Published online Jun 26, 2016. doi: 10.4330/wjc.v8.i6.362
Peer-review started: July 5, 2015
First decision: August 16, 2015
Revised: March 15, 2016
Accepted: April 7, 2016
Article in press: April 11, 2016
Published online: June 26, 2016
In patients with ST-elevation myocardial infarction, recurrent cardiovascular events still remain the main cause of morbidity and mortality, despite significant improvements in antithrombotic therapy. We sought to review data regarding coronary thrombus analysis provided by studies using manual aspiration thrombectomy (AT), and to discuss how insights from this line of investigation could further improve management of acute coronary disease. Several studies investigated the fresh specimens retrieved by AT using techniques such as traditional morphological evaluation, optical microscopy, scanning electron microscopy, magnetic resonance imaging, and immunohistochemistry. These approaches have provided a better understanding of the composition and dynamics of the human coronary thrombosis process, as well as its relationship with some clinical outcomes. Recent data signaling to new antithrombotic therapeutic targets are still emerging.
Core tip: This paper describes the importance of coronary thrombosis as a direct effector of ST-elevation acute myocardial infarction, reviewing important data provided by coronary aspiration thrombectomy regarding thrombus composition and its relationship with clinical variables. The knowledge of such data is an important basis for improving antithrombotic therapy, as it signals for potential new therapeutic targets.
- Citation: Ribeiro DRP, Cambruzzi E, Schmidt MM, Quadros AS. Thrombosis in ST-elevation myocardial infarction: Insights from thrombi retrieved by aspiration thrombectomy. World J Cardiol 2016; 8(6): 362-367
- URL: https://www.wjgnet.com/1949-8462/full/v8/i6/362.htm
- DOI: https://dx.doi.org/10.4330/wjc.v8.i6.362
Over the past years, improvements in antithrombotic and reperfusion therapies have been associated with decreasing mortality in the setting of ST-elevation acute myocardial infarction (STEMI)[1]. However, coronary artery disease (CAD) remains the leading cause of death worldwide[2], so that efforts are still needed in order to better treat this condition. In most cases, STEMI is caused by the disruption of vulnerable atherosclerotic plaques associated with intense inflammatory activity of a dysfunctional endothelium. Such rupture is the trigger for platelet activation and aggregation and thrombin formation, culminating with total occlusion of the coronary artery by thrombus[3].
Because of the pivotal role of thrombus as a final effector of coronary occlusion and ischemic injury in most cases of acute coronary syndromes, many efforts have been made to improve antithrombotic therapy. For example, antithrombotic drugs like prasugrel and ticagrelor, as compared to clopidogrel, have shown to reduce ischemic events and even mortality in STEMI patients[4,5]. Recently, a large clinical trial demonstrated that double antiplatelet therapy with ASA and ticagrelor significantly reduced the risk of cardiovascular death, myocardial infarction (MI), or stroke in patients with previous MI[6].
Despite of these significant improvements in the medical treatment of patients with CAD, recurrent cardiovascular events still remain the main cause of morbidity and mortality, which justifies further studies to better understand the physiopathology of human coronary thrombosis.
Percutaneous coronary intervention (PCI) has been shown to be the preferred method of reperfusion in patients with ST-elevation acute MI[7]. The high thrombotic burden and the subsequent compromise of coronary flow after dilatation and stent implantation in many cases stimulated the development of adjunctive devices designed to remove thrombi. The manual aspiration thrombectomy (AT) technique was the most successful of such approaches, and it has gained widespread use after the demonstration of improved angiographic results and clinical outcomes in the TAPAS trial[8]. On the other hand, enthusiasm over this technique has substantially waned after recent reports of lack of benefit in the large TASTE and TOTAL trials[9-11].
The demonstration of lack of clinical benefit of AT in these trials is not fully understood yet. One possible explanation is that manual thrombectomy was not effective enough, which is supported by a recent TOTAL substudy using optical coherence tomography[12]. In this analysis, there was no difference between the two groups of patients randomized (routine upfront manual thrombectomy vs PCI alone) with respect to the mean amount of thrombus, although this residual amount was relatively low on average. Another substudy, evaluating angiographic variables, found a 30% reduction in the distal embolization in favor of the thrombectomy group, being this surrogate endpoint an independent predictor of mortality[13]. Assuming that for every 10 patients who have distal embolization, maybe one or two will die related to that, we would expect a reduction of mortality in the range of 10% or 15%, a difference which no trial was powered to detect.
Regardless of the clinical appropriateness of AT in current practice, its development has made possible a new line of investigation, with the opportunity of analyzing fresh specimens of in vivo coronary thrombi, assessing morphology, histology, immunohistochemistry and others[14,15]. Before the availability of this procedure, studies of coronary thrombi were performed mainly by post-mortem analyzes, angioscopy or ex-vivo analysis[16-20]. The information derived from post-mortem studies is reliable, but it is always limited by the selection bias that occur when studying only patients who died. Angioscopy provides in vivo information of thrombi morphology and color, but it has been used rarely due to technical difficulties of the method. Experimental studies, like the Badimon chamber[21] and others, are limited by not evaluating the process of human coronary thrombosis in vivo.
On the other hand, AT is limited by the relative frequent occurrence of unsuccessful procedures, which have been reported in approximately 25% of the patients[8]. Potential causes for failing to retrieve thrombotic material are partial lyses of thrombi by pharmacological therapy administered before arrival in the catheterization laboratory, non-thrombotic lesions, distal embolization before aspiration and limitations of the current aspiration devices. Challenging anatomies for performing AT include tortuous and/or calcified vessels, bifurcations, very distal lesions and small vessels[22].
Thrombus varies widely in shape and size. Arterial thrombi usually are about one centimeter long, arising at the site of an endothelial injury (for example, an atherosclerotic ruptured plaque) in the retrograde direction from the point of anchorage. It generally consists of a tangled network of variable amounts of platelets, fibrin, erythrocytes and degenerate leukocytes[23].
In patients with acute coronary syndromes, there are several factors associated with thrombus size, such as the intensity of anticoagulant and antithrombotic therapy[24,25], the age of the thrombus[14,26], and the presence of flow in the infarct-related artery before primary PCI[18]. Thrombus burden is an established predictor of complications during PCI with or without stents[27,28].
Another condition that may influence the characteristics of coronary thrombi is the presence of diabetes mellitus (DM). In this setting, thrombus area seems to be greater[21] and coronary plaques present greater total and distal plaque load than in those subjects without DM[16]. Moreno et al[29], evaluating coronary tissue retrieved by atherectomy, found a large content of lipid-rich atheroma, macrophage infiltration and subsequent thrombosis in patients with DM.
According to the macroscopic appearance, thrombi can be classified as white, red or mixed. White thrombi are mainly composed of platelets and fibrin[30]. Mizuno and cols showed that white thrombi occur when blood flow was not completely interrupted in the vessel[18]. In patients with STEMI, we have previously demonstrated that white thrombus has a smaller size when compared to red thrombus, and is associated with high fibrin infiltration, shorter ischemic times and lower mortality[31]. Red thrombi are wet, gelatinous and resemble a blood clot being formed by fibrin, erythrocytes and platelets[30], causing complete occlusion of the vessel[18].
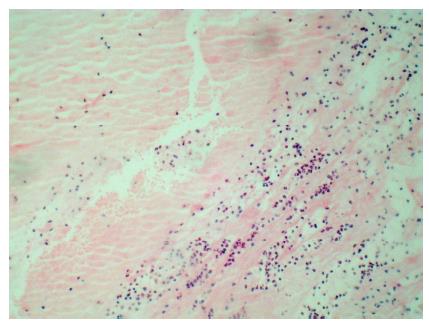
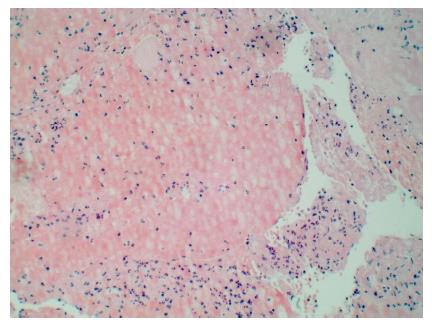
Thrombi can also be classified according to its age: (1) recent (newly formed), composed primarily of fibrin, white blood cells and red blood cells (Figure 1); (2) lytic (intermediate), characterized by the presence of apoptosis of leukocytes (Figure 2); and (3) organized thrombi, classified mainly by presenting collagen and connective soft tissue[14,17].
Rittersma et al[14] assessed coronary thrombi age in 199 STEMI patients submitted to AT within 6 h after onset of chest pain. The authors found that in at least 50% of patients, coronary thrombi were days or weeks old, indicating a variable period of plaque instability and thrombus formation initiated before onset of symptoms. These findings were later confirmed by another report by Kramer et al[26]. In an important study with more than 1300 STEMI patients, fresh thrombus was identified in approximately 30% of the patients. The mortality rates at the 4-year follow-up were significantly higher in patients with older thrombi (16%) when compared to those with fresh thrombus (7%)[32].
Silvain et al[15] used magnetic resonance imaging to evaluate the composition of coronary thrombus and its association with ischemic time. It was found that fibrin content increased with ischemic time, ranging from 48% (< 3 h) up to 67% (> 6 h), whereas platelet content decreased from 21% (< 3 h) to 9% (> 6 h). Multivariate analysis indicated that ischemic time was the only predictor of thrombus composition, with a 2-fold increase of fibrin content per ischemic hour[15].
Immunohistochemistry detects surface proteins in the cells of tissues using the principle of antibodies binding specifically to antigens. It is used in specimens removed surgically or in autopsies. In the assessment of thrombi retrieved by AT, this can also be an additional tool to histopathology, in order to increase the sensitivity for recognition of thrombus components[33,34].
Ikuta et al[35] compared thrombotic material from individuals with stable or unstable angina with immunohistochemistry analysis. The patients with unstable coronary syndromes presented higher platelet aggregation and activation, and also increased immunoreactivity of GP IIb/IIIa and P-selectin[35].
Iwata et al[36] analysed the cellular constituents of 108 thrombi aspirated from coronary lesions in 62 patients who underwent emergent intervention for the treatment of acute (< 24 h) or recent (24-72 h) STEMIs. The content of platelets, as determined by immunostaining for CD42a, presented a negative correlation with the time since the onset of chest pain. The ratio of CD34-positive cells in intracoronary thrombi had a significant positive correlation with restenosis at follow-up coronary angiography. This finding indicates that the early accumulation of primitive cells in platelet aggregates may play a role in neointimal growth after successful coronary intervention in patients with acute coronary syndromes.
Sambola et al[37] compared the content of thrombotic and fibrinolytic factors in thrombi of patients submitted to rescue PCI to those with successful thrombolysis. Thrombi resistant to lysis showed higher content of platelets, fibrin, P-selectin and Von Willebrand Factor, demonstrating a disturbance in thrombus structure of these patients.
Yamashita et al[38] examined thrombi removed within 24 h of acute MI with immunohistochemistry techniques, focusing on possible mechanisms of thrombosis in patients with DM. There was a paucity of CD34-positive cells in the specimens analyzed, suggesting that the ability of these cells to down-regulate thrombus formation and facilitate thrombus organization was compromised in diabetic patients. On the other hand, the higher expression of HMGB-1 found in those with DM, in association with the thrombin-induced microvascular thrombosis accelerated by HMGB-1, may contribute to the adverse events frequently seen in these patients[38].
In the previous sections of this paper, we have described several studies that aimed to investigate the physiopathology of human coronary thrombosis by studying specimens of thrombi retrieved by AT (Table 1). The majority of those studies used techniques such as traditional morphological evaluation, optical microscopy, scanning electron microscopy, magnetic resonance imaging, and immunohistochemistry. More recently, novel approaches have been described.
| Ref. | Main comparison/subject | n | Results |
| Quadros et al[31] | White vs red thrombus | 113 | Mortality (0% vs 10.1%; P = 0.05), size (0.4 ± 0.2 vs 0.6 ± 0.4 mm, P < 0.001), fibrin (68% ± 19% vs 44% ± 18%, P < 0.001), ischemic time (4.5 ± 2.3 h vs 6.1 ± 3.1 h, P = 0.01) |
| Rittersma et al[14] | Age of intracoronary thrombi | 199 | Organized: 9%, lytic changes: 35%, fresh: 49%, both fresh and organized: 7% |
| Kramer et al[26] | Older vs fresh thrombus | 1315 | All-cause mortality at 4 yr (16.2% vs 7.4%, hazard ratio: 1.82, 95%CI: 1.17-2.85, P = 0.008) |
| Silvain et al[15] | Composition of coronary thrombus and its association with ischemic time | 45 | Fibrin content: 48.4% ± 21% (< 3 h) up to 66.9% ± 9% (> 6 h) (P = 0.02) |
| Iwata et al[36] | Restenosis vs without Restenosis | 108 | CD34-positive primitive cells (5.10% ± 0.66% vs 1.88% ± 0.24%, P < 0.01) |
| Sambola et al[37] | Thrombus resistant to fibrinolysis vs sensible to lysis | 20 | Rescue PCI: Significantly higher levels of fibrin (P = 0.016), P-selectin (P = 0.03) and VWF (P = 0.03) than patients who were underwent to primary PCI |
| Yamashita et al[38] | Thrombosis in diabetics vs non diabetics | 50 | Paucity of CD34-positive cells and higher expression of HMGB-1 in diabetics |
Ramaiola et al[39] applied principles of proteomics and advanced cellular microscopy to evaluate retrieved coronary thrombi. The authors showed that profilin-1 (Pfn-1) levels in the systemic circulation are directly correlated to the duration of coronary artery thrombotic occlusion. Thrombus age is an independent predictor of long-term mortality[32], and these results may suggest that measuring Pfn-1 levels could be used to assess ongoing thrombosis and occlusion time in clinical practice[39].
The immune response mediated by lymphocytes is involved in the pathogenesis of the acute coronary syndromes[3], but there is few evidence of the role of T cells in thrombus composition. Regulatory T cells (Treg) are an inherent anti-inflammatory component of adaptive immunity which exerts atheroprotective effects[40-44]. Treg were frequently identified among T cell subsets present in coronary thrombi of patients presenting with ACS[45], which raises the hypothesis of a local compensatory mechanism to attenuate inflammation[46]. The concept of expanding antigen-specific Treg to diminish vascular inflammation and atherothrombosis by immunotherapy is appealing and may represent a new line of investigation[45].
Thrombosis plays a central role in acute coronary syndromes. A better understanding of the human coronary thrombosis process in vivo and its relationship with clinical outcomes could be obtained by analyzes of specimens obtained by AT. Recent data signaling to new therapeutic targets has been recently provided, and insights from this line of investigation will help to further improve management of acute coronary disease.
P- Reviewer: Lazzeri C, Landesberg G S- Editor: Kong JX L- Editor: A E- Editor: Jiao XK
| 1. | Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, Wallentin L. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | WHO Fact sheet N° 310. [updated 2014 May]. Available from: http: //www.who.int/mediacentre/factsheets/fs310/en/. [Cited in This Article: ] |
| 3. | Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004-2013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 781] [Cited by in F6Publishing: 751] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 4. | Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4952] [Cited by in F6Publishing: 4881] [Article Influence: 325.4] [Reference Citation Analysis (0)] |
| 5. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4971] [Cited by in F6Publishing: 4719] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 6. | Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1314] [Cited by in F6Publishing: 1279] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 7. | Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20. [PubMed] [Cited in This Article: ] |
| 8. | Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 799] [Cited by in F6Publishing: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 9. | Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, Kedev S, Thabane L, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemelä K, Steg PG, Bernat I, Xu Y, Cantor WJ, Overgaard CB, Naber CK, Cheema AN, Welsh RC, Bertrand OF, Avezum A, Bhindi R, Pancholy S, Rao SV, Natarajan MK, ten Berg JM, Shestakovska O, Gao P, Widimsky P, Džavík V. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372:1389-1398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 417] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 10. | Fröbert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 771] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 11. | Lagerqvist B, Fröbert O, Olivecrona GK, Gudnason T, Maeng M, Alström P, Andersson J, Calais F, Carlsson J, Collste O. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemelä K, Fung A, Cheema AN, Meeks B, Alexopoulos D. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: the optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J. 2015;36:1892-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Overgaard CB. Angiographic Sub-study of the TOTAL trial: a randomized trial of manual thrombectomy during PCI for STEMI. Paper presented at: EuroPCR; 2015; May 20-23; Paris, France. [Cited in This Article: ] |
| 14. | Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M, de Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 17. | Henriques de Gouveia R, van der Wal AC, van der Loos CM, Becker AE. Sudden unexpected death in young adults. Discrepancies between initiation of acute plaque complications and the onset of acute coronary death. Eur Heart J. 2002;23:1433-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Mizuno K, Satomura K, Miyamoto A, Arakawa K, Shibuya T, Arai T, Kurita A, Nakamura H, Ambrose JA. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992;326:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 468] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Abela GS, Eisenberg JD, Mittleman MA, Nesto RW, Leeman D, Zarich S, Waxman S, Prieto AR, Manzo KS. Detecting and differentiating white from red coronary thrombus by angiography in angina pectoris and in acute myocardial infarction. Am J Cardiol. 1999;83:94-97, A8. [PubMed] [Cited in This Article: ] |
| 20. | Uchida Y, Masuo M, Tomaru T, Kato A, Sugimoto T. Fiberoptic observation of thrombosis and thrombolysis in isolated human coronary arteries. Am Heart J. 1986;112:691-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Viswanathan GN, Marshall SM, Schechter CB, Balasubramaniam K, Badimon JJ, Zaman AG. Thrombus and antiplatelet therapy in type 2 diabetes mellitus. A prospective study after non-ST elevation acute coronary syndrome and a randomised, blinded, placebo-controlled study in stable angina. Thromb Haemost. 2012;108:937-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Vink MA, Kramer MC, Li X, Damman P, Rittersma SZ, Koch KT, van der Wal AC, Tijssen JG, de Winter RJ. Clinical and angiographic predictors and prognostic value of failed thrombus aspiration in primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:634-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Hemodynamic Disorders, Thromboembolic Disease and Shock. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease, Professional Edition. 9th ed. Philadelphia: Elsevier Saunders 2015; 113-135. [Cited in This Article: ] |
| 24. | Niccoli G, Spaziani C, Marino M, Pontecorvo ML, Cosentino N, Bacà M, Porto I, Leone AM, Crea F. Effect of chronic Aspirin therapy on angiographic thrombotic burden in patients admitted for a first ST-elevation myocardial infarction. Am J Cardiol. 2010;105:587-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Goto S. Propagation of arterial thrombi: local and remote contributory factors. Arterioscler Thromb Vasc Biol. 2004;24:2207-2208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kramer MC, van der Wal AC, Koch KT, Rittersma SZ, Li X, Ploegmakers HP, Henriques JP, van der Schaaf RJ, Baan J, Vis MM. Histopathological features of aspirated thrombi after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. PLoS One. 2009;4:e5817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Mabin TA, Holmes DR, Smith HC, Vlietstra RE, Bove AA, Reeder GS, Chesebro JH, Bresnahan JF, Orszulak TA. Intracoronary thrombus: role in coronary occlusion complicating percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;5:198-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 281] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Sianos G, Papafaklis MI, Daemen J, Vaina S, van Mieghem CA, van Domburg RT, Michalis LK, Serruys PW. Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J Am Coll Cardiol. 2007;50:573-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 29. | Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180-2184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 286] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 30. | Pasternack RC, Braunwald E, Sobel BE. Acute myocardial infarction. Heart Disease: a Textbook of Cardiovascular Medicine, Vol 2. 3rd ed. Philadelphia: W.B. Saunders 1988; 1222-313. [Cited in This Article: ] |
| 31. | Quadros AS, Cambruzzi E, Sebben J, David RB, Abelin A, Welter D, Sarmento-Leite R, Mehta RH, Gottschall CA, Lopes RD. Red versus white thrombi in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: clinical and angiographic outcomes. Am Heart J. 2012;164:553-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Kramer MC, van der Wal AC, Koch KT, Ploegmakers JP, van der Schaaf RJ, Henriques JP, Baan J, Rittersma SZ, Vis MM, Piek JJ. Presence of older thrombus is an independent predictor of long-term mortality in patients with ST-elevation myocardial infarction treated with thrombus aspiration during primary percutaneous coronary intervention. Circulation. 2008;118:1810-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Arbustini E, Dal Bello B, Morbini P, Gavazzi A, Specchia G, Viganò M. Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation. 2000;69:1095-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Fukuchi M, Watanabe J, Kumagai K, Katori Y, Baba S, Fukuda K, Yagi T, Iguchi A, Yokoyama H, Miura M. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J Am Coll Cardiol. 2001;37:1436-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Ikuta T, Naruko T, Ikura Y, Ohsawa M, Fukushima H, Shirai N, Itoh A, Haze K, Ehara S, Sasaki Y. Immunolocalization of platelet glycoprotein IIb/IIIa and P-selectin, and neutrophil-platelet interaction in human coronary unstable plaques. Int J Mol Med. 2005;15:573-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Iwata H, Sata M, Ando J, Fujita H, Morita T, Sawaki D, Takahashi M, Hirata Y, Takanashi S, Tabata M. Impact of primitive cells in intracoronary thrombi on lesion prognosis: temporal analysis of cellular constituents of thrombotic material obtained from patients with acute coronary syndrome. Heart. 2010;96:748-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Sambola A, Francisco J, Del Blanco BG, Ruiz-Meana M, Martí G, Otaegui I, Serra V, Barrabes JA, Figueras J, García-Dorado D. Immunohistochemical and molecular characteristics of coronary thrombus resistant to fibrinolysis. J Am Coll Cardiol. 2012;59:E448-E448. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Yamashita A, Nishihira K, Matsuura Y, Ito T, Kawahara K, Hatakeyama K, Hashiguchi T, Maruyama I, Yagi H, Matsumoto M. Paucity of CD34-positive cells and increased expression of high-mobility group box 1 in coronary thrombus with type 2 diabetes mellitus. Atherosclerosis. 2012;224:511-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Ramaiola I, Padró T, Peña E, Juan-Babot O, Cubedo J, Martin-Yuste V, Sabate M, Badimon L. Changes in thrombus composition and profilin-1 release in acute myocardial infarction. Eur Heart J. 2015;36:965-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1387] [Cited by in F6Publishing: 1501] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 41. | Björkbacka H, Fredrikson GN, Nilsson J. Emerging biomarkers and intervention targets for immune-modulation of atherosclerosis - a review of the experimental evidence. Atherosclerosis. 2013;227:9-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. Eur Heart J. 2009;30:2838-2844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178-180. [PubMed] [Cited in This Article: ] |
| 45. | Klingenberg R, Brokopp CE, Grivès A, Courtier A, Jaguszewski M, Pasqual N, Vlaskou Badra E, Lewandowski A, Gaemperli O, Hoerstrup SP. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur Heart J. 2015;36:1041-1048. [PubMed] [Cited in This Article: ] |
| 46. | Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli FR. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J. 2010;31:1457-1469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |