Published online Jul 26, 2010. doi: 10.4330/wjc.v2.i7.205
Revised: June 11, 2010
Accepted: June 18, 2010
Published online: July 26, 2010
AIM: To assess coronary endothelial function of conduit and resistance vessels in patients with metabolic syndrome (MS).
METHODS: Seventy-eight men (mean age, 57 years) with chest pain and angiographically normal coronary arteries were included in the study. Patients with coronary spastic angina were excluded. Changes in coronary artery diameter and coronary blood flow (CBF) in response to acetylcholine (ACh) were determined using quantitative coronary angiography and Doppler velocity measurements. Coronary flow reserve was calculated as the ratio of coronary blood velocity after adenosine triphosphate infusion relative to baseline values. Patients were divided into two groups based on the presence or absence of MS.
RESULTS: There were 24 patients in the MS group (31%). The increase in CBF in response to ACh infusion was impaired in the MS group (P < 0.0001) compared to the non-MS group, whereas changes in coronary artery diameter in response to ACh infusion did not differ between the two groups. Multivariate regression analysis revealed that MS was a significant factor associated with the lesser change in CBF induced by ACh infusion at 30 μg/min (P < 0.0001, r2 = 0.46).
CONCLUSION: Coronary endothelial dysfunction was present at the level of resistance vessels but not conduit vessels in the MS patients included in our study.
- Citation: Teragawa H, Mitsuba N, Nishioka K, Ueda K, Kono S, Higashi Y, Chayama K, Kihara Y. Impaired coronary microvascular endothelial function in men with metabolic syndrome. World J Cardiol 2010; 2(7): 205-210
- URL: https://www.wjgnet.com/1949-8462/full/v2/i7/205.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i7.205
The metabolic syndrome (MS) is characterized by abdominal obesity, elevated blood pressure, hypertriglycemia, low high-density lipoprotein (HDL) cholesterolemia, and hyperglycemia[1]. Several studies have revealed that MS is a factor that may be responsible for future cardiovascular events, independent of racial differences[2-6].
Studies have also indicated the presence of endothelial dysfunction assessed using several modalities, such as positron emission tomography, flow-mediated dilation in the brachial artery, and venous occlusion plethysmography, in MS patients[7-13]. However, no studies have investigated coronary endothelial function using quantitative coronary angiography and Doppler velocity measurements, which can provide insight into the dynamic biology of the endothelium of coronary arteries at the level of conduit and resistance vessels and can also provide prognostic information for risk stratification in the later clinical phase[14,15]. Therefore, we investigated the relationship between coronary endothelial function at the level of conduit and resistance vessels and the presence of MS in patients with chest pain and angiographically normal coronary arteries.
Seventy-eight Japanese men who underwent coronary angiography to evaluate chest pain were included in this study. All patients had angiographically normal epicardial coronary arteries, normal left ventricular function (contrast ventriculographic ejection fraction ≥ 60%), and normal coronary flow reserve (CFR > 2.0). We excluded patients with coronary spastic angina, previous myocardial infarction, left ventricular hypertrophy (LVH), moderate-severe valvular disease detected by echocardiography (UCG), heart failure or other serious diseases. Written informed consent was obtained from all patients prior to entry into the study. The protocol was approved by the Ethics Committee of our institution.
All anti-anginal agents were discontinued at least 48 h prior to catheterization, except for the unrestricted use of sublingual nitroglycerin, which was withheld for 1 h prior to catheterization. Diagnostic left heart catheterization and coronary angiography were performed using a standard percutaneous brachial approach. A 6F guide catheter was introduced into the left main coronary artery. A 0.0014-inch Doppler flow guidewire (Volcano FloWire; Volcano Therapeutics Inc., Rancho Cordova, CA) was advanced through the guide catheter into the proximal segment of the left anterior descending coronary artery. The wire tip was positioned in a straight segment of the vessel to obtain a reliable flow-velocity signal.
After baseline control conditions were established, incremental doses of acetylcholine (ACh) were infused into the left coronary artery (3 μg/min and 30 μg/min) for 2 min with 5-min intervals between consecutive doses. After re-establishment of control conditions, nitroglycerin was infused intracoronarily at the rate of 200 μg/min for 1 min. Finally, adenosine triphosphate (20 μg) was infused. ACh and nitroglycerin were infused directly into the left coronary ostium using an infusion pump (TE-311; Terumo, Tokyo, Japan) at the rate of 1 mL/min.
Coronary angiography was performed under controlled conditions and at the end of each drug infusion. Coronary blood flow (CBF) velocity was monitored continuously using a 12-MHz pulsed Doppler velocimeter (FloMap; Volcano Therapeutics Inc. Rancho Cordova, CA). Arterial pressure, heart rate and ECG were monitored continuously and recorded using a multichannel recorder (Polygraph 1600; Nihon Electric Corporation, Tokyo, Japan).
The method for measuring coronary diameter has been previously described in detail[16,17]. The coronary segment 2 mm distal to the Doppler wire tip was selected for quantitative analysis. In each patient, luminal diameters of selected segments of the left anterior descending coronary artery were measured by a single investigator blinded to angiographic and clinical data in order to determine the effects of different drugs on epicardial coronary diameter. Luminal diameters were measured on an end-diastolic frame using a computer-assisted coronary angiographic analysis system (CAAS II/QUANTCOR; Siemens, Berlin and Munich, Germany). Means of triplicate measurements of luminal diameter were used for analysis. Changes in coronary diameter in response to ACh and nitroglycerin infusions are expressed as percent change from the baseline measurement on the angiogram obtained prior to infusion. Intra- and inter-observer variability have previously been reported to be excellent[18].
CBF was calculated as the product of CBF velocity and vessel diameter using the following formula: π× average peak velocity × 0.125 × diameter2. For CBF calculations, the internal diameter of the vessel at the location of the flow measurements (2 mm distal to the wire tip) was measured using the method described above. CFR was calculated as the ratio of CBF velocity after an adenosine triphosphate infusion to the baseline velocity.
The presence of MS was determined according to the final report of the National Cholesterol Education Program’s Adult Treatment Panel III criteria[1]. The above-mentioned criteria may not be suitable for determining abdominal obesity in Japanese patients; therefore, we adopted the Japanese criteria for abdominal obesity (waist circumference ≥ 85 cm in men) in the present study. Consequently, we defined MS as the presence of at least three of the following factors: (1) waist circumference ≥ 85 cm; (2) fasting triglycerides > 150 mg/dL; (3) HDL cholesterol < 40 mg/dL; (4) hypertension (systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg, or use of antihypertensive drug therapy); and (5) fasting glucose ≥ 110 mg/dL. The patients were divided into the following two groups based on the presence or absence of MS: the MS group comprising patients with MS and the non-MS group comprising patients without MS. In addition, the MS score was defined as the sum of the MS factors (0-5) that were present.
In each patient, total cholesterol, triglycerides, HDL-cholesterol, low-density lipoprotein cholesterol, glucose, insulin, hemoglobin A1C, and high-sensitive C-reactive protein (CRP) were measured. The homeostasis model assessment-insulin resistance (HOMA-IR) index was calculated as the fasting glucose (mg/dL) × fasting insulin level (μU/mL)/405.
All data were expressed as mean ± SE. Baseline characteristics of the two groups were compared with Student’s unpaired t-test or by χ2 analysis, as appropriate. Serial changes in hemodynamic variables and changes in coronary vasoreactivity in response to drug infusion were compared using a one-way analysis of variance. If the analysis of variance showed a significant difference between means, the level of significance was determined by contrast analysis. Serial percent changes in the coronary vascular response to ACh infusion were compared between groups using a two-way analysis of variance. We used Spearman’s rank correlation to investigate the relationship between the MS score and change in CBF induced by ACh infusion. We performed uni- and multivariate regression analyses to identify factors associated with percent changes in CBF induced by ACh. A P value < 0.05 indicated statistical significance.
The patients’ characteristics are indicated in Table 1. There were 24 patients with MS (31%). Body mass index, waist circumference and the frequency of having each MS factor were higher in the MS group than in the non-MS group. The average MS score was significantly higher in the MS group than in the non-MS group.
| Variables | MS group | Non-MS group | P value |
| n (%) | 24 (31) | 56 (69) | |
| Age (yr) | 57 ± 2 | 57 ± 2 | NS |
| Body mass index (kg/m2) | 26.4 ± 0.6 | 24.3 ± 0.3 | 0.0089 |
| Waist circumference (cm) | 90 ± 1 | 86 ± 1 | 0.0114 |
| MS risk factors, n (%) | |||
| Abdominal obesity | 22 (92) | 32 (59) | 0.0042 |
| Elevated triglyceride | 18 (75) | 22 (41) | 0.0012 |
| Low HDL cholesterol | 11 (46) | 5 (9) | 0.0002 |
| Hypertension | 17 (71) | 15 (28) | 0.0004 |
| Hyperglycemia | 11 (46) | 8 (15) | 0.0032 |
| Average MS score | 3.3 ± 0.1 | 1.5 ± 0.1 | < 0.0001 |
Data for other conventional risk factors, including biochemical parameters and medications taken, are also indicated in Table 2. The triglyceride, fasting blood sugar, hemoglobin A1C, and HOMA-IR levels were higher in the MS group and the level of HDL cholesterol was lower in the MS group compared with the non-MS group. The frequency of medication intake was similar in the two groups.
| Variables | MS group | Non-MS group | P value |
| Other risk factors | |||
| Smoking | 13 (54) | 16 (35) | NS |
| Hypercholesterolemia1 | 6 (25) | 10 (18) | NS |
| Total cholesterol (mg/dL) | 206 ± 7 | 194 ± 5 | NS |
| Triglyceride (mg/dL) | 205 ± 17 | 157 ± 11 | 0.0218 |
| HDL cholesterol (mg/dL) | 44 ± 3 | 52 ± 2 | 0.0204 |
| LDL cholesterol (mg/dL) | 121 ± 6 | 111 ± 4 | NS |
| Diabetes mellitus | 5 (21) | 5 (9) | 0.0189 |
| Fasting blood sugar (mg/dL) | 107 ± 3 | 98 ± 2 | 0.0189 |
| Hemoglobin A1C | 5.7 ± 0.1 | 5.4 ± 0.1 | 0.0445 |
| HOMA-IR | 2.5 ± 0.2 | 1.7 ± 0.1 | 0.0046 |
| C-reactive protein (mgl/L) | 1.7 ± 0.3 | 1.3 ± 0.2 | NS |
| Medications | |||
| Statins | 1 (4) | 6 (11) | NS |
| ACI or ARB | 2 (8) | 1 (2) | NS |
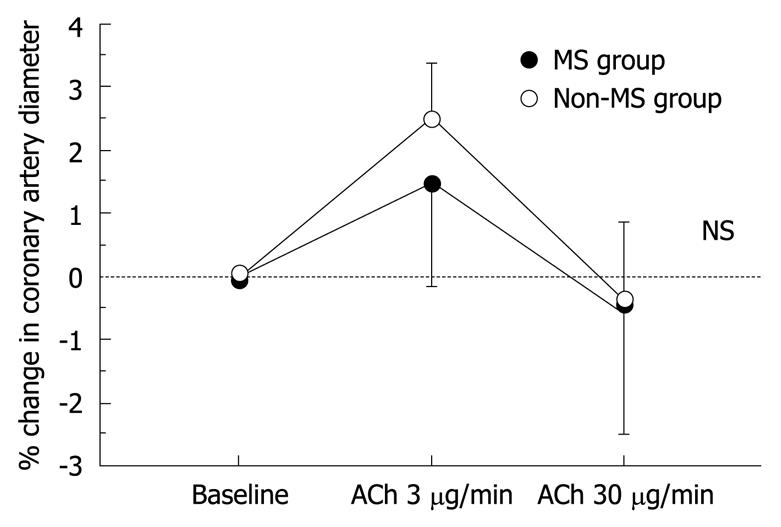
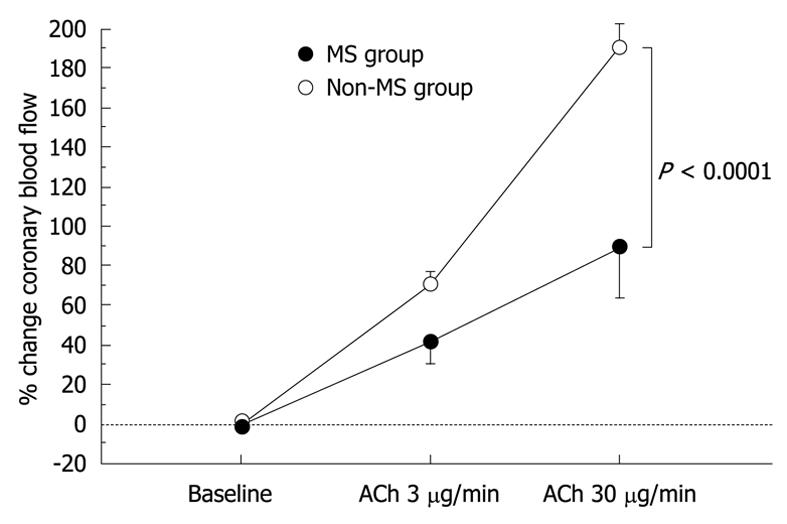
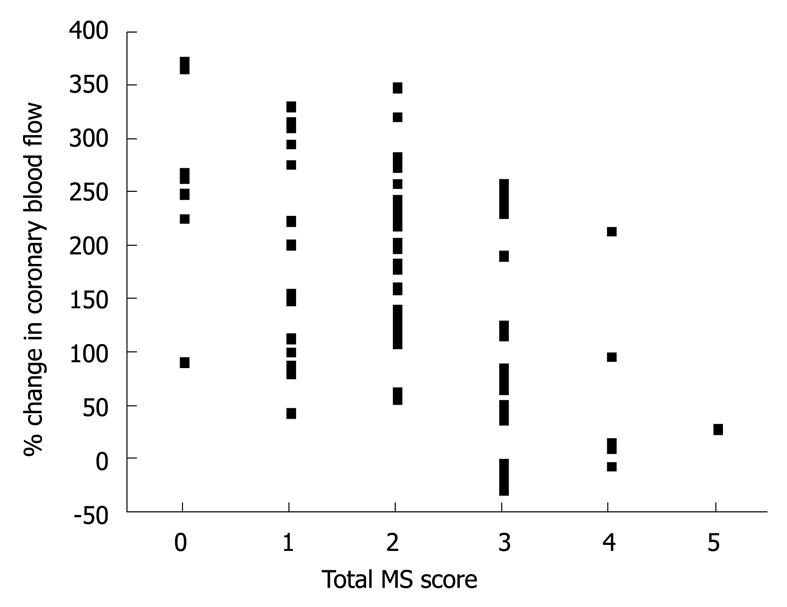
The results of hemodynamics and coronary vasoreactivity are indicated in Table 3. The mean blood pressure was higher in the MS group. The baseline coronary artery diameter and CBF were similar in the two groups. Changes in coronary artery diameter in response to ACh infusion and NTG-induced coronary dilation also did not differ between the two groups (Figure 1 and Table 3). However, the increase in CBF in response to ACh infusion was impaired in the MS group compared to the non-MS group (P < 0.0001, Table 3 and Figure 2). However, CFR did not differ between the two groups (Table 3). Statistical significance between the MS and non-MS groups was more prominent in percent change in CBF induced by ACh infusion at a dose of 30 μg/min, and subsequent analyses were performed using this value. The total MS scores were negatively associated with the increase in CBF in response to infusion of ACh at 30 μg/min (r = -0.51, P < 0.0001, Figure 3).
| Variables | MS group | Non-MS group | ||
| Value | % change | Value | % change | |
| Baseline mean blood pressure (mmHg) | 111 ± 2a | 104 ± 2 | ||
| Baseline heart rate (/min) | 67 ± 2 | 64 ± 1 | ||
| Coronary diameter (mm) | ||||
| Baseline | 3.26 ± 0.11 | 0 | 3.11 ± 0.07 | 0 |
| ACh at 3 μg/min | 3.30 ± 0.12 | 1.6 ± 1.4 | 3.18 ± 0.08 | 2.6 ± 0.7 |
| ACh at 30 μg/min | 3.23 ± 0.13 | -0.5 ± 1.9 | 3.08 ± 0.09 | -0.5 ± 1.3 |
| Nitroglycerin | 3.67 ± 0.12 | 13.9 ± 2.3 | 3.54 ± 0.08 | 14.7 ± 1.6 |
| Coronary blood flow (mL/min) | ||||
| Baseline | 77 ± 6 | 0 | 65 ± 4 | 0 |
| ACh at 3 μg/min | 110 ± 14 | 38 ± 9b | 114 ± 9 | 73 ± 6 |
| ACh at 30 μg/min | 137 ± 20a | 82 ± 18d | 190 ± 13 | 198 ± 12 |
| Coronary flow reserve | 3.3 ± 0.2 | 3.6 ± 0.1 | ||
Analysis of individual MS factors indicated that elevated triglycerides (P = 0.0246), low HDL cholesterol (0.0409), elevated blood pressure (0.0032), and hyperglycemia (P = 0.0309) were associated with a lower change in CBF in response ACh infusion at 30 μg/min. Univariate analysis revealed that the presence of MS (P < 0.0001), reduced CFR (P = 0.0003) and an elevated CRP level (P = 0.0027) were associated with a lower CBF response induced by ACh infusion at 30 μg/min; a high heart rate at baseline also tended to be associated with the reduced response. Multivariate regression analysis using these parameters demonstrated that the presence of MS (P < 0.0001) as well as reduced CFR (P = 0.0005) and elevated CRP (P = 0.0234) were significant factors associated with the reduced CBF response induced by ACh infusion at 30 μg/min (r2 = 0.46, Table 4).
| Variables | % change in coronary blood flow induced by ACh 30μg/min | |
| t value | P value | |
| Presence of MS | -5.07 | < 0.0001 |
| Coronary flow reserve | 3.62 | 0.0005 |
| C-reactive protein | -2.76 | 0.0073 |
| Baseline heart rate | -1.13 | 0.262 |
The present study revealed that coronary endothelial function at the level of resistance vessels is impaired in MS patients while that at the level of conduit vessels is similar among both MS and non-MS patients. Multivariate regression analysis demonstrated that the presence of MS was a significant factor associated with impaired coronary endothelial function at the level of resistance vessels.
Many reported studies have used several modalities to investigate the relationship between MS and coronary microvascular circulation. PET analysis has revealed that the increase in myocardial blood flow in response to a cold pressor test is impaired in MS patients[7], indicating the presence of coronary microvascular endothelial dysfunction; this is in accordance with the results obtained in the present study. On the other hand, Pirat et al[19], using UCG, have reported an impaired CFR in the LAD of coronary arteries in patients with MS. Furthermore, Turhan et al[20] reported an impaired CBF using the Thrombolysis in Myocardial Infarction frame count method in MS patients with angiographically normal coronary arteries. The purpose of the present study was to assess ACh-induced coronary vasomotion and circulation, and thus our study protocol excluded patients with several conditions, such as severely reduced CFR (< 2.0) or LVH, which are frequently observed in MS patients. Therefore, differences in patient selection and other patient characteristics may contribute to the discrepancy in the results. In all the above-mentioned studies, the presence of coronary endothelial dysfunction at the level of the resistance vessels was shown in MS patients; however, no studies have investigated coronary endothelial function at the level of the conduit vessels in MS patients. The present study revealed that coronary endothelial function at the level of the conduit vessels was not reduced in response to ACh. This finding suggests that coronary endothelial function at the level of the resistance vessels is impaired earlier and is more prominent than that at the level of conduit vessels. The patients in our study exhibited chest symptoms, even in the non-MS group, and it was not clarified whether coronary endothelial function, especially at the level of the conduit vessels, was preserved in such patients. Nonetheless, the finding that coronary microvascular endothelial dysfunction was severely impaired in the MS group is certain and clarifies the impact of MS on pathogenesis of the coronary artery vasculature. If the degree of MS is severe and its duration is longer, coronary endothelial dysfunction at the level of conduit vessels may be evident after the establishment of coronary microvascular endothelial dysfunction.
There are several possible mechanisms responsible for MS-induced coronary endothelial dysfunction. Several studies have revealed that insulin resistance may induce endothelial dysfunction mediated by oxidative stress[21,22] and decreases in insulin-dependent activation of endothelial nitric oxide synthase (eNOS)[23]. Furthermore, it has been reported that adiponectin may play a role in the phosphorylation of eNOS[24] and reduced adiponectin, which is often recognized in MS patients, may lead to endothelial dysfunction. In addition, it has been reported that pericardial fat tissue, which is increased in MS patients, discharges several cytokines systemically and locally[25,26], indicating the possibility that impairment of coronary endothelial function may be caused by pericardial fat tissue. These mechanisms may solely and/or multifactorially contribute to coronary endothelial dysfunction in MS patients.
The present study revealed that coronary endothelial dysfunction at the level of resistance vessels was more prominent than that at the level of conduit vessels in MS patients. Until now, there has been no data available indicating which is the stronger factor; i.e. coronary endothelial dysfunction at the level of conduit vessels or at that of resistance vessels, affecting future cardiovascular events. However, Halcox et al[15] have reported that if coronary endothelial dysfunction is present either at the level of conduit or resistance vessels, future cardiovascular events occur more frequently. Thus, the finding that coronary microvascular endothelial dysfunction is more prominent in MS patients may provide important information clarifying the pathogenesis of MS-induced cardiovascular events. The present study also demonstrated that coronary microvascular endothelial function declined in association with increased total MS score, even in non-MS patients with a moderate MS score such as 2; careful follow-up may be needed for such subjects.
There are several limitations to the present study. First, all the patients in our study had chest symptoms and had undergone coronary angiography; thus, they may represent a specific group. In addition, MS patients met the minimal criteria for MS and several non-MS patients also had a moderate MS score. Therefore, the results of the present study may not always represent endothelial function in all MS patients. Second, our data showed that CFR was not different in the two groups. However, we excluded patients with a CFR < 2.0 and/or LVH in order to accurately measure ACh-induced coronary circulation. However, in general, many such patients may be regarded as MS patients. If we had added them in the MS group in the present study, CFR in the MS group might have been lower than that in the non-MS group. There have been many studies showing impaired CFR in patients with MS[19,27] and we do not mean to imply that CFR is preserved in MS patients. Finally, we did not measure biochemical parameters associated with MS, such as adiponectin, interleukin-6 and tumor necrotizing factor-α. Therefore, we cannot report on the precise mechanisms of MS-induced coronary microvascular endothelial dysfunction in the present study.
In conclusion, these findings suggest that coronary microvascular endothelial dysfunction is present in MS patients who have chest pain but angiographically normal coronary arteries. Such coronary microvascular endothelial dysfunction may be involved in the pathogenesis of MS-induced cardiovascular events.
Metabolic syndrome (MS) is a major cause of future cardiovascular events. Endothelial dysfunction is thought to be involved in the pathogenesis of MS-induced cardiovascular events. However, coronary endothelial function in patients with MS remains to be elucidated.
The purpose of the present study was to assess the coronary endothelial function of the conduit and resistance vessels in patients with MS and angiographically normal coronary arteries.
Several studies investigating coronary endothelial function in patients with MS have been reported and their results have identified coronary endothelial dysfunction at the level of the resistance vessels. However, no study has investigated coronary endothelial function at the level of the conduit vessels. Quantitative coronary angiography and Doppler velocity measurements, which we adopted in the present study, can assess coronary endothelial function at the levels of the conduit and resistance vessels simultaneously. Our study demonstrates that the increase in coronary blood flow in response to acetylcholine (ACh) infusion was impaired in MS patients compared with non-MS patients, whereas changes in coronary artery diameter in response to ACh infusion did not differ between the two groups. These findings suggest that coronary endothelial function at the level of the resistance vessels is impaired earlier and is more prominent than that at the level of the conduit vessels.
These findings suggest that coronary microvascular endothelial dysfunction is present in MS patients who have chest pain but angiographically normal coronary arteries. Such coronary microvascular endothelial dysfunction may provide a vital evidence for the pathogenesis of MS-induced cardiovascular events.
This is an interesting study confirming previous findings on how MS affects coronary function.
Peer reviewer: Qinglin Yang, MD, PhD, Associate Professor, Department of Nutrition Sciences, University of Alabama at Birmingham, 1675 University Blvd, Birmingham, AL 35294, United States
S- Editor Cheng JX L- Editor Lutze M E- Editor Yang C
| 1. | Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. |
| 2. | Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716. |
| 3. | Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245-1250. |
| 4. | Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066-1076. |
| 5. | McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385-390. |
| 6. | Takeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohhata J, Isobe T, Shimamoto K. Metabolic syndrome and cardiac disease in Japanese men: applicability of the concept of metabolic syndrome defined by the National Cholesterol Education Program-Adult Treatment Panel III to Japanese men--the Tanno and Sobetsu Study. Hypertens Res. 2005;28:203-208. |
| 7. | Quiñones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, De La Rosa R, Chon Y, Yang H, Nicholas SB. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140:700-708. |
| 8. | Dell'Omo G, Penno G, Pucci L, Mariani M, Del Prato S, Pedrinelli R. Abnormal capillary permeability and endothelial dysfunction in hypertension with comorbid Metabolic Syndrome. Atherosclerosis. 2004;172:383-389. |
| 9. | Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32-38. |
| 10. | Bahia L, Aguiar LG, Villela N, Bottino D, Godoy-Matos AF, Geloneze B, Tambascia M, Bouskela E. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics (Sao Paulo). 2006;61:433-440. |
| 11. | Lind L. Endothelium-dependent vasodilation, insulin resistance and the metabolic syndrome in an elderly cohort: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2008;196:795-802. |
| 12. | Title LM, Lonn E, Charbonneau F, Fung M, Mather KJ, Verma S, Anderson TJ. Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vasc Med. 2008;13:263-270. |
| 13. | Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140-146. |
| 14. | Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285-1295. |
| 15. | Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653-658. |
| 16. | Teragawa H, Fukuda Y, Matsuda K, Ueda K, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart. 2004;90:750-754. |
| 17. | Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460-466. |
| 18. | Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001;86:212-216. |
| 19. | Pirat B, Bozbas H, Simsek V, Yildirir A, Sade LE, Gursoy Y, Altin C, Atar I, Muderrisoglu H. Impaired coronary flow reserve in patients with metabolic syndrome. Atherosclerosis. 2008;201:112-116. |
| 20. | Turhan H, Erbay AR, Yasar AS, Bicer A, Sasmaz H, Yetkin E. Impaired coronary blood flow in patients with metabolic syndrome: documented by Thrombolysis in Myocardial Infarction (TIMI) frame count method. Am Heart J. 2004;148:789-794. |
| 21. | Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159-165. |
| 22. | Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998-1005. |
| 23. | Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001;276:30392-30398. |
| 24. | Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021-45026. |
| 25. | Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. |
| 26. | Malavazos AE, Ermetici F, Coman C, Corsi MM, Morricone L, Ambrosi B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int J Cardiol. 2007;121:132-134. |
| 27. | Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188-1195. |