Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.174
Peer-review started: November 10, 2016
First decision: November 30, 2016
Revised: December 21, 2016
Accepted: January 11, 2017
Article in press: January 13, 2017
Published online: February 26, 2017
Processing time: 109 Days and 2.7 Hours
To investigate the association between carotid atherosclerosis and cystatin C (CysC) and to determine the optimal CysC cut-off value.
One hundred twenty-eight subjects were included in this study. Atherosclerosis was defined as a maximum carotid plaque thickness (MCPT) of greater than 2 mm. A receiver operating characteristic curve analysis was used to determine the diagnostic value of serum CysC for atherosclerosis. The subjects were divided into two groups according to the CysC cut-off value. We screened for diabetes, hypertension, dyslipidemia, smoking status, alcohol consumption, and exercise behavior. The association between atherosclerosis and CysC levels was assessed using multivariate analysis.
The subjects were then divided into two groups according to the CysC cut-off value (0.73 mg/L). The median age of the high CysC group was 72 years (85% males), whereas that of the low CysC group was 61 years (63% males). The CysC levels were significantly correlated with Cr and estimated glomerular filtration rate (eGFR) values. Body-mass index, visceral fat area, hypertension, diabetes mellitus, and MCPT were significantly higher in the high CysC group than in the low CysC group. Furthermore, the eGFR was significantly lower in the high CysC group. Regarding lifestyle habits, only the exercise level was lower in the high CysC group than in the low CysC group. Multivariate analysis, adjusted for age and sex, revealed that high CysC levels were significantly associated with an MCPT of ≥ 2 mm (odds ratio: 2.92; 95%CI: 1.13-7.99).
Higher CysC levels were associated with an MCPT of ≥ 2 mm. The CysC cut-off value of 0.73 mg/L appears to aid in the diagnosis of atherosclerosis.
Core tip: Atherosclerosis is a leading worldwide cause of morbidity and mortality. The association between cystatin C (CysC) and atherosclerotic disorders remains controversial, and the cut-off value of CysC for atherosclerosis is unknown. Our study revealed that the optimal CysC cut-off point was 0.73 mg/L by receiver operating characteristic curve analysis. Higher CysC levels were significantly and independently correlated with an maximum carotid plaque thickness of ≥ 2 mm in multivariate analysis. Our data indicate that CysC could be a useful laboratory tool for predicting atherosclerosis during health checkups.
- Citation: Kobayashi T, Yokokawa H, Fujibayashi K, Haniu T, Hisaoka T, Fukuda H, Naito T. Association between high cystatin C levels and carotid atherosclerosis. World J Cardiol 2017; 9(2): 174-181
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/174.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.174
Atherosclerosis is a leading worldwide cause of morbidity and mortality[1,2]. The incidence of cardiovascular diseases (CVDs), including cerebrovascular, peripheral arterial, and coronary artery disease, is increasing and accounts for approximately one-fourth of all deaths in World Health Organization member states[3]. More than 17 million people die annually from CVDs, and, by 2030, more than 23 million CVD-related deaths are expected to occur worldwide. In Japan, the age-standardized fraction of mortality from CVDs is approximately 30%.
The ankle-brachial index, pulse-wave velocity, flow-mediated dilation, and ultrasonic evaluation have been introduced as methods for assessing the structural and functional effects of atherosclerosis[4-6]. Carotid atherosclerosis, estimated by intima-media thickness (IMT), is a sensitive surrogate marker for CVD and can now be non-invasively measured by B-mode ultrasonography[7,8]. IMT is a marker for systemic subclinical atherosclerosis and a strong predictor of incident myocardial infarction and ischemic stroke[9,10]. Carotid plaque may be an even more powerful predictor of vascular outcomes than IMT[11,12]. Maximum carotid plaque thickness (MCPT), widely used for assessing atherosclerotic change, is associated with an increased risk of vascular morbidity[13].
High plasma adiponectin independently predicted death and major adverse cardiovascular events in a large community-based population[14]. High-sensitivity C-reactive protein serum levels were reported to be significantly related to the severity of coronary atherosclerosis[15]. In addition to these markers, serum cystatin C (CysC) has recently been proposed as a more reliable biomarker for atherosclerosis and chronic renal disease. Furthermore, high CysC levels are indicated as a useful marker for identifying an elevated risk of CVD and a higher total mortality among patients assessed as being at low risk by both creatinine (Cr) and estimated glomerular filtration rate (eGFR) values[16,17]. A previous study revealed that atherosclerotic changes associated with inflammation could be one mechanism by which CysC is associated with CVD[16]. However, the association between CysC and atherosclerotic disorders remains controversial, the cut-off values of CysC for atherosclerosis are unknown, and previous reports on this association as well as the association between CysC and MCPT are limited[18-20]. A diagnostic CysC cut-off value has not been determined. In this study, we examined the association between CysC levels and atherosclerotic changes in Japanese subjects.
The present cross-sectional study included 133 Japanese subjects who underwent an inpatient medical health checkup at Juntendo University Hospital, Tokyo from October 2010 to January 2013. Among these subjects, five were excluded because of missing laboratory data. Thus, 128 subjects [98 men and 30 women; median age, 70 years (age range, 39-87 years)] were included.
The subjects were asked to complete a self-administered questionnaire about their sociodemographic characteristics, past medical history (diabetes, hypertension, and dyslipidemia), and lifestyle behaviors (alcohol consumption, current smoking status, and daily exercise activity).
The body weight, height, and waist circumference of the patients were measured, and the body-mass index [BMI (kg/m2)] was calculated. Systolic and diastolic blood pressure were measured in a sitting position after a 15-min rest using a standard mercury sphygmomanometer. Venous blood samples were collected following overnight fasting. Plasma glucose concentrations, hemoglobin A1c (HbA1c), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), Cr, and CysC levels were also measured. Low-density lipoprotein cholesterol was estimated using the Friedewald equation [TC-HDL-C-(TG/5)]. For the assessment of visceral fat accumulation, abdominal fat areas were measured from abdominal CT scans taken at the umbilical level while in the supine position and during late expiration, according to the Japanese Guidelines for Obesity Treatment[21].
The following parameters were calculated: eGFR was calculated using the Japanese GFR inference formula, which was developed by the Japanese Society of Nephrology[22]: eGFR (mL/min per 1.73 m2) = 194 × serum Cr (mg/dL) - 1.094 × age (years) - 0.287 (× 0.739 if female).
HbA1c was calculated as the National Glycohemoglobin Standardization Program (NGSP) value (%), which was developed by the Japan Diabetes Society[23]: HbA1c = NGSP (%) × 1.02 + 0.25.
Lifestyle-related diseases were defined using several criteria: (1) diabetes mellitus was defined as an HbA1c level of ≥ 6.5%, a fasting plasma glucose level of ≥ 126 mg/dL, or current antidiabetic therapy[24]; (2) hypertension was defined by a systolic blood pressure of ≥ 140 mmHg, a diastolic blood pressure of ≥ 90 mmHg, or current antihypertensive therapy[25]; and (3) dyslipidemia was defined as a fasting TG level of ≥ 150 mg/dL, a low-density lipoprotein cholesterol level of ≥ 140 mg/dL, or an HDL-C level of < 40 mg/dL[26]. Three unhealthy lifestyle behaviors were evaluated in this study: Drinking alcohol more than once a week, current smoking, and no regular physical activity.
A detailed protocol for measuring carotid artery atherosclerosis has been published[27]. Carotid plaque and IMT were measured using high-resolution B-mode ultrasonography to estimate atherosclerosis in the carotid artery. Eight technicians who were trained by a supervisor physician and who were certified in the protocol assessed carotid plaque and the mean IMT of the common carotid artery. A plaque was defined as a maximum IMT of > 1.0 mm. MCPT was measured at the peak plaque prominence in any of the carotid artery segments. Atherosclerosis was defined on the basis of the severity of carotid atherosclerosis by MCPT at a cut-off level of 2 mm. As previously reported, an MCPT of ≥ 2 mm is defined as an atherosclerotic change[13].
The results were expressed as medians of the test parameters. The Youden index, a point on the receiver operating characteristic (ROC) curve, was used to determine the diagnostic values of serum CysC levels that were indicative of atherosclerosis.
In a second analysis, the subjects were divided into two groups according to CysC levels above and below the cut-off value. Their demographic characteristics were then compared using the t test for continuous variables and the chi-square test for categorical variables. Multiple logistic regression analysis with adjustments for age and sex was conducted to determine the correlations between an MCPT of ≥ 2 mm and metabolic variables including CysC level. Our study included only 128 subjects, of whom 52 had arteriosclerosis. Because there is a limit to the number of adjusted variables, we combined several metabolic related variables in one item. Variables that were significantly associated with an MCPT of ≥ 2 mm were then investigated with multiple logistic regression analysis.
Statistical test results were considered significant when the P value was < 0.05. All calculations were performed using JMP Pro, version 11 (SAS Institute, Cary, NC, United States). The study protocol was approved by the Human Ethics Committee of Juntendo University. The participants’ clinical data were retrospectively retrieved from an institutional database. All of the examinations included in this study were performed as a routine part of the program, and none were aimed at specifically collecting data for the current study. The study protocol was approved by the institutional ethics committee, so it was not necessary to obtain informed consent from the participants. A biostatistician reviewed the study.
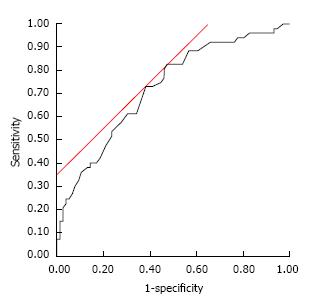
The subject characteristics are shown in Table 1. The median age was 70 years (77% males). Twenty-three (18%) subjects smoked, 78 (61%) were alcohol consumers, and 90 (70%) did not exercise regularly. The median visceral fat area was 125.2 cm2. Sixty-one (48%) subjects were diagnosed with hypertension, 72 (56%) with dyslipidemia, and 29 (23%) with diabetes mellitus. The ROC analysis conducted to determine the cut-off value of CysC revealed a significantly higher risk of atherosclerosis at 0.73 mg/L (Figure 1) (sensitivity: 82.7%, specificity: 52.6%).
| Variables | Median (min, max) or n (%) |
| Age (yr) | 70 (39, 87) |
| Sex (male) | 98 (77) |
| Body-mass index (kg/m2) | 24.2 (15.1, 38) |
| Lifestyle-related items | |
| Current smokers | 23 (18) |
| Alcohol consumers | 78 (61) |
| No exercise habits | 90 (70) |
| Visceral fat area (cm2) | 125.2 (22.9, 281.7) |
| Clinical history | |
| Ischemic heart disease, n (%) | 6 (5) |
| Blood pressure | |
| Systolic blood pressure (mmHg) | 122 (92, 156) |
| Diastolic blood pressure (mmHg) | 68 (50, 86) |
| Diagnosed hypertension | 61 (48) |
| Lipid metabolism | |
| Total cholesterol (mg/dL) | 194.5 (115, 727) |
| High-density lipoprotein cholesterol (mg/dL) | 54 (30, 96) |
| Low-density lipoprotein cholesterol (mg/dL) | 111 (41, 205) |
| Triglycerides (mg/dL) | 99.5 (37, 593) |
| Diagnosed dyslipidemia | 72 (56) |
| Glucose metabolism | |
| Fasting plasma glucose (mg/dL) | 93.5 (74, 226) |
| Hemoglobin A1c (%) | 5.3 (4.5, 8.3) |
| Diagnosed diabetes mellitus | 29 (23) |
| Kidney function | |
| Creatinine (mg/dL) | 0.75 (0.38, 1.36) |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 78.4 (38.9, 122.6) |
| Cystatin C (mg/L) | 0.78 (0.49, 1.45) |
| Carotid ultrasonography | |
| Right common carotid artery plaque thickness (mm) | 0 (0, 3.6) |
| Right carotid bulb-internal carotid artery plaque thickness (mm) | 1.5 (0, 5.5) |
| Left common carotid artery plaque thickness (mm) | 0 (0, 2.8) |
| Left carotid bulb-internal carotid artery plaque thickness (mm) | 1.5 (0, 4.2) |
| Right common carotid artery maximum intima- media thickness (mm) | 1.0 (0.6, 1.9) |
| Left common carotid artery maximum intima- media thickness (mm) | 1.0 (0.7, 2.3) |
The subjects were then divided into two groups according to the CysC cut-off value (0.73 mg/L). The subjects’ characteristics according to the CysC level are shown in Table 2. The median age of the high CysC group was 72 years (85% males), whereas that of the low CysC group was 61 years (63% males). The CysC levels were significantly correlated with Cr and eGFR values. BMI, visceral fat area, hypertension, diabetes mellitus, and MCPT were significantly higher in the high CysC group than in the low CysC group. Furthermore, the eGFR was significantly lower in the high CysC group. Regarding lifestyle habits, only the exercise level was lower in the high CysC group than in the low CysC group. In addition, sensitivity, specificity, positive predictive value, and negative predictive value as calculated from the data in Table 2 were 83%, 53%, 54% and 82%, respectively.
| Variables | Median (min, max) or n (%) | P value | |
| Higher cystatin C (≥ 0.73) (n = 79) | Lower cystatin C (< 0.73) (n = 49) | ||
| Age (yr) | 72 (46, 87) | 61 (39, 80) | < 0.011 |
| Sex (male) | 67 (85) | 31 (63) | < 0.012 |
| Body-mass index (kg/m2) | 24.9 (17.0, 38.0) | 23.5 (15.1, 30.2) | < 0.011 |
| Visceral fat area (cm2) | 142.7 (48.3, 281.7) | 103.7 (22.9, 249.2) | < 0.011 |
| Lifestyle habits | |||
| Current smokers | 14 (23) | 9 (19) | 0.882 |
| Alcohol consumers | 47 (59) | 31 (63) | 0.672 |
| No exercise habits | 28 (35) | 10 (20) | 0.072 |
| Diagnosed hypertension | 48 (61) | 13 (27) | < 0.012 |
| Diagnosed dyslipidemia | 45 (57) | 27 (55) | 0.842 |
| Diagnosed diabetes mellitus | 24 (30) | 5 (10) | < 0.012 |
| Kidney function | |||
| Creatinine (mg/dL) | 0.81 (0.45, 1.36) | 0.62 (0.38, 0.97) | < 0.011 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 70.6 (38.9, 110.2) | 88.7 (59.7, 122.6) | < 0.011 |
| Carotid ultrasonography | |||
| Maximum carotid plaque thickness ≥ 2 mm | 43 (54) | 9 (18) | < 0.012 |
Next, we compared differences in demographics and clinical variables between subjects with MCPTs of ≥ 2 mm or < 2 mm (Table 3). Age, visceral fat area, hypertension, diabetes mellitus, Cr, eGFR, and CysC were significantly higher in the MCPT of ≥ 2 mm group than the < 2 mm group. Furthermore, the eGFR was significantly lower in the MCPT of ≥ 2 mm group. The two groups did not differ with regard to lifestyle habits.
| Variables | Median (min, max) or n (%) | P value | |
| MCPT ≥ 2 mm (n = 52) | MCPT < 2 mm (n = 76) | ||
| Age (yr) | 72 (51, 87) | 66 (39, 83) | < 0.011 |
| Sex (male) | 42 (81) | 56 (74) | < 0.352 |
| Body-mass index (kg/m2) | 24.1 (17.0, 38.0) | 24.3 (15.1, 31.7) | < 0.351 |
| Visceral fat area (cm2) | 138.9 (30.5, 281.7) | 115.8 (22.9, 249.2) | < 0.101 |
| Lifestyle habits | |||
| Current smokers | 9 (17) | 14 (18) | 0.852 |
| Alcohol consumers | 28 (54) | 50 (66) | 0.172 |
| No exercise habits | 18 (35) | 20 (26) | 0.312 |
| Diagnosed hypertension | 32 (62) | 29 (38) | < 0.012 |
| Diagnosed dyslipidemia | 29 (56) | 43 (57) | 0.932 |
| Diagnosed diabetes mellitus | 18 (35) | 11 (14) | < 0.012 |
| Kidney function | |||
| Creatinine (mg/dL) | 0.80 (0.45, 1.36) | 0.75 (0.38, 1.17) | < 0.031 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 73.3 (38.9, 111.9) | 80.2 (47.9, 122.6) | < 0.021 |
| Cystatin C (mg/L) | 0.83 (0.55, 1.45) | 0.72 (0.49, 1.22) | < 0.011 |
The factors associated with an MCPT of ≥ 2 mm are shown in Table 4. Multivariate analysis, adjusted for age and sex, revealed that high CysC levels were significantly associated with an MCPT of ≥ 2 mm (odds ratio: 2.92; 95%CI: 1.13-7.99).
| Variables | Univariate | Multivariate1 | ||
| Odds ratio | 95%CI | Odds ratio | 95%CI | |
| Cystatin C | 5.31 | 2.27-12.39 | 2.92 | 1.13-7.99 |
| Diabetes mellitus | 3.13 | 1.33-7.37 | 1.82 | 0.70-4.86 |
| Hypertension | 2.59 | 1.26-5.36 | 1.56 | 0.69-3.53 |
| Dyslipidemia | 0.97 | 0.48-1.97 | ||
| Current smoking | 0.91 | 0.36-2.30 | ||
| Alcohol consumers | 0.61 | 0.29-1.25 | ||
| Exercise habits | 0.67 | 0.31-1.45 | ||
| Visceral fat area ≥ 100 cm2 | 1.31 | 0.62-2.78 | ||
| AICc2 | 158 | |||
| 3R2 | 0.16 | |||
In this study, multivariate analysis revealed that higher CysC levels were significantly associated with carotid atherosclerosis, as defined by an MCPT of ≥ 2 mm, in middle-aged and elderly Japanese subjects. The cut-off CysC value (0.73 mg/L) could aid in the diagnosis of atherosclerosis. To our knowledge, this is the first report demonstrating an association between CysC and carotid atherosclerosis as assessed by MCPT. The CysC cut-off level potentially has promising clinical value in the diagnosis of atherosclerosis.
Our results revealed a significant association between high CysC levels and an MCPT of ≥ 2 mm. A meta-analysis previously revealed that CysC is strongly and independently correlated with the risk of subsequent cardiovascular disease[28]. Although several studies have revealed an association between high CysC levels and atherosclerosis, their results differed from ours because of the different targets and indicators used. A previous study, which analyzed 637 Japanese subjects without chronic kidney disease, revealed that CysC was positively correlated with the cardio-ankle vascular index in women[19]. In a study of 60 Japanese hypertensive patients, serum CysC levels were positively correlated with carotid IMT[29]. In data collected via 64-slice CT coronary angiography, a high CysC level was found to be significantly correlated with early-stage coronary atherosclerotic plaques in 405 Japanese patients without established chronic kidney dysfunction[18]. Our results are in agreement with the previous hypothesis that CysC level is a reliable marker for atherosclerosis.
There are several possible explanations for the association between CysC and atherosclerotic change. First, inflammation may be associated with both CysC and atherosclerosis. The Cardiovascular Health Study[30], which analyzed 4637 ambulatory elderly patients, revealed a significant linear association between CysC and C-reactive protein but not Cr or eGFR[31]. It is well known that inflammation plays a role in atherogenesis, atherosclerotic plaque progression, and acute coronary syndrome. Second, CysC plays an important role in maintaining atherosclerotic plaque stability. A previous study[32] analyzed 31 plaques removed by endarterectomy, demonstrating with immunohistochemistry that CysC in human carotid plaques localized with collagen and elastin. An imbalance between cysteine proteases and CysC in arterial wall remodeling occurs in vascular diseases, such as atherosclerosis and abdominal aortic aneurysm[33].
Imaging assessments, such as ultrasound and CT, are often performed for assessing arteriosclerotic vascular disease. However, not all institutions can practice such assessments because of the lack of sonographers or appropriate devices. Therefore, it is potentially important that atherosclerosis can be evaluated using a blood test, such as for CysC levels. A diagnostic CysC cut-off value has not been previously determined. Our study revealed that the CysC cut-off value of 0.73 mg/L could contribute to the diagnosis of atherosclerosis.
Our study had a few limitations. First, the subjects were selected from a single institution, the sample size was small, and > 70% of our subjects were healthy men. Selection bias may have affected the analysis, as the investigated cohort did not accurately represent the Japanese population. Thus, future large-scale cohort studies are required. Second, lifestyle habits were evaluated using a self-administered questionnaire, and the subjects may have stated that they had a healthier lifestyle than they actually did. Further evaluations of lifestyle habits based on a validated questionnaire are necessary. Third, causal inferences cannot be made because of the cross-sectional nature of the study design. A prospective study is required for determining whether higher CysC levels are associated with the development of atherosclerosis-related diseases or death.
In conclusion, higher CysC levels were correlated with carotid atherosclerosis as defined by an MCPT of ≥ 2 mm among middle-aged and elderly Japanese subjects. Higher CysC levels have a low specificity but a high sensitivity and can therefore help exclude atherosclerosis. The CysC cut-off value of 0.73 mg/L appears to aid in the diagnosis of atherosclerosis. Our data indicate that CysC could be a useful laboratory tool for predicting atherosclerosis during health checkups.
The authors would like to thank Enago (https://www.enago.jp/) for the English language review.
Atherosclerosis is a leading worldwide cause of morbidity and mortality. Carotid plaque may be a powerful predictor of vascular outcomes. Maximum carotid plaque thickness (MCPT), widely used for assessing atherosclerotic change, is associated with an increased risk of vascular morbidity.
Serum cystatin C (CysC) has recently been proposed as a reliable biomarker for atherosclerosis and chronic renal disease. However, the association between CysC and atherosclerotic disorders remains controversial, the cut-off values of CysC for atherosclerosis are unknown, and previous reports on this association as well as the association between CysC and MCPT are limited. A diagnostic CysC cut-off value has not been determined.
Higher CysC levels were associated with an MCPT of ≥ 2 mm. The CysC cut-off value of 0.73 mg/L appears to aid in the diagnosis of atherosclerosis.
It may be difficult for an institution to practice imaging assessment because of the lack of sonographers or appropriate devices. Therefore, it is potentially important that atherosclerosis can be evaluated using a blood test, such as CysC levels. The CysC cut-off value of 0.73 mg/L could contribute to the diagnosis of atherosclerosis.
CysC is a 13-kD protease inhibitor which is produced by all nucleated cells. It is mainly used as a biomarker of kidney function. Recently, it has been studied for its role in predicting new-onset or deteriorating cardiovascular disease.
This is a well-written article investigating the association between CysC and carotid atherosclerosis.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Schoenhagen P, Tarantino G, Zhang ZH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5211] [Article Influence: 521.1] [Reference Citation Analysis (0)] |
| 2. | Laslett LJ, Alagona P, Clark BA, Drozda JP, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 3. | Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. USA: World Health Organization 2011; 2-14 Available from: http://www.world-heart-federation.org/fileadmin/user_upload/images/CVD_Health/Global_CVD_Atlas.pdf. |
| 4. | Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463-e654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2327] [Cited by in RCA: 2220] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 5. | Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3382] [Cited by in RCA: 3064] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 6. | Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 705] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 7. | Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 8. | Kitagawa K, Hougaku H, Yamagami H, Hashimoto H, Itoh T, Shimizu Y, Takahashi D, Murata S, Seike Y, Kondo K. Carotid intima-media thickness and risk of cardiovascular events in high-risk patients. Results of the Osaka Follow-Up Study for Carotid Atherosclerosis 2 (OSACA2 Study). Cerebrovasc Dis. 2007;24:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478-487. [PubMed] |
| 10. | O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3371] [Cited by in RCA: 3350] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 11. | Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 514] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33:2916-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 446] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 13. | Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology. 2008;70:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 14. | Lindberg S, Mogelvang R, Pedersen SH, Bjerre M, Frystyk J, Flyvbjerg A, Galatius S, Jensen JS. Relation of serum adiponectin levels to number of traditional atherosclerotic risk factors and all-cause mortality and major adverse cardiovascular events (from the Copenhagen City Heart Study). Am J Cardiol. 2013;111:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Assadpour Piranfar M. The Correlation between High-Sensitivity C-Reactive Protein (hsCRP) Serum Levels and Severity of Coronary Atherosclerosis. Int Cardiovasc Res J. 2014;8:6-8. [PubMed] |
| 16. | Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55:1932-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Dupont M, Wu Y, Hazen SL, Tang WH. Cystatin C identifies patients with stable chronic heart failure at increased risk for adverse cardiovascular events. Circ Heart Fail. 2012;5:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Imai A, Komatsu S, Ohara T, Kamata T, Yoshida J, Miyaji K, Shimizu Y, Takewa M, Hirayama A, Deshpande GA. Serum cystatin C is associated with early stage coronary atherosclerotic plaque morphology on multidetector computed tomography. Atherosclerosis. 2011;218:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Yamashita H, Nishino T, Obata Y, Nakazato M, Inoue K, Furusu A, Takamura N, Maeda T, Ozono Y, Kohno S. Association between cystatin C and arteriosclerosis in the absence of chronic kidney disease. J Atheroscler Thromb. 2013;20:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Garimella PS, Ix JH, Katz R, Shlipak MG, Criqui MH, Siscovick DS, Kramer H, Sibley CT, Sarnak MJ. Association of albumin-creatinine ratio and cystatin C with change in ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2015;65:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | [Definition and the diagnostic standard for metabolic syndrome--Committee to Evaluate Diagnostic Standards for Metabolic Syndrome]. Nihon Naika Gakkai Zasshi. 2005;94:794-809. [PubMed] |
| 22. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5325] [Cited by in RCA: 5225] [Article Influence: 326.6] [Reference Citation Analysis (0)] |
| 23. | Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 24. | Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, Ito C, Inagaki N, Iwamoto Y, Kasuga M. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;1:212-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1026] [Cited by in RCA: 1124] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 25. | Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res. 2009;32:3-107. [PubMed] |
| 26. | Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, Daida H, Biro S, Hirobe K, Funahashi T. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Oike M, Yokokawa H, Fukuda H, Haniu T, Oka F, Hisaoka T, Isonuma H. Association between abdominal fat distribution and atherosclerotic changes in the carotid artery. Obes Res Clin Pract. 2014;8:e448-e458. [PubMed] [DOI] [Full Text] |
| 28. | Lee M, Saver JL, Huang WH, Chow J, Chang KH, Ovbiagele B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: a meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Watanabe S, Okura T, Liu J, Miyoshi K, Fukuoka T, Hiwada K, Higaki J. Serum cystatin C level is a marker of end-organ damage in patients with essential hypertension. Hypertens Res. 2003;26:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 604] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 31. | Shlipak MG, Katz R, Cushman M, Sarnak MJ, Stehman-Breen C, Psaty BM, Siscovick D, Tracy RP, Newman A, Fried L. Cystatin-C and inflammatory markers in the ambulatory elderly. Am J Med. 2005;118:1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Gonçalves I, Ares MP, Moberg A, Moses J, To F, Montan J, Pedro LM, Dias N, Fernandes E Fernandes J, Fredrikson GN. Elastin- and collagen-rich human carotid plaques have increased levels of the cysteine protease inhibitor cystatin C. J Vasc Res. 2008;45:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 338] [Article Influence: 13.0] [Reference Citation Analysis (0)] |