanced CT or MRI (CECT/CEMRI) or both were performed in all patients and correlation of findings on either or both was done. CEMRI was performed using hepatocyte specific Gadobenate Dimeglumine (Gd BOPTA) and Iomeron (400 mg, w/v) were used for dynamic CT examinations.
After baseline B mode US examination and Doppler application (in cases wherever needed), a repeated bolus of 1.0-1.5 mL of SonoVue (BR1, Bracco, Italy) was administered intravenously through an 18 gauge iv catheter in the right forearm or arm, followed by a saline flush bolus of approximately 5 mL. CEUS images were captured in a cine loop obtained with the timer switched on after the saline flush during arterial, portal venous and delayed phases. Low-MI real-time continuous scanning in the contrast-specific mode was done. The US machine used was iU22 (Philips Medical Systems, Bothell, WA) with a convex array transducer (1-6 mHz) and a continuous low-MI (< 0.2) contrast specific imaging mode. The focal zone was set below the region of interest, with a minimum frame rate of 10 Hz. Real time scanning with observation of the contrast enhancement pattern was made. Where needed, still frames were acquired and stored in the hard disc so as to be reviewed again. Flash filling technique was used to study the pattern of early enhancement phase, without reinjection, when required. Reinjection with contrast was also done for studying a particular vascular pattern when required.
Patients were recruited (1) in consultation with the referring physician after a routine B mode scan when an incidental nodule or focal space occupying lesion was detected; (2) after direct referral from the clinicians, most commonly after a raised AFP level or with reports of an indeterminate mass from outside/internal imaging examination, including CT/MRI/USG; (3) for surveillance of patients with chronic liver disease or full blown cirrhosis at risk for HCC; and (4) for follow up of post radiofrequency ablation (RFA), tumors and post trans-arterial chemo-embolization (TACE) lesions. The CEUS diagnosis was compared with observations on other imaging modalities, histopathology and clinical follow up.
CT-scan and MRI were performed as per the current optimized protocols. The multi detector CT examination was dynamic and done with three phases after a non contrast scan. Contrast agent with a low osmolarity, Iomeron (1.5-2.0 mL/kg bodyweight, 400 mg/mL) was administered at a rate of 4.0-4.5 mL/s. The acquisition during the arterial phase was synchronized with a bolus tracking technique. It was followed by a portal-venous phase acquisition starting 60-70 s after the start of the injection, followed by an equilibrium phase at 180 s. The MR imaging protocol included at least a T2-weighted spin-echo sequence, a T1-weighted spin-echo or gradient-echo sequence and a dynamic T1-weighted gadolinium-enhanced study during a breath hold. Post-contrast arterial, portal and delayed scans were acquired. The slice thickness was below 5 mm and the inter-slice gap was limited to below 15% of the slice thickness.
Both examinations were read by an experienced radiologist blinded to sonographic findings. Patients were followed up for a mean period of 6 mo. Determination of positive or neutral influence was assessed and included the underlying criteria. Positive results included: (1) correct CEUS diagnosis of an incidentally detected mass on imaging by USG/CT/MRI; (2) decrease in time for further management and second line investigations on the basis of the CEUS report; (3) confident diagnosis on CEUS when indeterminate on other modalities such as CT, MRI or done outside the institute; (4) unsuspected diagnosis on CEUS; and (5) successful monitoring for RFA and TACE lesions post procedure. Absence of any of the above criteria put the patient in the neutral/negative results category. This also included a difference in the diagnosis on CEUS and CT/MRI. Agreement of CEUS with CT and MRI scans was reviewed and in cases of disagreement, analyzed in light of the final diagnosis.
The diagnostic accuracy of CEUS was determined in 50 patients with reference standards of CT/MR imaging. Final diagnoses were compounded based on clinical information, biopsy (n = 5, all miscellaneous lesions) and correlation with CT/MRI imaging (n = 45).
About 50 patients were examined on CEUS and then followed up by CT/MRI (Table 1). Single lesions were evaluated in 47 patients, more than 2 lesions were observed in 3 patients (2 in post TACE group and 1 in post RFA group). Multiple lesions/masses with the same diagnosis were counted as a single entity. The patients with chronic liver disease were divided into 2 subgroups on CEUS. One subgroup showed hyper-vascular, enhancing lesions on the arterial phase and the other did not show any hypervascular/enhancing nodule.
| FD | Total number | True positive diagnosis | TP ref | FP | FP ref | MP | UMP |
| HCC | 17 | 15 | 17 | 2 | 0 | 15 | 2 |
| CLD (no enhancing nodule) | 10 | 7 | 10 | 3 | 0 | 7 | 3 |
| CLD (enhancing nodule) | 5 | 1 | 4 | 4 | 1 | 1 | 4 |
| Post-RFA HCC | 5 | 5 | 5 | 0 | 0 | 5 | 0 |
| Post-TACE HCC | 3 | 3 | 3 | 0 | 0 | 3 | 0 |
| Others | 10 | 5 | 9 | 5 | 1 | 5 | 5 |
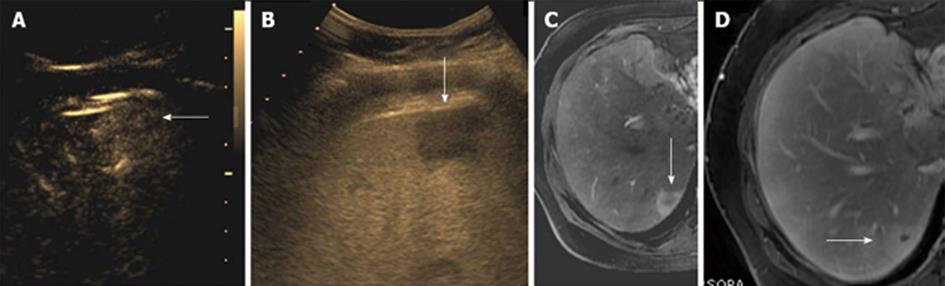
In the chronic liver disease (CLD) subgroup with enhancing nodules on CEUS, only 1 lesion was diagnosed correctly as dysplastic nodule/well differentiated HCC (Figure 1). The other 4 enhancing nodules also diagnosed as dysplastic nodules on CEUS showed only a faint positive arterial blush on CT without subsequent washout and were more likely to be regenerative nodules and hence were given a final diagnosis of the same. Hence, all 4 of these lesions were false positive on CEUS. In the non hypervascular (non enhancing) CLD subgroup, 7 nodules were detected by CEUS compared to 10 nodules on CT/MRI. Interestingly these nodules did not show any enhancement on contrast US. However, few of these nodules showed mild enhancement/arterial blush in the hepatic arterial phase on CT/MRI without obvious washout and iso-density/iso-intensity on the equilibrium phase on CT/MRI. A final diagnosis of regenerative etiology was made for this subgroup.
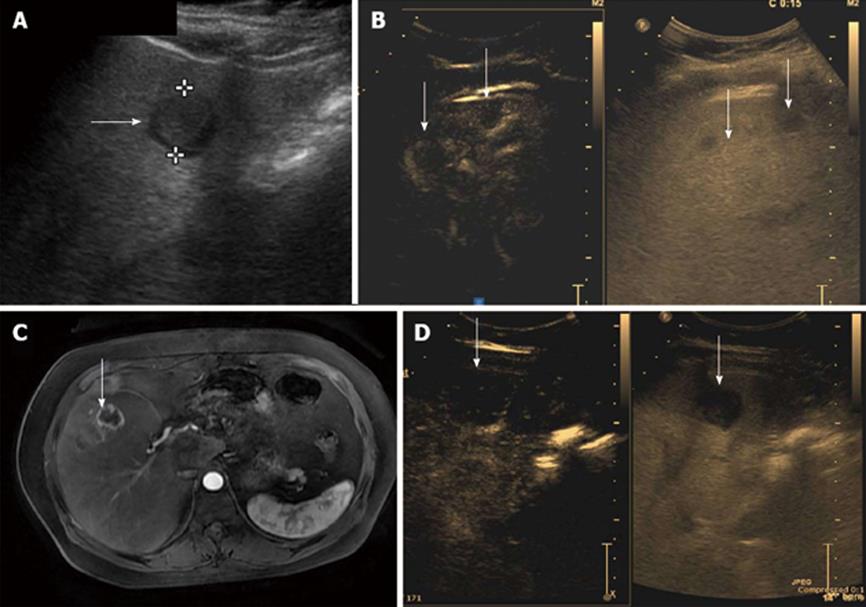
Diagnoses in the miscellaneous group included 3 hemangiomas (Figure 2), 5 metastatic lesions (from carcinoma breast, neuroendocrine tumor and carcinoma gall bladder) (Figure 3) and 2 regenerative nodules in a non-documented cirrhotic. These 2 nodules were diagnosed on USG and later a liver biopsy showed changes of early liver parenchymal disease. Out of the 3 hemangiomas, CEUS could only diagnose 2 correctly. The 3rd lesion did not show complete fill-in on the CEUS scan and hence was diagnosed as metastatic vs atypical hemangioma.
The 2 regenerative nodules in patients with early liver parenchymal disease were diagnosed correctly; however, the liver biopsy was awaited to get a confirmation of status of liver disease. These were also correlated with CT diagnosis. One of the metastases from neuro-endocrine tumors could not be diagnosed on CEUS and was diagnosed as an atypical hemangioma. This was confirmed later on CT, followed by biopsy.
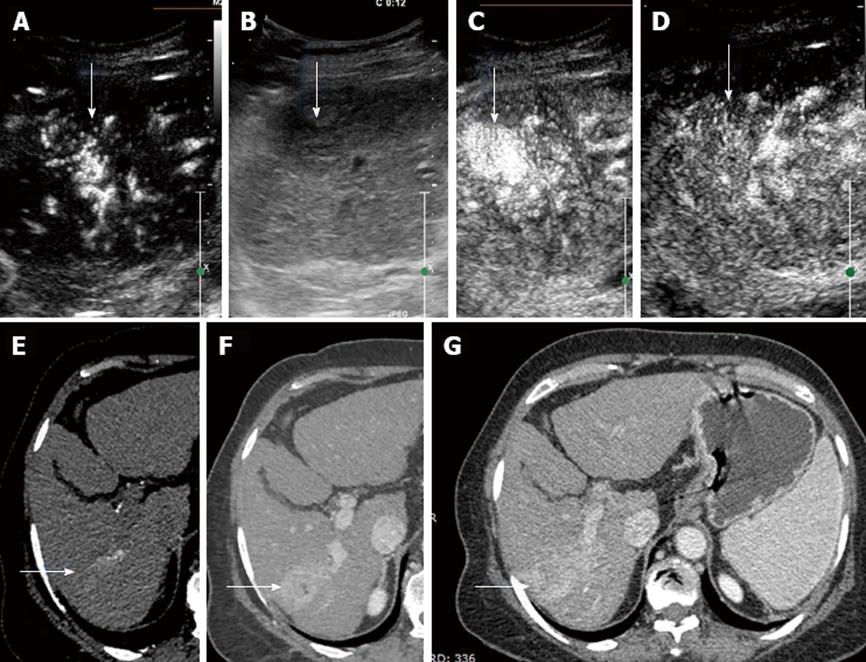
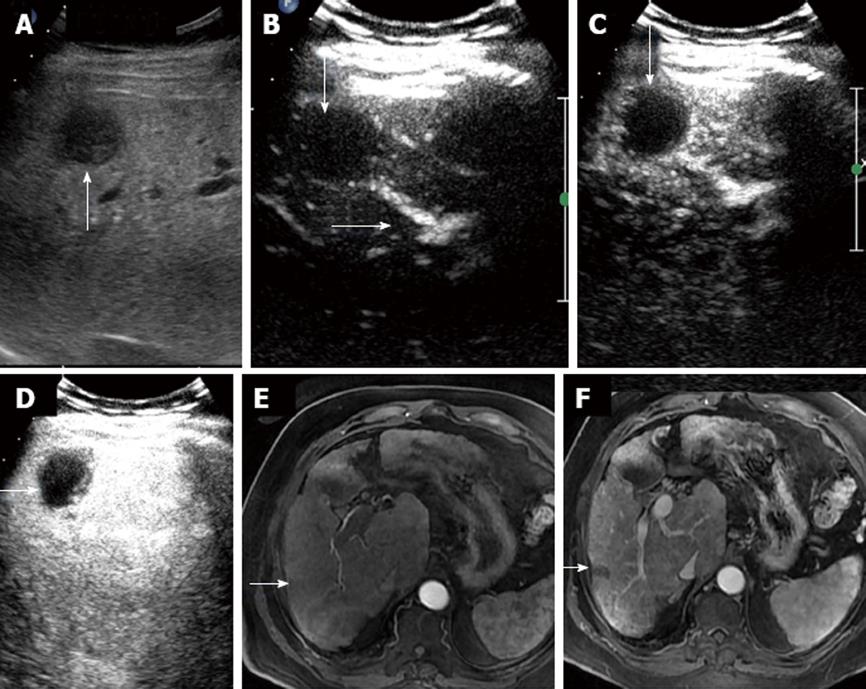
Of the 17 malignant hepatocellular lesions diagnosed on CEUS, 15 showed classical enhancement pattern of HCC on CT/MRI with positive AFP correlation (Figures 4 and 5). The 2 lesions that were characterized as HCC on CEUS did not show adequate washout and hypervascularity on cross sectional imaging modalities and were kept under follow up as dysplastic nodules. These lesions remained unchanged at the 6 mo follow up on MRI. Five lesions were assessed post RFA to look for enhancing residual tumor (Figure 6). No residual enhancing tumor margin/tissue were found in any of the lesions. Patients who were status post TACE (3 in number) were also examined for residual/recurrent disease. Mild peripheral vascularity was seen in all 3 of the lesions without obvious residual tumor tissue. Masses ranged in size from 1-10 cm in diameter in the post RFA and TACE groups. The results were corroborated with CT/MRI and were found to show excellent correlation.
Twenty (40% of the total examinations) were referred from clinicians for characterization of a mass from previous imaging done outside or within the institute, fifteen (30% of total exams) were performed for surveillance in chronic liver disease, two (4% of total exams) were performed on patients admitted to the intensive care with raised AFP and too critically sick to be shifted to CT/MRI room. Three (6% of total exams) were patients with h/o iodinated contrast allergy who underwent a CEUS rather than CT after being given a choice between the 2 modalities and having the advantages and disadvantages of both procedures explained. Two (4% of total exam) patients were severely claustrophobic, five (10% of total examinations) were performed for monitoring lesions after RFA and three (only single large mass, 6% of total exams) were done post TACE for routine follow up. All patients underwent an uneventful evaluation without significant adverse effects. The number of patients referred from clinicians steadily increased from 12 in the first 12 mo of the study to 38 in the last 12 mo. CEUS was able to give matched correct results (compared to the reference standard) for a total number of 36 out of 50 referred patients.
CEUS diagnosed 88% HCC (n = 15/total 17 cases) which were confirmed after correlation with second imaging and raised AFP (Figure 7). Biopsy was not required in any of the cases.
χ2 test was used to determine the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the CEUS data and the results were as follows (Table 2). In the CLD surveillance group, specificity of CEUS was 53.3% vs 100% by CT/MRI. Post RFA and TACE, specificity of lesion characterization by CEUS was 100% in single, large mass assessment, similar to CT/MRI. For non HCC lesions, the specificity of CEUS diagnosis was 50% vs 90% by CT/MRI.
| χ2 | Estimate | 95%CI |
| Sensitivity | 0.861 | 0.639-0.956 |
| Specificity | 0.500 | 0.055-0.945 |
| PPV | 0.969 | 0.759-0.997 |
| NPV | 0.167 | 0.018-0.69 |
| LR+ | 1.722 | 0.240-12.334 |
| LR- | 0.278 | 0.029-2.696 |
CEUS has been acknowledged as a widely known imaging modality for characterization of liver lesions since the time of the formulation of international guidelines for its usage, at Copenhagen in March 2003[3]. Since the first version of these guidelines dated January 2004, which focused on the evaluation of known or suspected FLL, the literature has ample proof that we have come a long way in our understanding of CEUS, especially in the field of hepatic lesions[2,3].
Diagnosis and surveillance of HCC is a major concern of the various international groups such as AASLD, European Association for the Study of the Liver (EASL) and APASL which have formulated consensus guidelines for imaging, diagnosis, treatment and surveillance of HCC so as to effectively maximize and conserve healthcare resources for the benefit of the entire community at risk[18,20]. The non invasive imaging diagnosis is doubly important in the case of HCC due to the significant risk of needle track tumor seedling on biopsy[10]. Due to this very reason, none of the patients in our study group, especially with solitary lesions or otherwise suitable for transplant, were biopsied at the time of evaluation. This could also be attributed to the fact that all modalities required for multi level evaluation were available to us at a tertiary care level. CEUS, using Kupffer cell specific contrast agent (which is currently available only in Japan), has been included by the APASL group, amongst one of the two imaging modalities which are used to diagnose HCC in indeterminate or atypical HCCs. The AASLD group does not recommend CEUS for diagnosis of HCC because of lack of availability of contrast agents in the United States[18]. At our institute, CEUS using Sonovue (the only available contrast agent in India) has been considered under protocol as one of the two modalities for determination of the final diagnosis of liver cancer in indeterminate masses not characterized by a single modality.
Sonovue and other contrast agents have been used in characterization of FLL and studies have shown the benefits of contrast US above non contrast B mode US[4,21-23]. Sonovue contains stabilized aqueous suspension of sulfur hexafluoride microbubbles with a phospholipid shell and a small diameter of approximately 2.5 mm. These bubbles are blood pool agents with both transpulmonic and transinusoidal passage. They diffuse into the blood pool unlike CT and MRI contrast agents which are extra vascular and hence have no equilibrium phase. Due to the wideband response of UCA at low MI, there is good visualization of micro and macro vasculature in all three phases of liver hemodynamics.
Quaia et al[22] and Hohmann et al[23] studied the effect of contrast US on FLL compared to non contrast US and were able to characterize malignant and benign lesions better than B mode US, in agreement with the reference standard. Strobel et al[24] conducted a multicenter trial to evaluate the diagnostic efficacy of CEUS for the differential diagnosis of liver tumors in clinical practice in 1349 patients over a span of two years. The PPV of contrast-enhanced US for the diagnosis of malignant tumors was 95.4% and the NPV was 95.7%. They clearly proved that CEUS improves the differential diagnosis of hepatic tumors, especially if B mode scan or power Doppler morphological criteria are not in consideration. Wilson et al[10] compared the diagnostic accuracy and confidence level, recommended management of focal liver masses after CEUS with non contrast US in 167 patients and found that CEUS improves the accuracy of diagnosis of FLL and reduces recommendations for further investigations. However, in the above study, the “benign and others (including metastases)” group comprised of double the number of lesions compared to the HCC group. “The benign and others” group was characterized with higher sensitivity and specificity and further imaging was less recommended. The classical HCC group was also less recommended for more work up. In our study group, HCC was further divided into indeterminate subgroup, surveillance subgroup and lesions requiring staging for transplant as well as the post therapy sub-groups. Further investigation was hence recommended more often for the various subgroups due to inadequacy of CEUS to accurately confirm the number of lesions and involvement of other segments of the liver. Ding et al[7] studied a group of 147 tumors using established diagnostic criteria and reported a high sensitivity of 96.3% and 97.5% specificity for the diagnosis of benign lesions like hemangioma and low sensitivity and specificity of 92% and 86.7% respectively for HCC. This group also studied the role of CEUS in small (< 3 cm in diameter) FLL amongst 200 patients. The results showed significant improvement in differentiating between malignant and benign small FLLs with contrast US compared to baseline non contrast scans.
The specificity of CEUS for non HCC lesions in our group was only 50% vs 90% by CT. We could attribute this to the small sample size and sample bias. The specificity for diagnosis of HCC by CEUS in this group was 85%. Our group observed that the recommendations for a second investigation were most commonly made only for cases under surveillance and for small indeterminate nodules in cirrhotics under investigation. Rettenbacher[11] discussed the contrast uptake patterns of liver lesions as well as important clinical indications for CEUS in his paper. He emphasized the role of CEUS as an excellent diagnostic modality for dynamic imaging of FLL, especially in the context of cirrhotics and HCC. He emphasized the fact that CEUS is helpful in patients with a pre-identified nodule on B mode imaging. Our group also observed that surveillance was not the forte of CEUS since our specificity and sensitivity for this group was the poorest of all subgroups. However, the use of CEUS in pre identified nodules, indeterminate masses and as a problem solving tool for smaller than 1 cm sized lesions was definitely worthwhile. Quaia et al[8] also conducted a study to look at different patterns of contrast enhancement in different focal hepatic lesions (approximately 47) after injection of the microbubble contrast agent SonoVue using high or low acoustic power imaging. They used unenhanced gray-scale US followed by color Doppler US with spectral analysis of tumoral vessels. Lesions were subsequently evaluated by US contrast (Sonovue) using dynamic imaging and were evaluated and compared with CT and biopsy results as the reference points. Results showed that CEUS was able to identify differential enhancement patterns in different focal hepatic lesions.
This study group included 22 HCCs which showed diffuse arterial enhancement followed by washout in the delayed phase. The regenerative nodules showed dotted enhancement and did not show washout or hypo-echogenicity compared to the liver on the delayed phase. In our group of enhancing nodules without obvious washout in patients with underlying cirrhosis, we observed (n = 4, false positive) lesions which we labeled as dysplastic nodules vs well-differentiated HCC. These nodules showed variable enhancement on CT/MRI without and were given a final diagnosis of regenerative nodules. von Herbay et al[14] studied enhancement post SonoVue administration in patients with HCC in correlation to both lesion diameter and histological differentiation of the lesion. They discovered that the well-differentiated HCCs displayed hyperechoic enhancement in the arterial phase and hypoechoic demarcation in the late phase. These features also showed significant correlation to the diameter of these lesions. Leen et al[9] studied the enhancement patterns of HCC with CEUS and its relationship with the degree of histological differentiation. They observed that moderately differentiated HCC exhibited classic enhancement features, while well- and poorly differentiated tumors showed most atypical enhancement patterns (approximately 60%-70%). They advocated an extended observation in the portal phase to look for a late washout seen to occur more frequently in some liver cancers. We observed the same in our group. The 2 small HCC lesions which were missed (false negatives) were small nodules (< 2 cm) and were not well visualized on either B mode or contrast US. They appeared as arterial blushes on MRI with delayed hepato-biliary phase washout and normal AFP levels. These were given a diagnosis of well differentiated HCC lesions. Also, four lesions which were seen as arterial blushes on CT/MRI and did not show obvious features of washout of liver cancer were kept in the regenerative nodules group. These were diagnosed as dysplastic nodules on contrast US. All nodules were less than 1 cm in size. Dietrich[25] described liver tumor characterization with the use of US contrast agents. They advocated that the uniqueness of liver lesions lay in their vascular pattern and their enhancement differences makes the liver a unique area to be explored with the use of UCAs rather than CT or MRI. Contrast sonography allows for real time examination of detailed and specific vascularity in about 80% of liver tumors due to typical perfusion patterns. Tranquart et al[12] conducted a multicenter trial in France involving 15 centers, proving the efficacy and competitiveness of Sonovue and CEUS as a diagnostic tool compared to CT and MRI imaging. However, we found out on our analysis that this multicenter trial involved different types of liver lesions, including benign and metastatic nodules which formed the bulk of the lesions. Also, no baseline US was performed for these patients. Interesting observations in contrast to the above group was seen in our population. The sub-group of indeterminate malignant lesions which were large in size and in the post therapy sub group of patients, the efficacy of CEUS in diagnosis was definitely comparable to CT/MRI[12]. We also had a large subset of surveillance group for liver cancer which did not show results comparable to CT/MRI. Dietrich[25] were pioneers in studying the medico-economics of CEUS, which they later observed to be a cost effective modality compared to CT and MRI. We observed that the medical reimbursement system in France is policy and insurance based. On the contrary, in India, most outpatient procedures are self funded by the patient and in such a scenario, the economics for the patient to get a contrast CT or a contrast US is almost similar. In such a situation, one has to weigh the affordability vs need for further investigation, radiation risks as well as willingness of the patient to get more than one investigation just to get a final diagnosis. Bhayan et al[26] studied the importance of washout on CEUS. They observed that most malignant lesions show washout except atypical cases of HCC. However, washout as a phenomenon was also seen in benign lesions. Rapid washout was more indicative of secondaries and HCCs showed more variable, gradual washout. Amongst our study group, washout of all HCC lesions diagnosed on contrast US was seen in portal venous/delayed phases. However, a few benign nodules were also seen to enhance initially with apparent washout on (the dysplastic nodule group on CEUS, found to be regenerative on CT) the delayed phase. This increased the diagnostic dilemma in the differential diagnosis of HCC, especially in patients with underlying cirrhosis. Various study groups[27,28] studied the importance of contrast US in cirrhotic livers exclusively and found that, for screening purposes and for lesion characterization, CEUS definitely showed an edge over routine B mode US. Bolondi et al[19] wrote in their review article that CEUS enables the characterization of FLL, irrespective of chronic liver disease. They reviewed the guidelines of EFSUMB and the observations made by the federation which contemplated that CEUS is expected to considerably increase as a modality of choice and replace many computed tomography and MRI examinations in the near future. EFSUMB has advocated the use of CEUS in staging and follow-up of cancer patients and in monitoring local ablative treatment. At our tertiary hospital study group, the pattern seen in patients with CLD showed that it was more difficult to characterize focal lesions with underlying CLD than without it. The assessment of a vast number of innocuous looking nodules which have the potential to become malignant at different stages of evolution in a cirrhotic liver definitely seemed challenging on contrast US. This was obvious with the specificity and sensitivity of our surveillance group. However, evaluation of predetermined lesions or further characterization of lesions observed on B mode or other investigations with CEUS definitely held various advantages as discussed below.
Soye et al[13] studied the use of CEUS in characterization of FLL in 68 patients with clinico-radiological and clinico-pathological follow up, which revealed a sensitivity of 95.0% and specificity of 97.9% in diagnosis of malignant lesions. They advocated the use of CEUS in the setting of a general hospital with an in house general radiology department. In our group, we have tried to assess the similar situation at a tertiary care level, where more focused and advanced diagnostic therapeutic modalities are available and observed that CEUS has 88% specificity and 86% sensitivity in diagnosis of HCC with a PPV of 97%. A recent study by Lencioni et al[29] studied the use of Sonazoid (Daiichi Sankyo, Tokyo, Japan), a second-generation of lipid-stabilized suspension of a perfluorobutane gas microbubble contrast agent being used exclusively in Japan. They used the contrast for observing the enhancement pattern of HCC and the current developments and applications of Sonazoid-enhanced US and Sonazoid-enhanced 3D US for diagnosing and treating HCC. Sonazoid typically has a late and a vascular phase. The HCC lesions appear as a defect on the delayed scan and reinjection of contrast in these lesions helps confirm lesions which show hypervascularity as malignant. This phase can also be used for therapeutic interventions. Sonazoid scanning is typically done at high MI. This contrast agent definitely appeared to cover the existing lacunae faced while scanning with Sonovue. A study by Jang et al[6] assessed the clinical value and potential impact of real-time SonoVue-enhanced sonography using low MI (0.1-0.3) with real time dynamic imaging in 127 patients (both benign and malignant lesions) to characterize FLL. The final diagnosis was based on comparison with CT, MRI and histopathology. The observations made by the group showed that CEUS did not rely on availability of clinical history to enable the diagnoses and it reduced the need for further imaging investigations from 23.7% to 90.4%. Our group also concurred with the reduction in need for further investigation. We did require correlation with clinical history, lab parameters and tumor markers for all patients. It was also observed that a few US scans were performed after a CT or MRI as a problem solving tool. The time for further management was definitely reduced. Furthermore, CT/MRI was required in most of our patients because the bulk of our cases were cirrhotics and non visualization of a hypervascular nodule did not necessarily mean that another segment of the liver which could not be simultaneously assessed on contrast US was free of an early HCC or dysplastic nodule. Hence, the specificity of the surveillance group in HCC was only 53.3% vs 100% on CT/MRI. The overall specificity of CEUS was subsequently decreased to 50% compared to CT/MRI. However the specificity of CEUS was definitely found to be more than routine B mode or Doppler US. Wilson et al[15] studied the obstacles and the evidence to support the use of UCA for imaging and diagnosis, due to the unavailability of US contrast in the United States. The group concluded that the evidence outshone the obstacles and CEUS is definitely an impressive tool which should be used without doubt for imaging and lesion characterization. Despite the obstacles of regulatory and practice patterns, the evidence indicated that radiologists and patients would miss an effective imaging option if strong support was not extended to US contrast agents by the FDA.
Various other studies have been performed to study and compare the efficacy of contrast US with other imaging modalities. The first Indian experience of CEUS guided radio frequency ablation was published by Kapoor et al[30] in which they demonstrated the advantages of CEUS vs CT. They showed the effectiveness of its real time accuracy, monitoring throughout the procedure and immediate effect of ablation by looking at the contrast enhancement at the residual margins. This allowed the operator to assess the result of the procedure and whether a repeat ablation session was required before terminating the procedure. We found excellent correlation of CEUS in post ablation and post TACE group of HCC patients and definitely advocate the use of contrast US in the follow up of this subgroup.
To our knowledge, our analysis is the first Indian study to evaluate and assess the role of CEUS in the context of screening, diagnosis and management of liver cancer in a tertiary liver center which is fully equipped with other high end modalities. Hence, notwithstanding the existing specificity and sensitivity of contrast US, the main focus of our study was to observe the use and importance of contrast US in a specialized setting of liver transplant oriented management approach and clinical referral base. The wholesome positive impact of CEUS in HCC related patients at our institute included: (1) Provisional urgent diagnosis of an incidentally detected mass (outside referrals/CT/MRI inconclusive results) by CEUS was able to allay patient and clinician anxiety to some extent. Almost half (40%) of the referrals in our study group were made for an immediate diagnosis of an existing indeterminate mass; (2) There was significant decrease in referral time for second line investigations which included a CT/MRI for correlation and confirmation. CT/MRI was performed in all the patients of our study group for staging of HCC which required cross sectional imaging, assessment of feasibility for liver transplant and evaluation of diagnostic sensitivity and specificity of this modality in comparison with existing CT and MRI; (3) Time needed for decision to consider for liver transplant, TACE, RFA was shortened on the basis of CEUS report that was provided for all the patients in the study group; (4) The clinician confidence levels towards CEUS increased since confident diagnoses were being made on CEUS in cases of indeterminate masses, when the patient was unfit for CT/MRI, allergic to contrast, claustrophobic etc. This group comprised of at least 14% of the total exams performed. Contrast US acted as an excellent add-on solution to the existing radiological set up; (5) Monitoring of post RFA or TACE by CEUS proved to be a cost effective and radiation free method. Almost 16% of referrals comprised this subgroup. The post procedure disadvantages of reactive peri-lesional hyperemia and pneumoperitoneum seen on CT/MRI were not observed on CEUS; (6) Contrast US was found to have various advantages compared to dynamic CT and MRI scans. It is radiation free, portable and can be performed at the patient’s bed side; (7) The US contrast is non nephrotoxic and hence can be used for patients with concomitant compromised renal function, especially in cases of hepato renal syndrome; (8) The patients are also reported to have more discomfort on the CT gantry compared to routine US evaluation; (9) The software of contrast enhanced sonography can be incorporated into pre existing US hardware and hence can be more cost effective than installation of CT/MRI in general/tertiary hospital settings; and (10) The information provided by the US studies is comparable to dynamic CT and MRI. Our study group showed that CEUS correctly diagnosed 88% of the total primary liver cancers with PPV of approximately 97%. The sensitivity was 86%; however, the specificity was relatively low (53%).
The results of our study demonstrated that CEUS has a definite role in a specialized liver care center which is also equipped with high end multi detector CT and 3T MRI. The portability of the US equipment was able to provide urgent diagnosis of an incidentally detected mass so as to further direct treatment and decrease patient and clinician anxiety in hemodynamically unstable patients. Time to further imaging for second line CT or MRI when required was also reduced in the presence of a documented lesion. On the other hand, the action time for decision making with reference to management of the patient also decreased on the basis of CEUS. There were a few areas of bias in the study. The study group was small with limited referrals to our super specialized center. Biopsy was not performed for any of the HCCs or dysplastic nodules, keeping in mind the significantly high percentage of needle track seedling. The post TACE lesions in the study were large masses only and multiple small lesions could not be studied.
The progressive increase in referrals over 2 years from clinicians to the contrast US department showed a slow build of confidence in cases of indeterminate lesions, where the patient was unfit for CT/MRI, contrast allergy, claustrophobia, etc. CEUS actually became an excellent add on solution to the existing radiological set up. On the management side, monitoring of post RFA or TACE (small number of well visualized lesions only) by CEUS was a cost effective and radiation free method, which seemed more appropriate for these patients who had been undergoing multiple CT/MRI scans earlier. To summarize, it would be appropriate to say that CEUS has carved its niche in a tertiary liver care hospital, along with other stalwarts of imaging, and is definitely here to stay.
Contrast enhanced ultrasound (CEUS) was subsequently introduced as a potential tool for improvised liver imaging. Since then CEUS has brought about many significant changes to the diagnostic paradigm of hepatocellular focal lesions, particularly hepatocellular carcinoma (HCC).
The specificity and sensitivity of CEUS was compared with both computer tomography (CT) and magnetic resonance imaging (MRI) by various study groups and found to be clinically significant even in multimodality centers; characterization of focal liver lesions was found to be in the order of 90%-95% concordance with other imaging modalities.
CEUS is a valuable augmentation to the practice of ultrasonography and an irreplaceable modality for confounding cases and interpretation of indeterminate lesions in imaging of HCC.
The results demonstrated that CEUS has a definite role in a specialized liver care center which is also equipped with high end multi detector CT and 3T MRI.
The authors constructively proposed the hypothesis that it is likely that in the future percutaneous thermal ablations (particularly microwave ablation) should be considered the first option for the treatment of HCC measuring 2 cm or smaller (radiofrequency ablation), or even up to 4-5 cm (microwave ablation).
P- Reviewer Chen F S- Editor Zhai HH L- Editor Roemmele A E- Editor Yan JL
| 1. | Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E, Lencioni R, Lindsell D, Martegani A, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T; EFSUMB Study Group. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249-256. [PubMed] [Cited in This Article: ] |
| 2. | Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 181] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Goldberg BB. Ultrasound contrast agents. London: Martin Dunitz 1997; . [Cited in This Article: ] |
| 4. | Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 134] [Cited by in F6Publishing: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Ricci P, Laghi A, Cantisani V, Paolantonio P, Pacella S, Pagliara E, Arduini F, Pasqualini V, Trippa F, Filpo M. Contrast-enhanced sonography with SonoVue: enhancement patterns of benign focal liver lesions and correlation with dynamic gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2005;184:821-827. [PubMed] [Cited in This Article: ] |
| 6. | Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, Li CL. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285-297. [PubMed] [Cited in This Article: ] |
| 8. | Quaia E, Degobbis F, Tona G, Mosconi E, Bertolotto M, Pozzi Mucelli R. [Differential patterns of contrast enhancement in different focal liver lesions after injection of the microbubble US contrast agent SonoVue]. Radiol Med. 2004;107:155-165. [PubMed] [Cited in This Article: ] |
| 9. | Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Wilson SR, Jang HJ, Kim TK, Burns PN. Diagnosis of focal liver masses on ultrasonography: comparison of unenhanced and contrast-enhanced scans. J Ultrasound Med. 2007;26:775-787; quiz 788-790. [PubMed] [Cited in This Article: ] |
| 11. | Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol. 2007;64:173-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Tranquart F, Correas JM, Ladam Marcus V, Manzoni P, Vilgrain V, Aube C, Elmaleh A, Chami L, Claudon M, Cuilleron M. [Real-time contrast-enhanced ultrasound in the evaluation of focal liver lesions: diagnostic efficacy and economical issues from a French multicentric study]. J Radiol. 2009;90:109-122. [PubMed] [Cited in This Article: ] |
| 13. | Soye JA, Mullan CP, Porter S, Beattie H, Barltrop AH, Nelson WM. The use of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Ulster Med J. 2007;76:22-25. [PubMed] [Cited in This Article: ] |
| 14. | von Herbay A, Vogt C, Westendorff J, Häussinger D, Gregor M. Correlation between SonoVue enhancement in CEUS, HCC differentiation and HCC diameter: analysis of 130 patients with hepatocellular carcinoma (HCC). Ultraschall Med. 2009;30:544-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Molins IG, Font JM, Alvaro JC, Navarro JL, Gil MF, Rodríguez CM. Contrast-enhanced ultrasound in diagnosis and characterization of focal hepatic lesions. World J Radiol. 2010;2:455-462. [PubMed] [Cited in This Article: ] |
| 17. | Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748-3756. [PubMed] [Cited in This Article: ] |
| 18. | Tan CH, Low SC, Thng CH. APASL and AASLD Consensus Guidelines on Imaging Diagnosis of Hepatocellular Carcinoma: A Review. Int J Hepatol. 2011;2011:519783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Bolondi L, Correas JM, Lencioni R, Weskott HP, Piscaglia F. New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis. 2007;39:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349-361. [PubMed] [Cited in This Article: ] |
| 21. | Solbiati L, Cova L, Kirn V, Tonolini M. Contrast-enhanced sonography is highly reliable and potentially cost-effective for characterizing focal liver lesions initially detected by unenhanced sonography. Chicago: RSNA 2004; 426. [Cited in This Article: ] |
| 22. | Quaia E, Bertolotto M, Calderan L, Mosconi E, Mucelli RP. US characterization of focal hepatic lesions with intermittent high-acoustic-power mode and contrast material. Acad Radiol. 2003;10:739-750. [PubMed] [Cited in This Article: ] |
| 23. | Hohmann J, Skrok J, Puls R, Albrecht T. [Characterization of focal liver lesions with contrast-enhanced low MI real time ultrasound and SonoVue]. Rofo. 2003;175:835-843. [PubMed] [Cited in This Article: ] |
| 24. | Strobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A, Friedrich-Rust M, Kunze G, Becker D, Will U. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 2008;29:499-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Dietrich CF. Characterisation of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol. 2004;51 Suppl:S9-S17. [PubMed] [Cited in This Article: ] |
| 26. | Bhayana D, Kim TK, Jang HJ, Burns PN, Wilson SR. Hypervascular liver masses on contrast-enhanced ultrasound: the importance of washout. AJR Am J Roentgenol. 2010;194:977-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Wong GL, Xu HX, Xie XY. Detection of focal liver lesions in cirrhotic liver using contrast-enhanced ultrasound. World J Radiol. 2009;1:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Maruyama H, Takahashi M, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound for characterisation of hepatic lesions appearing non-hypervascular on CT in chronic liver diseases. Br J Radiol. 2012;85:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Kapoor A, Kapoor A, Mahajan G. Technical note: Radiofrequency ablation of hepatocellular carcinoma with contrast-enhanced ultrasound guidance: First Indian experience. Indian J Radiol Imaging. 2011;21:121-123. [PubMed] [Cited in This Article: ] |