Published online Apr 26, 2014. doi: 10.4330/wjc.v6.i4.140
Revised: March 5, 2014
Accepted: March 13, 2014
Published online: April 26, 2014
Processing time: 150 Days and 2.4 Hours
In population-based studies, including diabetic and nondiabetic cohorts, glycated hemoglobin A1c (HbA1c) has been reported as an independent predictor of all-cause and cardiovascular disease mortality. Data on the prognostic role of HbA1c in patients with acute myocardial infarction (MI) are not univocal since they stem from studies which mainly differ in patients’ selection criteria, therapy (thrombolysis vs mechanical revascularization) and number consistency. The present review is focused on available evidence on the prognostic significance of HbA1c measured in the acute phase in patients with ST-elevation myocardial infarction (STEMI) submitted to primary percutaneous coronary intervention (PCI). We furthermore highlighted the role of HbA1c as a screening tool for glucose intolerance in patients with STEMI. According to available evidence, in contemporary cohorts of STEMI patients submitted to mechanical revascularization, HbA1c does not seem to be associated with short and long term mortality rates. However, HbA1c may represent a screening tool for glucose intolerance from the early phase on in STEMI patients. On a pragmatic ground, an HbA1c test has several advantages over fasting plasma glucose or an oral glucose tolerance test in an acute setting. The test can be performed in the non-fasting state and reflects average glucose concentration over the preceding 2-3 mo. We therefore proposed an algorithm based on pragmatic grounds which could be applied in STEMI patients without known diabetes in order to detect glucose intolerance abnormalities from the early phase. The main advantage of this algorithm is that it may help in tailoring the follow-up program, by helping in identifying patients at risk for the development of glucose intolerance after MI. Further validation of this algorithm in prospective studies may be required in the contemporary STEMI population to resolve some of these uncertainties around HbA1c screening cutoff points.
Core tip: Data on the prognostic role of glycated hemoglobin A1c (HbA1c) in patients with acute myocardial infarction (MI) are not univocal since they stem from studies which mainly differ in patients' selection criteria, therapy (thrombolysis vs mechanical revascularization) and number consistency. According to available evidence, in contemporary cohorts of ST-elevation myocardial infarction (STEMI) patients submitted to mechanical revascularization, HbA1c does not seem to be associated with short and long term mortality. However, in STEMI patients, HbA1c, even measured in the early phase, may represent a screening tool for glucose intolerance since its measurement can be performed in the non-fasting state and reflects average glucose concentration over the preceding 2-3 mo. We therefore proposed an algorithm based on pragmatic grounds which could be applied in STEMI patients without known diabetes in order to detect glucose intolerance abnormalities from the early phase. The main advantage of this algorithm is that it may help in tailoring the follow-up program, by helping in identifying patients at risk for the development of glucose intolerance after MI.
- Citation: Lazzeri C, Valente S, Chiostri M, D’Alfonso MG, Gensini GF. Clinical significance of glycated hemoglobin in the acute phase of ST elevation myocardial infarction. World J Cardiol 2014; 6(4): 140-147
- URL: https://www.wjgnet.com/1949-8462/full/v6/i4/140.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i4.140
Discovered more than forty years ago by Rahbar et al[1], the breakthrough for glycated hemoglobin A1c (HbA1c) was achieved when it was discovered in the Diabetes Control and Complications Trial in 1993 that the concentration of HbA1c was an excellent predictor of diabetes-related long-term complications[2].
In population-based studies[3], including diabetic and nondiabetic cohorts, HbA1c has been reported as an independent predictor of all-cause and cardiovascular disease (CDV) mortality[4-6]. Among individuals with diabetes, every 1% rise in HbA1c is associated with a 30% increase in all-cause mortality and a 40% increase in CVD mortality[7]. In the Reykjavik Study and in a meta-analysis of other Western prospective studies, fasting and post-load glucose levels were modestly associated with coronary heart disease (CHD) risk in people without diabetes[8], while associations of HbA1c with CHD risk in such people appeared somewhat stronger (a RR for CHD of 1.20 per 1% higher HbA1c). In a community-based population study, elevated HbA1c has been recently reported to be predictive for CDV and mortality in patients without diabetes mellitus, regardless of fasting glucose levels[9].
Data on the prognostic role of HbA1c in patients with acute myocardial infarction (AMI) stem from studies which mainly differ for patients’ selection criteria, therapy (thrombolysis vs mechanical revascularization) and number consistency.
The present review is focused on available evidence on the prognostic significance of HbA1c measured in the acute phase in patients with ST-elevation myocardial infarction (STEMI) submitted to primary percutaneous coronary intervention (PCI). We furthermore highlighted the role of HbA1c as a screening tool for glucose intolerance in these patients.
Only small studies assessed the prognostic role of HbA1c in STEMI patients without a history of diabetes and results are not univocal due to differences in patients’ selection criteria and methods[10-13]. In 150 non diabetic patients with myocardial infarction (MI), mortality rate and the risk of cardiogenic shock increased with HbA1c[10]. In a high-risk MI population[12]. HbA1c was a risk marker of death at follow-up in patients without a history of diabetes and not in diabetic patients, while, in a small group of MI patients (diabetic and not diabetic) treated with thrombolysis[11], there were significant relationships between admission glucose, HbA1c level and mortality at follow-up. Similarly, in 374 STEMI patients (diabetic and not diabetic), after adjusting for baseline characteristics, HbA1c remained a strong independent predictor of in-hospital mortality (OR = 1.412; 95%CI: 1.031-1.935, P = 0.03)[14].
On the other hand, in 504 unselected, consecutive non diabetic STEMI patients submitted to PCI, hyperglycemia (not glycated hemoglobin) was a predictor of 30-d outcome[13]. We recently[15] assessed the prognostic role of HbA1c for mortality at short and long terms in 518 consecutive STEMI patients without previously known diabetes, all submitted to mechanical revascularization. Patients with HbA1c ≥ 6.5% showed higher values of admission, peak and discharge glucose (P < 0.001, P < 0.001 and P < 0.001, respectively) and a higher incidence of acute insulin resistance [as inferred by the Homeostatic Model Assessment index (HOMA)] (P = 0.001) as well as higher values of fibrinogen (P < 0.001) and triglycerides (P = 0.001) and lower values of HDL (P = 0.018). No differences in short and long-term mortality rates and in the use of devices were detectable between patients with HbA1c < 6.5% and those with HbA1c ≥ 6.5%. At multivariate backward logistic regression analysis HbA1c was not associated with in-hospital death (OR = 7.210, 95%CI: 0.75-69.69, P = 0.088). At follow-up [median 39.7 (22.2-57.1) mo], a Kaplan-Meier survival curve documented no significant differences between patients with HbA1c < 6.5% and those with HbA1c ≥ 6.5%. In our study population, patients with HbA1c levels higher than 6.5% did not show a higher infarct size (as indicated by TnI and left ventricular ejection fraction) or a more critical illness (as inferred by the use of devices). Discrepancies with previous papers are mainly related to number consistency[10], population selection criteria[11] and type of revascularization[13]. As a difference from previous studies[10,11,13], we observed that higher HbA1c values help in identifying a subset of patients who, in the early phase of STEMI, show an abnormal glucose response to stress as indicated by higher values of glucose, worse glycemic control during Intensive Cardiac Care Union (ICCU) stay (peak glycemia) and a higher incidence of acute insulin resistance (HOMA index). All these factors have been associated with increased risk of early death as reported by Deedwania et al[16] and by us in previous reports[17-21]. Patients with HbA1c > 6.5% also showed an increased inflammatory activation (increased values of fibrinogen), suggesting a link between acute glucose dysmetabolism and inflammatory activation in the early phase of STEMI[16].
Similar results were recently reported by Tian et al[22] in an observational multicenter study performed in 608 STEMI patients submitted to primary PCI. The study population was stratified according to the new American Diabetes Association criteria, into three groups: I, HbA1c 5.6% or less (n = 262); II, HbA1c 5.7%-6.4% (n = 182); and III, HbA1c at least 6.5% (n = 164). The 7-d mortality was similar (P = 0.179) between groups I (1.9%), II (2.2%), and III (0.0%) as well as the 30-d mortality (P = 0.241) between groups I (3.8%), II (2.2%), and III (1.2%). Major adverse cardiac events at the 7-d and 30-d follow-up were not significantly different between the three groups either (P > 0.05). After adjusting the baseline characteristics, HbA1c was not an independent predictor of short-term outcomes (HR = 0.431; 95%CI: 0.175-1.061, P = 0.067).
In patients with AMI and diabetes, the two Diabetes Insulin Glucose in AMI studies both showed that increasing HbA1c levels increased mortality in diabetic patients with MI[23,24]. Conversely[12], in Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan trial (including patients with MI complicated by heart failure) the level of HbA1c had no impact on mortality among the patients with well-known diabetes. Similarly, in consecutive diabetic patients undergoing PCI[25], HbA1c was not a predictor of cardiac events at one-year follow-up.
In a recent investigation[26], which includes the largest series of consecutive STEMI patients with known diabetes submitted to mechanical revascularization, we observed that HbA1c was not associated with mortality in either the short or the long term. Nevertheless, higher HbA1c values (which were detectable in about half of the entire population) helped to identify a subset of patients who, in the early phase of STEMI, showed an abnormal glucose response to stress as indicated by higher values of glucose, a worse glycemic control during ICCU stay (as inferred by peak glycemia) and a higher incidence of acute insulin resistance (as indicated by HOMA index). This subset of patients may deserve a more aggressive treatment for glucose management, since previous studies performed by other intestigators[16] and by us[17-21,26,27] showed that admission glycemia and peak glycemia are independent predictors for in-hospital mortality in STEMI patients.
In the thrombolytic era, in two small studies both excluding patients with newly diagnosed diabetes[28,29], an independent effect on mortality of HbA1c was reported in nondiabetic patients with MI. HbA1c levels higher than 6.5% were associated with higher ischemic score in patients with MI (diabetic and non diabetic) submitted to thrombolysis[13], and significant relationships were observed between admission glucose, HbA1c level and mortality at follow-up. Glycated hemoglobin was a potent risk marker of death at follow-up only in MI patients without a history of diabetes but not in diabetic patients[12]. Conversely, elevated admission glucose (and not glycated hemoglobin) was an important predictor of 30-d outcome after STEMI in 504 unselected, consecutive non diabetic patients with STEMI submitted to PCI[11]. Chan et al[30] reported, in a small cohort of 317 diabetic patients with acute coronary syndrome, that HbA1c levels before admission were not associated with short-term cardiovascular outcome (all-cause mortality, cardiovascular mortality, symptom driven revascularization, rehospitalization for angina, and hospitalization for heart failure).
On the other hand, Timmer et al[31] observed that increasing quartiles of HbA1c (even below the diagnostic threshold for diabetes mellitus) were associated with increased mortality rates over an average 3.3 years of follow-up in 4176 consecutive STEMI patients without known diabetes submitted to PCI. This finding was partially related to the fact that increasing HbA1c levels were associated with adverse baseline characteristics such as a higher cardiovascular risk profile.
In a large contemporary cohort of 1205 consecutive patients with STEMI submitted to PCI, we recently[32] assessed the impact of increased HBA1c (≥ 6.5%) on long term mortality. In our series 276 patients with previously diagnosed diabetes (276/1205, 22.9%, Group A), 78 patients without previously known diabetes and HbA1c ≥ 6.5% (78/1205, 6.5%, Group B) and 851 patients without previously known diabetes and HbA1c < 6.5% (851/1205, 70.1%, Group C). At Cox regression analysis, HbA1c ≥ 6.5% was not related to 1-year post discharge mortality in patients with previously diagnosed diabetes (Group A) nor in those without previously known diabetes (Group B and C). Kaplan-Meier survival curve analysis showed that patients in Group A exhibited the lowest survival rate, while patients in Group B (that is patients without previously known diabetes and with HbA1c ≥ 6.5%) showed a significant reduction in their survival rate since 6-mo after discharge. In conclusion, in our investigation HBA1c levels were not related with outcomes at multivariable analysis in a large cohort of unselected STEMI patients submitted to PCI.
More than 18 million people in the United States have diabetes mellitus, and approximately 35% of the population is prediabetic[33]. Another 7 million Americans have undiagnosed diabetes and are at high risk of developing diabetic complications, including CDV[34,35]. These numbers are expected to continue to rise in the United States and worldwide in large part due to the growing obesity epidemic[36-38]. In 2010, an estimated 6.4% of the world’s adult population (approximately 285 million individuals) had diabetes, and the prevalence is projected to increase to 7.7% (approximately 439 million individuals) by 2030[39].
In the glucose tolerance in AMI study[40], HbA1c independently predicted glucose intolerance (OR = 2.58 95%CI: 1.17-6.09, P = 0.024) in people with acute coronary syndrome without known diabetes, correlating closely with the 2-h plasma glucose in an oral glucose tolerance test (r = 0.39, P < 0.0001). Furthermore, an HbA1c ≥ 30 mmol/mol (4.9%) had sensitivity and specificity of 79% and 49% for detecting undiagnosed diabetes, respectively, with the area under curve of 0.685 (P = 0.001). In the Euro Heart Survey on diabetes, 22% of people admitted to hospital as emergency cases because of coronary artery disease were found to have undiagnosed diabetes after a glucose tolerance test, with a further 36% found to have impaired glucose tolerance[41].
It has been recently observed among patients with high-risk non-ST-segment elevation acute coronary syndrome (NSTE ACS)[42] that a substantial proportion of patients admitted with high-risk NSTE ACS had previously undiagnosed diabetes mellitus (12.2%) or prediabetes (10.8%) as defined by fasting glucose or HbA1c after hospital admission.
Table 1 shows the prevalence of glucose intolerance according to existing investigations on this topic in patients with AMI. These studies were selected by a PubMed search matching “acute myocardial infarction/STEMI/acute coronary syndrome” and “glucose intolerance/hyperglycemia/glycated hemoglobin”.
| Ref. | Patients | Methods | Prevalence | Results |
| Norhammar et al[40], 2002 | 81 non diabetic AMI patients | OGTT | Diabetes: 31% IGT: 35% | HbA1c on admission was independent predictor of glucose intolerance at 3 mo (P = 0.024) |
| Ishihara et al[53], 2006 | 200 non diabetic patients with AMI | OGTT | Diabetes: 27% | Fasting glucose and HbA1c were independent predictors of abnormal glucose tolerance, but admission glucose was not. |
| Gustafsson et al[12], 2007 | 2841 patients with heart failure complicating AMI | HbA1c | History of diabetes: 17% HbA1c < 4.9%: 58% HbA1c 4.9%-5.1%: 15% HbA1c > 5.1%: 10% | In non diabetic patients, a 1% absolute increase in HbA1c level at baseline resulted in a 24% increase in mortality In diabetic patients, the level of HbA1c had no impact on mortality |
| Rasoul et al[11], 2007 | 504 non diabetic STEMI | HbA1c | HbA1c < 6.0%: 82.5% HbA1c > 6.0%: 17.5% | HbA1c was not associated with 30-d mortality |
| Cakmak et al[13], 2008 | 100 non diabetic patients with AMI treated with thrombolysis; patients on antidiabetic therapy excluded | HbA1c | HbA1c 4.5-6.4%: 25% HbA1c 6.5-8.5%: 28% HbA1c > 8.5%: 47% | Admission HbA1c was significantly correlated with mortality (P = 0.009) |
| Knudsen et al[47], 2009 | 224 non diabetic STEMI | OGTT | Abnormal glucose regulation: 46.9% in the early phase 24.9% at 3 mo | High levels of HbA1c and admission plasma glucose in-hospital significantly predicted abnormal glucose regulation at 3 mo (P < 0.001) |
| Timmer et al[31], 2011 | 4176 non diabetic STEMI patients | HbA1c quartiles | IQR1 ≤ 5.35%: 27% IQR2 5.6%-5.54%: 24% IQR3 5.55%-5.80%: 25% IQR4 ≥ 5.81%: 24% | HbA1c (hazard ratio, 1.2 per interquartile range; P < 0.01), but not glucose, was independently associated with long-term mortality |
| Lazzeri et al[15], 2012 | 518 non diabetic STEMI patients | HbA1c | HbA1c < 6.5%: 90.4% HbA1 c ≥ 6.5%: 9.6% | HbA1c was not associated with short and long term mortality |
| Tian et al[22], 2013 | 608 STEMI | Hb1c groups | I: HbA1c ≤ 5.6%: 43% II: HbA1c 5.7%-6.4%: 30% III: HbA1c ≥ 6.5%: 27% | After adjusting the baseline characteristics, HbA1c was not an independent predictor of short-term outcomes (HR = 0.431; 95%CI: 0.175-1.061, P = 0.067) |
| Lazzeri et al[32], 2013 | 1204 STEMI patients | HbA1c | Diabetic patients: 22.9% patients without known diabetes: HbA1c < 6.5%: 70.1% HbA1c ≥ 6.5%: 6.5% | At Cox regression analysis, HbA1c ≥ 6.5% was not related to 1-yr post discharge mortality in diabetic and in non diabetic patients |
The prevalence of STEMI patients with glucose intolerance, as detected mainly by HbA1c measured in the early phase, varies, ranging from 10% to more than 40%. Differences can be mainly related to the chosen value of HbA1c. More recently, in an observational multicenter study, Tian et al[22] stratified the study population according to HbA1c values and observed that the percentage of patients with HbA1c > 5.7% accounted for more than 50%.
On a clinical ground, in STEMI patients, early diagnosis of unknown type 2 diabetes or impaired glucose regulation allows initiation of treatment or lifestyle interventions, including diet and exercise to prevent type 2 diabetes and associated complications. Gaining information on family history for diabetes could help in identifying subjects with undiagnosed diabetes or at risk[43,44].
However, in the acute phase of STEMI, the identification of glucose intolerance is quite difficult since the common finding of hyperglycemia, irrespective of underlying diabetic status, is to be related mainly to the acute stress response[16-21,26] to myocardial ischemia[45].
Recently, National Institute for Health and Clinical Excellence (NICE) guidelines on the management of hyperglycaemia in acute coronary syndrome have advocated any hyperglycaemia (blood glucose > 11.0 mmol/L) without known diabetes be followed up with an HbA1c measurement before discharge and fasting plasma glucose test 4 d after the onset of acute coronary syndrome[46]. NICE recommend against routine use of the oral glucose tolerance test in patients with acute coronary syndrome and with fasting plasma glucose and HbA1c in the normal range. However, guidance on categorization of glycaemic status of those with elevated HbA1c and fasting plasma glucose, as well as screening for diabetes in those without hyperglycaemia, is less clear. As a consequence, the lack of simple strategy for early identification of glucose intolerance in acute coronary syndrome is potentially leaving many people undiagnosed and under-treated, especially after the cardiac event.
The oral glucose tolerance test is performed infrequently in the acute setting[41], since it is time consuming, not always well tolerated and it does not seem to provide reliable information on long-term glucometabolic state[47].
In the early phase of STEMI, fasting plasma glucose can be acutely elevated and therefore unreliable in the first 2 d of an acute event and in a large MI[48]. NICE has suggested fasting plasma glucose testing should not be conducted within the first 4 d of the acute event. However, in the current era of early reperfusion therapies, many patients with acute coronary syndrome are discharged earlier.
On a pragmatic ground, an HbA1c test has several advantages over fasting plasma glucose or an oral glucose tolerance test in an acute setting. The test can be performed in the non-fasting state and reflects average glucose concentration over the preceding 2-3 mo. Therefore, in our opinion, glycated hemoglobin should be measured in all patients with STEMI.
Measuring HbA1c assumes International Federation of Clinical Chemistry standardized laboratory assays are used. Furthermore, conditions precluding accurate measurement of HbA1c concentration for diagnosis should be excluded, including abnormalities of red cell turnover, chronic renal or liver failure and chronic use of certain medications.
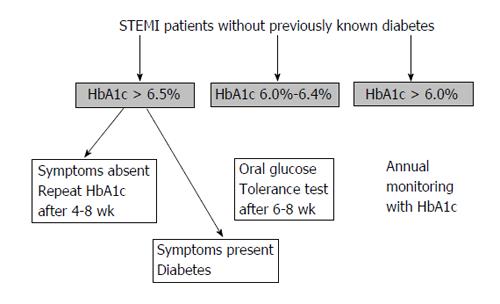
We therefore proposed an algorithm (Figure 1) based on pragmatic grounds (and our experience) which should be applied in STEMI patients without known diabetes in order to detect glucose intolerance abnormalities since the early phase.
Above HbA1c > 6.5%, individuals should be assessed for symptoms of diabetes (i.e., increased thirst, polyuria, unexplained weight loss, blurred vision, extreme fatigue), ruling out other causes, for example polyuria attributable to diuretic therapy. In those with unequivocal symptoms the diagnosis is confirmed[49]. Conversely, those with ambiguous or absent symptoms should undergo a confirmatory HbA1c measurement 4-8 wk post-discharge for consistency and to counteract any potential laboratory errors on the first occasion.
Patients with HbA1c between 6.0% and < 6.4% should undergo an oral glucose tolerance test after 6-8 wk.
STEMI patients without known diabetes and HbA1c < 6.0% should undergo annual surveillance with HbA1c as incident impaired glucose regulation and diabetes is higher compared with the general population[50].
The main advantage of this algorithm is that it may help in tailoring the follow-up program, by helping to identify patients at risk for the development of glucose intolerance after MI.
Further validation of this algorithm in prospective studies may be required in the contemporary STEMI population to resolve some of these uncertainties around HbA1c screening cut points.
Given the increasing focus on managing multiple coexisting illnesses affecting cardiovascular patients[51], the assessment of glycosylated hemoglobin (HbA1c) in patients with STEMI could be an important opportunity to improve care for these patients[52].
P- Reviewers: Ciccone MM, Hwang KC, Liu PY, Loffredo L, Sethi A, Wan Y S- Editor: Wen LL L- Editor: O’Neill M E- Editor: Liu SQ
| 1. | Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 219] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 2. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16227] [Article Influence: 507.1] [Reference Citation Analysis (3)] |
| 3. | Kishore P, Kim SH, Crandall JP. Glycemic control and cardiovascular disease: what’s a doctor to do? Curr Diab Rep. 2012;12:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Gall MA, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving HH. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Agewall S, Wikstrand J, Ljungman S, Fagerberg B. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol. 1997;80:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Goff DC, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, Buse JB, Genuth S, Probstfield JL, Simons-Morton DG. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:4i-20i. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Khaw KT, Wareham N, Luben R, Bingham S, Oakes S, Welch A, Day N. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ. 2001;322:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 617] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 8. | Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, Sigurdsson G, Danesh J, Gudnason V. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7:e1000278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1161] [Cited by in RCA: 1087] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 10. | Oswald GA, Corcoran S, Yudkin JS. Prevalence and risks of hyperglycaemia and undiagnosed diabetes in patients with acute myocardial infarction. Lancet. 1984;1:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Rasoul S, Ottervanger JP, Bilo HJ, Timmer JR, van ‘t Hof AW, Dambrink JH, Dikkeschei LD, Hoorntje JC, de Boer MJ, Zijlstra F. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med. 2007;65:95-100. [PubMed] |
| 12. | Gustafsson I, Kistorp CN, James MK, Faber JO, Dickstein K, Hildebrandt PR. Unrecognized glycometabolic disturbance as measured by hemoglobin A1c is associated with a poor outcome after acute myocardial infarction. Am Heart J. 2007;154:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 13. | Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, Kilic ID. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol. 2008;24:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Cicek G, Uyarel H, Ergelen M, Ayhan E, Abanonu GB, Eren M, Gibson CM. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Lazzeri C, Valente S, Chiostri M, Picariello C, Attanà P, Gensini GF. Glycated hemoglobin in ST-elevation myocardial infarction without previously known diabetes: its short and long term prognostic role. Diabetes Res Clin Pract. 2012;95:e14-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2008;117:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 319] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Lazzeri C, Chiostri M, Sori A, Valente S, Gensini GF. Postprocedural hyperglycemia in ST elevation myocardial infarction submitted to percutaneous coronary intervention: a prognostic indicator and a marker of metabolic derangement. J Cardiovasc Med (Hagerstown). 2010;11:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Lazzeri C, Sori A, Chiostri M, Gensini GF, Valente S. Prognostic role of insulin resistance as assessed by homeostatic model assessment index in the acute phase of myocardial infarction in nondiabetic patients submitted to percutaneous coronary intervention. Eur J Anaesthesiol. 2009;26:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Correlates of acute insulin resistance in the early phase of non-diabetic ST-elevation myocardial infarction. Diab Vasc Dis Res. 2011;8:35-42. [PubMed] |
| 20. | Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Acute glucose dysmetabolism in the early phase of ST-elevation myocardial infarction: the age response. Diab Vasc Dis Res. 2010;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. In-hospital peak glycemia and prognosis in STEMI patients without earlier known diabetes. Eur J Cardiovasc Prev Rehabil. 2010;17:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Hemoglobin A1c and short-term outcomes in patients with acute myocardial infarction undergoing primary angioplasty: an observational multicenter study. Coron Artery Dis. 2013;24:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 670] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 24. | Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 531] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Lemesle G, Bonello L, de Labriolle A, Maluenda G, Syed AI, Collins SD, Ben-Dor I, Torguson R, Kaneshige K, Xue Z. Prognostic value of hemoglobin A1C levels in patients with diabetes mellitus undergoing percutaneous coronary intervention with stent implantation. Am J Cardiol. 2009;104:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Lazzeri C, Valente S, Chiostri M, Picariello C, Attanà P, Gensini GF. The prognostic impact of glycated hemoglobin in diabetic ST-elevation myocardial infarction. Int J Cardiol. 2011;151:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Predictors of the early outcome in elderly patients with ST elevation myocardial infarction treated with primary angioplasty: a single center experience. Intern Emerg Med. 2011;6:41-46. [PubMed] |
| 28. | Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004;25:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Chan CY, Li R, Chan JY, Zhang Q, Chan CP, Dong M, Yan BP, Lam YY, Yu CM. The value of admission HbA(1c) level in diabetic patients with acute coronary syndrome. Clin Cardiol. 2011;34:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Timmer JR, Hoekstra M, Nijsten MW, van der Horst IC, Ottervanger JP, Slingerland RJ, Dambrink JH, Bilo HJ, Zijlstra F, van ‘t Hof AW. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Lazzeri C, Valente S, Chiostri M, Attanà P, Mattesini A, Nesti M, Gensini GF. Glycated hemoglobin and long term mortality in STEMI patients. JCM;. 2013;(in press). |
| 33. | Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3702] [Cited by in RCA: 3713] [Article Influence: 265.2] [Reference Citation Analysis (0)] |
| 34. | Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 838] [Cited by in RCA: 850] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 35. | Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1740] [Cited by in RCA: 1425] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 36. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8947] [Article Influence: 426.0] [Reference Citation Analysis (1)] |
| 37. | Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2447] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 38. | King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3616] [Cited by in RCA: 3339] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 39. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4361] [Article Influence: 290.7] [Reference Citation Analysis (4)] |
| 40. | Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc S, Rydén L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 717] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 41. | Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, Standl E, Soler-Soler J, Ohrvik J. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur Heart J. 2004;25:1880-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 42. | Giraldez RR, Clare RM, Lopes RD, Dalby AJ, Prabhakaran D, Brogan GX, Giugliano RP, James SK, Tanguay JF, Pollack CV. Prevalence and clinical outcomes of undiagnosed diabetes mellitus and prediabetes among patients with high-risk non-ST-segment elevation acute coronary syndrome. Am Heart J. 2013;165:918-925.e2. [PubMed] |
| 43. | Pannacciulli N, De Pergola G, Ciccone M, Rizzon P, Giorgino F, Giorgino R. Effect of family history of type 2 diabetes on the intima-media thickness of the common carotid artery in normal-weight, overweight, and obese glucose-tolerant young adults. Diabetes Care. 2003;26:1230-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | De Pergola G, Ciccone M, Pannacciulli N, Modugno M, Sciaraffia M, Minenna A, Rizzon P, Giorgino R. Lower insulin sensitivity as an independent risk factor for carotid wall thickening in normotensive, non-diabetic, non-smoking normal weight and obese premenopausal women. Int J Obes Relat Metab Disord. 2000;24:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Gholap N, Davies MJ, Mostafa SA, Squire I, Khunti K. A simple strategy for screening for glucose intolerance, using glycated haemoglobin, in individuals admitted with acute coronary syndrome. Diabet Med. 2012;29:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Senthinathan A, Kelly V, Dzingina M, Jones D, Baker M, Longson D. Hyperglycaemia in acute coronary syndromes: summary of NICE guidance. BMJ. 2011;343:d6646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Knudsen EC, Seljeflot I, Abdelnoor M, Eritsland J, Mangschau A, Arnesen H, Andersen GO. Abnormal glucose regulation in patients with acute ST- elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol. 2009;8:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Hage C, Malmberg K, Rydén L, Wallander M. The impact of infarct type on the reliability of early oral glucose tolerance testing in patients with myocardial infarction. Int J Cardiol. 2010;145:259-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | World Health Organization. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation. Geneva: World Health Organization 2011; 1-25. |
| 50. | Mozaffarian D, Marfisi R, Levantesi G, Silletta MG, Tavazzi L, Tognoni G, Valagussa F, Marchioli R. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet. 2007;370:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Werner RM, Greenfield S, Fung C, Turner BJ. Measuring quality of care in patients with multiple clinical conditions: summary of a conference conducted by the Society of General Internal Medicine. J Gen Intern Med. 2007;22:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Stolker JM, Sun D, Conaway DG, Jones PG, Masoudi FA, Peterson PN, Krumholz HM, Kosiborod M, Spertus JA. Importance of measuring glycosylated hemoglobin in patients with myocardial infarction and known diabetes mellitus. Am J Cardiol. 2010;105:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Hata T, Nakama Y, Kijima Y, Kagawa E. Is admission hyperglycaemia in non-diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance? Eur Heart J. 2006;27:2413-2419. [PubMed] |