Published online Aug 26, 2017. doi: 10.4330/wjc.v9.i8.673
Peer-review started: January 16, 2017
First decision: April 17, 2017
Revised: July 19, 2017
Accepted: July 21, 2017
Article in press: July 24, 2017
Published online: August 26, 2017
Processing time: 224 Days and 5.3 Hours
To investigate the role of interleukin-19 (IL-19) in a murine model of female-dominant heart failure (HF).
Expression of one copy of a phosphorylation-deficient cyclic adenosine monophosphate response-element binding protein (dnCREB) causes HF, with accelerated morbidity and mortality in female mice compared to males. We assessed expression of IL-19, its receptor isoforms IL-20R α/β, and downstream IL-19 signaling in this model of female-dominant HF. To test the hypothesis that IL-19 is cardioprotective in dnCREB-mediated HF, we generated a novel double transgenic (DTG) mouse of dnCREB and IL-19 knockout and assessed cardiac morbidity by echocardiography and survival of male and female mice.
IL-19 is expressed in the murine heart with decreased expression in dnCREB female compared to male mice. Further, the relative expression of the two IL-19 receptor isoforms manifests differently in the heart by sex and by disease. Male DTG mice had accelerated mortality and cardiac morbidity compared to dnCREB males, while female DTG mice showed no additional detriment, supporting the hypothesis that IL-19 is cardioprotective in this model.
Together, these data suggest IL-19 is an important cytokine mediating sex-specific cardiac (dys) function. Ongoing investigations will elucidate the mechanism(s) of sex-specific IL-19 mediated cardiac remodeling.
Core tip: Heart failure (HF) is a sexually dimorphic disease. In a female-dominant model of HF, the do-minant negative cyclic AMP response-element binding protein (dnCREB) mouse, female mice show accelerated cardiac morbidity and mortality alongside downregulated interleukin-19 (IL-19) expression, while male mice maintain IL-19 expression and are protected against cardiac dysfunction. We generated a novel double transgenic mouse with dnCREB and IL-19 knockout to test the hypothesis that IL-19 is cardioprotective. We show accelerated cardiac morbidity only in male mice, supporting the hypothesis that IL-19 is a sex-specific cardioprotective cytokine.
- Citation: Bruns DR, Ghincea AR, Ghincea CV, Azuma YT, Watson PA, Autieri MV, Walker LA. Interleukin-19 is cardioprotective in dominant negative cyclic adenosine monophosphate response-element binding protein-mediated heart failure in a sex-specific manner. World J Cardiol 2017; 9(8): 673-684
- URL: https://www.wjgnet.com/1949-8462/full/v9/i8/673.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i8.673
Heart failure (HF) is the leading cause of mortality within the United States, affecting more than 5 million Americans[1]. HF is frequently perceived as a disease with poorer prognosis in male patients, perhaps since women are protected against cardiovascular disease pre-menopausally. However, many large-scale epidemiological studies do not support this conclusion. Sexually divergent manifestation of HF is observed regarding etiological factors, in response to a variety of HF therapies, and in presentation of HF and its comorbidities. Although the prevalence of HF is lower in women compared to men, treatment and survival outcomes for female patients are poorer with females having disproportionally higher morbidity and mortality[1]. Women are more likely to be symptomatic and functionally limited[2], experience less improvement following hospitalization[3], and show higher level of disability at similar levels of left ventricular (LV) dysfunction with worse quality of life[4] than male counter-parts. Together, the sex differences in HF highlight the necessity to understand female disease to close the gap in treatment and prognosis.
The deleterious effects of inflammatory cytokines in the context of HF are well documented as elevated circulating levels of cytokines predict adverse outcomes in patients with HF[5]. Inflammatory cytokines directly affect cardiomyocyte contractility[6], as well as influence LV remodeling and hypertrophy. Few studies, however, have examined sex-specific differences in cytokine expression, though sparse data indicate inflammatory profiles may be sexually dimorphic[7]. Interleukin-19 (IL-19) is a member of the IL-10 subfamily of inter-leukins. IL-19 signals through an IL-20 receptor hete-rodimer IL-20Rα and β to activate cytoplasmic tyrosine kinases of the Janus family signal transducer activator of transcription (JAK-STAT)[8]. Differential relative expression of IL-20R subunits has been reported in different tissue types[9] and is suggested as a potential mechanism of differential downstream IL-19 signaling, though this hypothesis has not yet been tested in the heart. While initially thought to be restricted to immune cells, IL-19 expression has since been observed in a wide variety of tissue types. IL-19 exerts both proinflammatory and anti-inflammatory properties, depending on tissue and disease specific factors[10]. Previous work has demonstrated anti-inflammatory properties of IL-19 in endothelial and vascular smooth muscle cells[8], but its role in the heart remains unknown.
Here, we investigated the expression of IL-19 and its receptor complex in a female dominant model of HF. Cardiac-specific expression of a dominant negative cyclic AMP response element-binding protein (dnCREB) results in dilated cardiomyopathy with accelerated mortality in female mice[11]. In this model, the tran-scription factor CREB is rendered phosphorylation inactive via mutation of a critical Ser residue located in the kinase-inducible domain the protein[12]. In the unphosphorylated state, CREB can bind to DNA but cannot activate transcription, thus rendering the transcription factor inactive. This single nucleotide mutation results in significant cardiac dysfunction and accelerated morbidity and mortality in female mice compared to males. In comparison to male dnCREB mice, female dnCREB have significantly worse LV systolic function, higher heart rate, and diminished cardiac output, resulting in overall greater cardiac morbidity compared to male mice with the same genetic mutation[11]. This exaggerated pathology in female dnCREB is particularly interesting, as the majority of HF models involving genetically manipulated mice demonstrate more profound morbidity in the male sex[13]. In addition to this novel sexual divergence, the role of CREB in cardiac dysfunction is important, as CREB is functionally lost in rodent models of HF[14] and loss of CREB-regulated genes is observed early in the failing human heart[15]. Here, we show for the first time that IL-19 is expressed in the rodent heart, and is expressed in a sexually dimorphic manner in HF. Further, we demonstrate dysregulated downstream IL-19 signaling in female-dominant HF and suggest that IL-19 is cardioprotective in this model.
The mouse model used in this study was a hetero-zygous, phosphorylation-deficient (Ser133 to Ala133) mutant CREB (dnCREB) transgenic mouse[16] and non-transgenic (control, Con) littermates. dnCREB mice begin showing signs of contractile dysfunction at eight weeks of age. By 12 wk, female mice display significant mortality compared to males[11]. Homozygous IL-19 knockout (IL-19 KO) mice were generated as previously described[17] and show no overt signs of cardiac dysfunction or early morbidity and mortality. To have dnCREB and IL-19 KO mice on the same background, dnCREB mice were back-bred onto the C57 background for a minimum of 6 lineage passages. Male dnCREB mice were then crossed with female IL-19 KO to create heterozygous IL-19 KO. Male dnCREB IL-19 heterozygous mice from this F1 generation were then crossed with IL-19 KO female mice to create homozygous IL-19 KO and heterozygous dnCREB double transgenic male and female mice (DTG). Mice were housed at 4 per cage after weaning. Cages were inspected daily, and date of death noted for those mice found dead. Experiments were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”, and were approved by the Institutional Animal Care and Use Committee at the University of Colorado-Denver.
Cardiac function was assessed by two-dimensional transthoracic echocardiography using a VisualSonics Vevo 770 high-resolution ultrasound imager equipped with a 35-MHz transducer. Mice were lightly sedated with isoflurane and body temperature was maintained at 37 °C. Parasternal long- and short-axis B-mode videos and M-modes images (at the level of the midpapillary short axis) were routinely acquired. LV wall thicknesses and inner dimensions at diastole and systole were measured from the parasternal short-axis M-mode images.
Cardiomyocytes were isolated from C57BL/6 male and female mice (approximately 14 wk of age) by enzymatic dissociation of the whole heart on a Langendorff apparatus as previously described[18]. Briefly, hearts were rapidly removed and rinsed in a control buffer (133.5 mmol/L NaCl, 4mmol/L KCl, 1.2 mmol/L NaH2PO4, 10 mmol/L HEPES, 1.2 mmol/L MgSO4, and 1% bovine serum albumin) to remove blood, weighed and mounted on a Langendorff apparatus. The isolated heart was then perfused at 37 °C for 3 min with control buffer before switching to enzyme solution (control solution containing collagenase type II (2.4 mg/mL) and 25 μmol/L CaCl2). After perfusion, ventricles were removed, minced in control solution and incubated at 37 °C for an additional 3 min with titration. Dissociated cells were then filtered through a nylon mesh to remove big pieces of undigested tissues. Isolated cells were rinsed in control solution and allowed to settle by gravity to remove debris and non-cardiomyocytes. Calcium was added to the myocytes in step-wise fashion by settling/resuspension in 4 steps. Purified myocytes were resuspended in Medium 199 supplemented with 110 mg/L sodium pyruvate, 0.1 mmol/L β-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum and cultured on laminin-coated culture plates at a density of approximately 6000 cells/cm2 at 37 °C for 2 h before washing to remove dead and non-adherent cells. Cells were maintained overnight in serum-containing medium before experimentation. Ventricular fibroblasts were isolated following the first low-speed spin to sediment myocytes. Fibroblasts were plated in Dulbecco’s modified eagle medium (DMEM) plus 10% fetal calf serum and 1% penicillin/streptomycin and allowed to culture until confluence. Upon reaching confluence, the media was changed to serum-free DMEM for one hour. Cells were then washed one time with PBS and harvested for subsequent experimentation.
The LV was carefully dissected away from the right ventricle and atria, and flash-frozen in liquid nitrogen. LV were homogenized in isoelectric focusing buffer (8 mol/L urea, 2.5 mol/L thiourea, 4% Chaps, 2 mmol/L EDTA) containing 2 mmol/L tributylphosphine, 10 mmol/L DTT and protease inhibitors. The homogenate was centrifuged at 14000 g for 5 min, and the super-natant saved for protein analyses. Protein concentration was determined using a modified protein assay (Bio-Rad) and prepared in Laemmli sample buffer (Bio-Rad). Proteins were resolved on 7.5% SDS-PAGE gels and transferred to PVDF. Following blocking in 5% bovine serum albumin for one hour at room temperature, membranes were incubated with primary antibody overnight at 4 °C. The following primary antibodies were used: Phospho-Stat3 (Tyr705) (Cell Signaling 9131; 1:1000), Stat3 (Cell Signaling 9139; 1:1000). Membranes were washed and incubated with secondary antibody for one hour at room temperature. Protein bands were visualized using a chemiluminescent substrate and autoradiography. Membranes were probed first with phospho signal transducer and activator of transcription 3 (STAT3), stripped, and then re-probed for total STAT3. Equal loading of proteins was verified by Ponceau-S staining.
RNA was extracted from LV and isolated cells using standard TRIzol protocol (Thermo Fisher) and reverse transcribed using iScript cDNA synthesis kit (BioRad). For detection of murine IL-19 and IL-20R, real-time RT-PCR was performed with the iCycler My iQ using iQ SYBR Green Supermix (BioRad), normalized to the housekeeping gene 18S ribosomal RNA (18S). Primer sequences were as follows. IL-19 forward: 5’-GGCTAAAAGTATGTTCAGTTCTCC-3’, IL-19 reverse: 5’-AAATCTCTGGAGCGATGTCAG-3’, IL-20Rα forward: 5’-AACTGGCAGGCTGTGTATCC-3’, IL-20Rα reverse: 5’-TTGTCAGGTGCCTGGTTCTC-3’, IL-20Rβ forward: 5’-CGAGGAGGGACGGAAGAATG-3’, IL-20Rβ reverse: 5’-TACGGCCTCTCTCGATGTCA-3’, 18S forward: 5’-GCCGCTAGAGGTGAAATTCTTG-3’, 18S reverse: 5’-CTTTCGCTCTGGTCCGTCTT-3’. Myosin heavy chain β (MYHCβ), atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), myosin heavy chain α (MYHCα), and sarcoplasmic reticulum Ca2+ ATPase (SERCA) oligonucleotide sequences were used as previously published[19]. ΔCt were calculated relative to the housekeeping gene 18S to allow comparisons across all groups (genotype and sex). As such, a lower ΔCt indicates higher expression.
Significance was set a priori at P < 0.05. Data were analyzed by Students t-test using GraphPad and 2-way ANOVA (genotype by sex) using IBM SPSS Statistics Version 24. Data with unequal variance were log-transformed to meet assumptions for homoscedasticity. Data are expressed as means ± SE of the mean. Where statistical analyses trend towards significance (P < 0.1), values are also noted above figures. Kaplan-Maier survival curves were generated for survival data, and differences in survival were assessed with log rank test. The statistical methods of this study were reviewed by David Kao, MD from the University of Colorado-Denver.
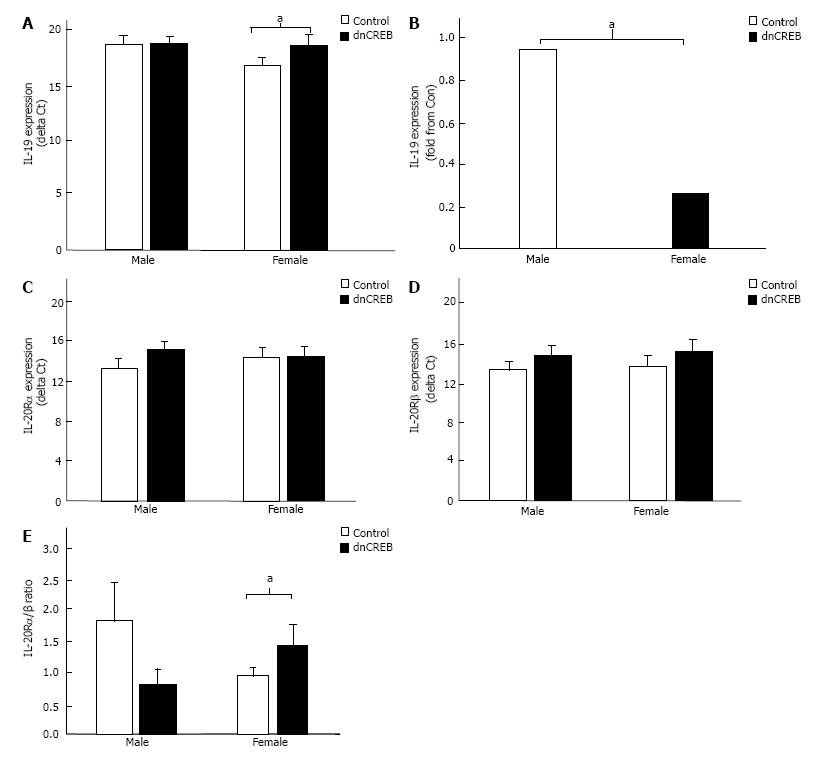
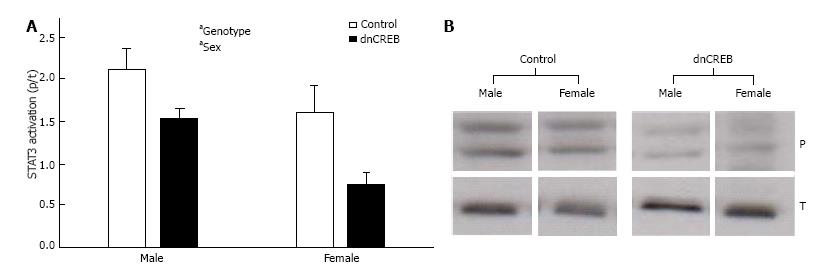
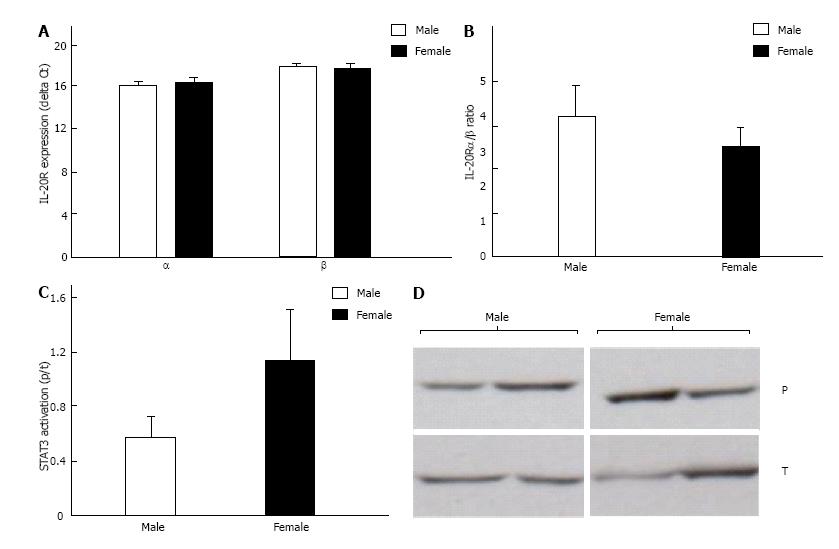
To begin to delineate the role of IL-19 in dnCREB-mediated HF, we assessed the expression of IL-19 and its receptor subunits in the LV from dnCREB mice compared to controls. Male dnCREB mice showed no change in IL-19 expression (Figure 1A); however, female dnCREB mice demonstrate significantly downregulated IL-19 expression (Figure 1A) with disease, a statistically different outcome in male and female mice (Figure 1B). Neither male nor female mice showed significant changes in expression of IL-20Rα or β with disease (Figure 1C and D). However, the ratio of α/β was significantly upregulated only in female dnCREB (Figure 1E). We then assessed activation of STAT3 as a downstream mediator of IL-19 signaling. Both male and female dnCREB mice showed downregulated STAT3 activation compared to control mice (Figure 2); however female dnCREB mice STAT3 activation was suppressed to 40% of control, while male dnCREB mice were suppressed to 70% of control.
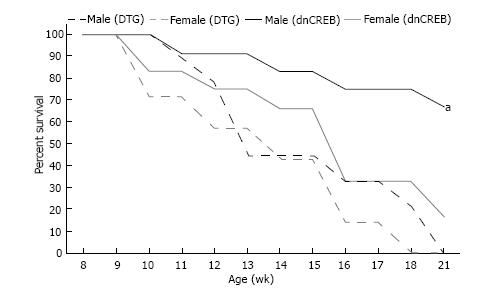
The sexual dimorphic regulation of IL-19 and its receptor subunits in dnCREB-mediated suggests that IL-19 is cardioprotective in the setting of dnCREB, since female mice in this model suffer premature morbidity and mortality compared to males and express significantly attenuated IL-19. To test this hypothesis, we generated a novel double transgenic model (DTG) of IL-19 knockout in dnCREB-mediated HF. Survival analyses show that DTG males died from HF earlier in the development of disease than dnCREB male mice; with nearly identical survival curves to dnCREB females and female DTG (Figure 3). During this time, there was no morbidity in the Con or IL-19 KO mice (data not shown). Thus, knockout of IL-19 in this model accelerates mortality only in male mice, with no additional effect in females.
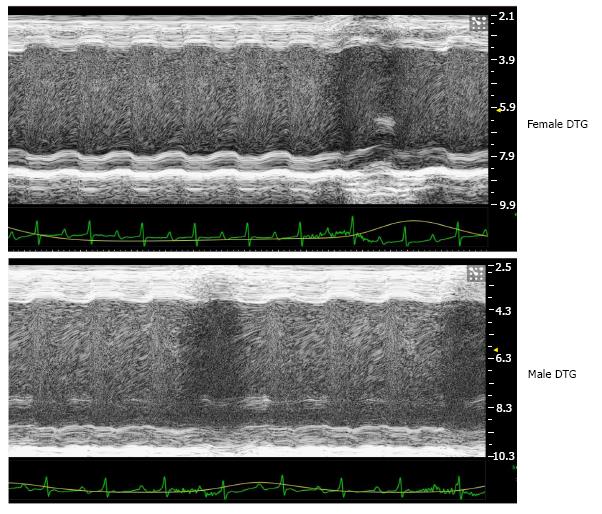
To confirm that accelerated mortality in DTG mice is due to cardiac dysfunction, we examined contractile function in male and female DTG mice at 10 wk of age by echocardiography. Representative M-mode echocar-diographs are shown in Figure 4, and quantification (no hyphen) of echocardiography is reported in Table 1. Previous reports of the dnCREB model of female-dominant HF reported diminished fractional shortening (19.4% and 8.79%) and cardiac output (17.4 and 14.6 mL/min) in male and female mice respectively, with a significant difference between sexes[11]. Consistent with the dnCREB model, our DTG mice showed evidence of significant cardiac contractile dysfunction, with low fractional shortening (14% and 12%), stroke volume (24 and 23 μL), and cardiac output (13.59 and 14.35 mL/min) in male and female mice, respectively. However, genetic ablation of IL-19 completely abrogated the previously reported sex difference in the dnCREB model. That is, male DTG mice display similar levels of cardiac morbidity as female DTG, suggesting that IL-19 is cardioprotective in the dnCREB model of HF.
| LVID; d (mm) | LVID; s (mm) | FS (%) | SV (μL) | HR (bpm) | CO (mL/min) | ||
| DTG | Male | 4.49 ± 0.47 | 3.90 ± 0.60 | 13.95 ± 4.2 | 24.10 ± 2.60 | 566 ± 30 | 13.59 ± 1.33 |
| Female | 4.53 ± 0.18 | 3.99 ± 0.29 | 12.02 ± 3.05 | 23.34 ± 3.11 | 621 ± 24 | 14.35 ± 1.30 |
We assessed the relative expression of IL-20Rα and β subunits in DTG male and female mice. We found male and female DTG mice to express similar levels of both subunits, with no difference in the ratio of the receptor subunits (Figure 5A and B). In addition, we assessed activation of STAT3 in male and female DTG mice and found STAT3 activation to be similar between sexes (Figure 5C and D). Expression of IL-20R and STAT3 activation in DTG male and female mice contrast with dnCREB mice, where downstream IL-19 signaling was significantly different between sexes.
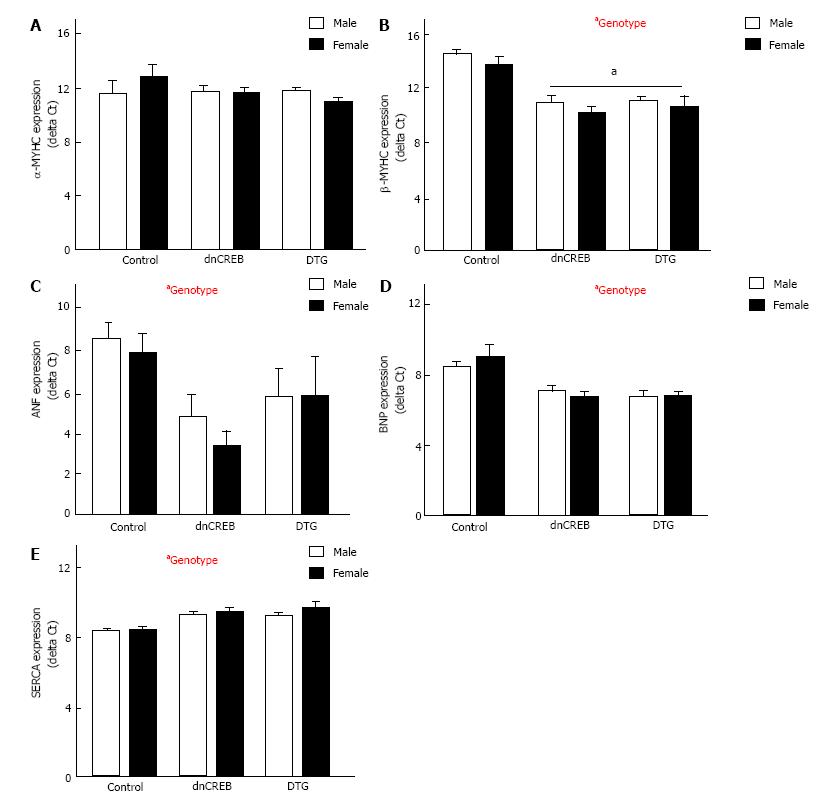
We assessed the expression of five genes in the hypertrophic gene program. These genes, components of the fetal gene program, are differentially expressed in established pathologic cardiac hypertrophy. While myosin heavy chain α (MYHCα) expression was unchanged by either sex or genotype (Figure 6A), myosin heavy chain β (MYHCβ), atrial natriuretic factor (ANF) and brain natriuretic peptide (BNP) were all significantly upregulated in dnCREB and DTG mice (Figure 6B-D), while sarcoplasmic reticulum Ca2+ ATPase (SERCA) expression was significantly downregulated (Figure 6E). Surprisingly, we observed no overall effect of sex on fetal gene program expression; however, BNP expression was more robustly induced in female dnCREB mice compared to male dnCREB (4.8 fold vs 2.6 fold, P < 0.05, data not shown).
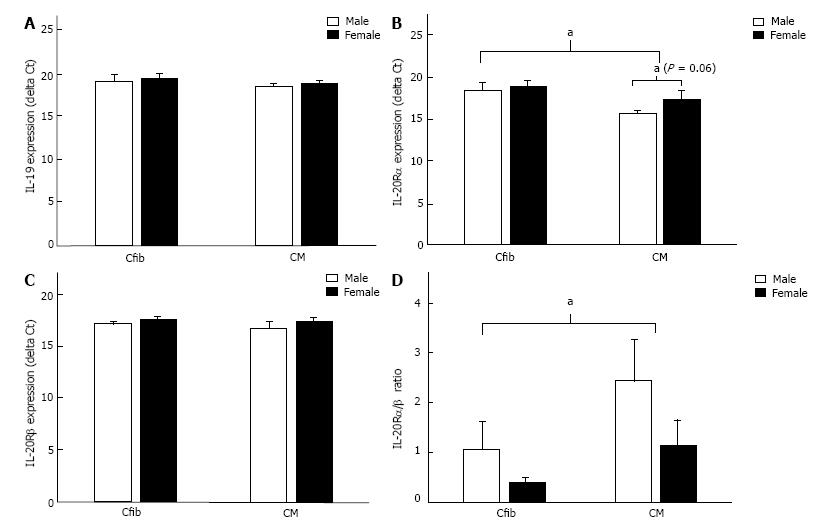
IL-19 signaling is uncharacterized in the heart. It is imperative we understand IL-19 signaling in the male and female heart to understand the dysregulation that occurs with disease. Therefore, we isolated primary cardiac myocytes and fibroblasts from male and female mice to assess IL-19 signaling in these two cell types. Both fibroblasts (Cfib) and myocytes (CM) expressed IL-19, with no differences between sexes in either cell type (Figure 7A). Both cell types also express IL-20Rα, with higher expression in CM than Cfib, and a trend towards lower expression in female myocytes than males (Figure 7B). Fibroblasts and myocytes similarly express IL-20Rβ, with no difference between sexes (Figure 7C), however the ratio of IL-20Rα/β was significantly higher in CM than Cfib (Figure 7D), suggesting the potential for cell-type specific responses to IL-19 signaling in the heart.
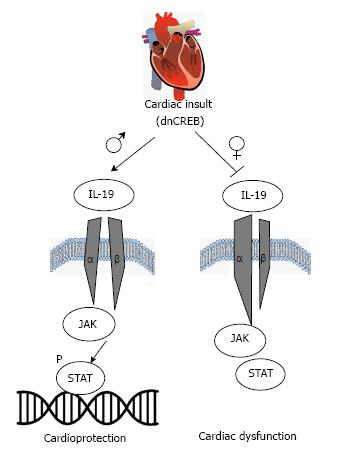
HF is a sexually dimorphic disease, adversely affecting female patients regarding morbidity and mortality. The maladaptive role of cytokines in HF is well documented; however, only a few studies have considered sex differences in cytokine expression or signaling. We show for the first time that IL-19, a previously undescribed cytokine in the heart, is expressed in rodent cardiac tissue and two previously unexamined cardiac cell types: Cardiac myocytes and fibroblasts. Further, we report dysregulation of IL-19 signaling in a female-dominant model of HF, suggesting a cardioprotective role of IL-19 in the heart. We propose the following model, as summarized in Figure 8, where IL-19 signaling through IL-20Rα/β activates STAT3 and canonical downstream cardioprotective mechanisms. This pathway is significantly downregulated in female dnCREB mice, resulting in attenuated STAT3 activation, LV remodeling, cardiac dysfunction, and premature mortality. However, male dnCREB mice maintain IL-19 expression with disease, resulting in heightened cardioprotection and significantly less morbidity and mortality compared to female mice. Ongoing investigations will elucidate further mechanistic insight into sexually divergent downstream IL-19 mediated cardiac signaling and whether this novel cytokine represents a new therapeutic target for the treatment of women’s heart disease.
IL-19 was first discovered over a decade ago and classified as a member of the IL-10 family based on structure and location of the IL-19 gene and the use of similar receptor complexes[20]. Since its discovery, however, the function of IL-19 has remained unclear. In a number of disease states including asthma[21], sepsis[22], and acute kidney injury[23] IL-19 acts as a pro-inflammatory factor. Conversely, in inflammatory bowel disease[17] and vascular disease[24], IL-19 appears to be anti-inflammatory and protect against disease progression. These data imply that IL-19 may function as either pro- or anti-inflammatory depending on the tissue and disease context. Further, it suggests that downstream IL-19 signaling including receptor subunit expression may be implicated in the disparate biological outcomes. A study examining IL-20R expression in 24 different human tissues reports significant differential α/β subunit expression between tissue types[9] suggesting differential relative expression of these two subunits may be implicated in the varying biological outcomes of IL-19 signaling. Various groups have attempted to define the binding kinetics and receptor requirements for IL-19 signaling. While most report a requirement for the IL-20R heterodimer and the rapid formation of a stable 1:1:1 complex in the presence of a ligand, other evidence also supports less stable homodimer formation[25]. In support of homodimer IL-20R signaling, IL-19 has clear effects on lymphocytes derived from IL-20Rβ knockout mice[26]. Thus the specific requirement for receptor dimerization remains controversial, as does the effect of subunit expression on IL-19 downstream function. We assessed IL-20R subunit expression in isolated primary cardiac myocytes and fibroblasts and found differential expression of the receptor subunits, suggesting that IL-19 may modulate IL-20R in a cell-type specific manner. These data are consistent with previous reports of IL-19 signaling in the vasculature which demonstrate that IL-19 stimulation of vascular smooth muscle cells induces expression of IL-20Rβ with no effect on endothelial cells[27]. Further, receptor subunit expression may be regulated in a sex-specific manner during disease progression as evidenced by differential IL-20Rα/β ratios in male and female dnCREB mice. Together, these data suggest that cell and context-specific regulation of IL-20 receptor isoforms may be important in disease, and may do so in a sexually dimorphic manner. Elucidation of the effects of IL-19 on specific cardiac cell populations (including inflammatory cells, endothelial, and vascular smooth muscle unexamined here) from male and female models warrants future investigation.
IL-19 treatment of IL-20Rα/β expressing cells leads to tyrosine phosphorylation of STAT3[28]. STAT3 is universally cardioprotective in response to a number of cardiac insults including ischemia-reperfusion injury[29] and hypertrophy[30]. Myocyte-specific STAT3 knockout mice develop cardiac fibrosis and myocardial dysfunction even in the absence of stress[31]. These mice are also more susceptible to inflammation-induced cardiac damage and greater contractile dysfunction[32], and ultimately lack of myocyte STAT3 leads to age-related HF[31,32]. Furthermore, human failing hearts exhibit reduced STAT3 levels and activity compared to heathy controls[33]. Thus, STAT3 is crucial for cardiac resistance to inflammation and other acute injuries. We show attenuated STAT3 activation in the LV from dnCREB male and female mice. Further, this effect is more robust in female dnCREB mice, correlating with the female-dominance of the dnCREB model and the early mortality of female mice. The mechanisms of STAT3-mediated cardiac protection in our model are not yet characterized; though canonical (Tyr705) STAT3 activation has proposed anti-oxidant, anti-apoptotic, and pro-angiogenic target genes (Reviewed in[34]). In addition, STAT3 also enhances mitochondrial respiration and acts on complex I to inhibit reactive oxygen species formation; mechanisms which are augmented by non-canonical phosphorylation of STAT3 at Ser727[35]. The action of STAT3 on mitochondrial function is particularly interesting and warrants future investigation, as dnCREB female mice have increased oxidant production, attenuated antioxidant defenses, and disrupted mitochondrial structure and function compared to male dnCREB[11].
In summary, we show for the first time that IL-19 demonstrates clear sexually dimorphic expression in the female-dominant dnCREB model of HF. Ablation of IL-19 in this model accelerates male mortality and causes severe cardiac morbidity, suggesting a cardioprotective role for IL-19 in the heart. Elucidation of IL-19 signaling in this model and in other models of female-dominant HF will facilitate the identification of novel therapeutics for women’s heart disease.
The authors thank Yanmei Du for technical assistance.
Heart failure (HF) is a sexually dimorphic disease, with worse morbidity and mortality in female patients compared to males. Inflammation is hypothesized to play a detrimental role in HF development, though whether it is regulated in a sexually dimorphic manner remains unknown. Interleukin-19 (IL-19) is an inflammatory cytokine with an unknown function in the healthy heart or in HF. Therefore, the authors set out to assess the role of IL-19 in female dominant HF.
HF is characterized by inflammatory signaling, though how this may be regulated in a sex-specific manner is unknown. Since HF is a sexually dimorphic disease, it is imperative that understand molecular mechanisms which contribute to disease in a sex-specific approach.
Although IL-19 has been studied in the vasculature, few reports exist regarding cardiac signaling. The authors show for the first time that IL-19 demonstrates clear sexually dimorphic expression in a model of female-dominant HF. Genetic ablation of IL-19 in this model accelerates male mortality, suggesting IL-19 is cardioprotective.
Development of sex-specific therapies will improve HF outcomes, and is a primary goal of personalized medicine. To develop sex-specific therapies, the authors must understand mechanisms which underlie HF in both males and females. Although the impact of IL-19 in the human heart remains unknown, the data identify sex-specific mediators of cardiac function, and suggest that therapies for the failing human heart be explored in both sexes.
This is a very interesting topic. The paper is well written, clear and interesting. The results provide adequate grounds for the conclusion.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin GM, Petretta M S-Editor: Kong JX L-Editor: A E-Editor: Lu YJ
| 1. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2893] [Cited by in RCA: 3392] [Article Influence: 282.7] [Reference Citation Analysis (0)] |
| 2. | Friedman MM. Gender differences in the health related quality of life of older adults with heart failure. Heart Lung. 2003;32:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care. 1998;36:1033-1046. [PubMed] |
| 4. | Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman-Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 384] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 6. | Radin MJ, Holycross BJ, Dumitrescu C, Kelley R, Altschuld RA. Leptin modulates the negative inotropic effect of interleukin-1beta in cardiac myocytes. Mol Cell Biochem. 2008;315:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 749] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 8. | Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol. 2008;173:901-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 432] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | England RN, Autieri MV. Anti-inflammatory effects of interleukin-19 in vascular disease. Int J Inflam. 2012;2012:253583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Watson PA, Birdsey N, Huggins GS, Svensson E, Heppe D, Knaub L. Cardiac-specific overexpression of dominant-negative CREB leads to increased mortality and mitochondrial dysfunction in female mice. Am J Physiol Heart Circ Physiol. 2010;299:H2056-H2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675-680. [PubMed] |
| 13. | Du XJ. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res. 2004;63:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Watson PA, Reusch JE, McCune SA, Leinwand LA, Luckey SW, Konhilas JP, Brown DA, Chicco AJ, Sparagna GC, Long CS. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol. 2007;293:H246-H259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J Clin Invest. 1998;101:2415-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis. 2010;16:1017-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Li D, Wu J, Bai Y, Zhao X, Liu L. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp. 2014;(87). [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Dockstader K, Nunley K, Karimpour-Fard A, Medway A, Nelson P, Port JD, Liggett SB, Bristow MR, Sucharov CC. Temporal analysis of mRNA and miRNA expression in transgenic mice overexpressing Arg- and Gly389 polymorphic variants of the β1-adrenergic receptor. Physiol Genomics. 2011;43:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Sabat R, Wallace E, Endesfelder S, Wolk K. IL-19 and IL-20: two novel cytokines with importance in inflammatory diseases. Expert Opin Ther Targets. 2007;11:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, Cheng KC, Lee MF, Chiang SR. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol. 2004;173:6712-6718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Hsing CH, Chiu CJ, Chang LY, Hsu CC, Chang MS. IL-19 is involved in the pathogenesis of endotoxic shock. Shock. 2008;29:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Hsu YH, Li HH, Sung JM, Chen WT, Hou YC, Chang MS. Interleukin-19 mediates tissue damage in murine ischemic acute kidney injury. PLoS One. 2013;8:e56028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Gabunia K, Jain S, England RN, Autieri MV. Anti-inflammatory cytokine interleukin-19 inhibits smooth muscle cell migration and activation of cytoskeletal regulators of VSMC motility. Am J Physiol Cell Physiol. 2011;300:C896-C906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Pletnev S, Magracheva E, Kozlov S, Tobin G, Kotenko SV, Wlodawer A, Zdanov A. Characterization of the recombinant extracellular domains of human interleukin-20 receptors and their complexes with interleukin-19 and interleukin-20. Biochemistry. 2003;42:12617-12624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Wahl C, Müller W, Leithäuser F, Adler G, Oswald F, Reimann J, Schirmbeck R, Seier A, Weiss JM, Prochnow B. IL-20 receptor 2 signaling down-regulates antigen-specific T cell responses. J Immunol. 2009;182:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Kako F, Gabunia K, Ray M, Kelemen SE, England RN, Kako B, Scalia RG, Autieri MV. Interleukin-19 induces angiogenesis in the absence of hypoxia by direct and indirect immune mechanisms. Am J Physiol Cell Physiol. 2016;310:C931-C941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545-3549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 314] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Bolli R, Dawn B, Xuan YT. Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2003;13:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Booz GW, Day JN, Baker KM. Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol. 2002;34:1443-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 32. | Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100:12929-12934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Zouein FA, Altara R, Chen Q, Lesnefsky EJ, Kurdi M, Booz GW. Pivotal Importance of STAT3 in Protecting the Heart from Acute and Chronic Stress: New Advancement and Unresolved Issues. Front Cardiovasc Med. 2015;2:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion. 2012;12:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |