Published online Dec 26, 2021. doi: 10.4330/wjc.v13.i12.745
Peer-review started: August 26, 2021
First decision: October 17, 2021
Revised: October 23, 2021
Accepted: December 3, 2021
Article in press: December 3, 2021
Published online: December 26, 2021
Evaluation of suspected stable angina patients with probable coronary artery disease (CAD) in the community is challenging. In the United Kingdom, patients with suspected stable angina are referred by community physicians to be assessed by specialists within the hospital system in rapid access chest pain clinics (RACPC). The role of a highly sensitive troponin I (uscTnI) assay in the diagnosis of suspected CAD in a RACPC in a “real-life” setting in a non-academic hospital has not been explored.
To examine the diagnostic value of uscTnI (detection limit 0.12 ng/L, upper reference range 8.15 ng/L, and detected uscTnI in 96.8% of the reference population), in the evaluation of stable CAD in a non-selected patient group, with several co-morbidities, who presented to the RACPC.
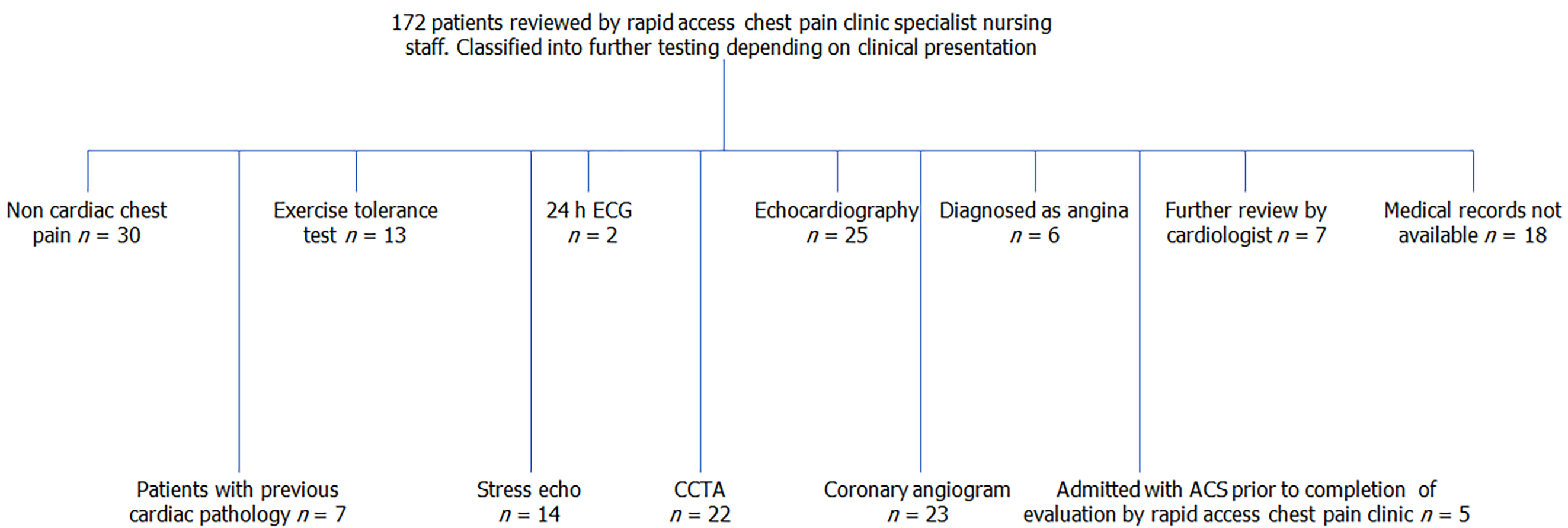
One hundred and seventy two RACPC patients were assigned to either functional or anatomical testing according to the hospital protocol.
The investigations offered to patients were exercise tolerance test 7.6%, 24 h ECG 1.2%, Echocardiogram 14.5%, stress echocardiogram 8.1%, coronary computed tomography angiography (CCTA) 12.8%, coronary angiogram 13.4%, 17.4% were diagnosed with non-cardiac chest pain, 3.5% treated as stable angina, 8.2% reviewed by cardiologists, electronic medical records were not available in 10.4%. Receiver operating characteristic curves for CAD used uscTnI values measured in patients who underwent functional testing, angiogram or CCTA. Values > 0.52 ng/L showed 100% sensitivity and at > 11.6 ng/L showed 100% specificity. In the range > 0.52-11.6 ng/L, uscTnI may not have the same diagnostic potential. In patients assigned to coronary angiogram higher concentrations of uscTnI was associated with severe CAD. Low levels of uscTnI and low pre-test probability of CAD (QRISK3) may decrease patient numbers assigned to CCTA.
The uscTnI diagnostic cut-off values in a RACPC will depend on patient population and their presenting co-morbidity. In the presence of clinical comorbidities and previous CAD the uscTnI needs to be used in conjunction with clinical assessment.
Core Tip: In the United Kingdom, patients with suspected stable angina are referred to rapid access chest pain clinic (RACPC) by community physicians for assessment by hospital specialist medical practitioners. We evaluated the value of a new highly sensitive cardiac troponin I assay in the management of patients with suspected coronary artery disease (CAD) in a RACPC. Patients admitted for further assessment and preselected for either coronary computed tomography angiography or coronary angiogram the assay may indicate the severity of CAD. The diagnostic cut-off values of the assay is determined by the patient population and existing co-morbidities.
- Citation: Ramasamy I. Highly sensitive troponin I assay in the diagnosis of coronary artery disease in patients with suspected stable angina. World J Cardiol 2021; 13(12): 745-757
- URL: https://www.wjgnet.com/1949-8462/full/v13/i12/745.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i12.745
Chest pain is a common presenting symptom in primary care, of which there are many possible causes. The most important of these in terms of subsequent morbidity and mortality are the acute chest pain coronary syndromes (acute coronary syndrome, ACS) comprising unstable angina and acute myocardial infarction (AMI). Current medical practice in the United Kingdom is to transfer patients with ACS urgently to hospital emergency care. In contrast, the differential diagnosis of chronic chest pain is wide and includes cardiac pain as exertional chest pain or stable angina. The diagnosis of coronary heart disease (CHD) may be missed in these patients[1].
New models of assessment have been introduced. In the United States, chest pain observation units provide short stay inpatient care where chest pain is monitored and investigated prior to either formal admission or discharge[1]. In England, the government’s National Service Framework guideline recommended outpatient rapid access chest pain clinic (RACPC) in which people who develop new symptoms that their community physician thinks might be due to stable angina can be assessed by a specialist within 2 weeks of referral[2]. Unlike patients with ACS, who are investigated in emergency care units, evaluation of patients with stable symptoms and suspected CHD (stable angina) in a RACPC may be a challenge. The ability to recognise the high-risk patient with stable angina and an increased risk of progression to AMI may be difficult on clinical grounds alone and may require further functional testing.
There can be considerable variation in which possible stable angina is assessed in RACPC. The American College of Cardiology (ACC)/American Heart Association (AHA)[3,4] and European Society of Cardiology (ESC) recommend clinical risk scores[5] which incorporate age, sex and chest pain presentation to calculate pre-test probability. Pre-test probability is determined by the Diamond-Forrester Coronary Artery Surgery Study[3,4] and the updated version the Coronary Artery Disease consortium (CADC) clinical risk scores. NICE guidelines, United Kingdom[6] suggest a symptom based clinical assessment followed by coronary computed tomography angiography (CCTA) for those in the possible angina group. CCTA identifies obstructive and nonobstructive coronary artery disease (CAD) and allows the quantification of coronary artery calcium[7]. Guidelines recommend a stepwise approach for decision making in suspected stable CAD. A clinical assessment of the probability of stable CAD is followed by non-invasive testing, based usually on the availability of non-invasive tests. Characteristics of tests, sensitivity and specificity, used to diagnose CAD are given in the ESC guidelines[5]. Some of the functional tests available are: Exercise electrocardiogram (ECG), exercise stress echocardiography, dobutamine stress echocardiography and coronary CCTA. A variety of factors affect test choice which include local availability of specific tests, local expertise in test performance and interpretation, or the presence of diagnostic or prognostic questions addressed better by one form of test. Local availability and tariff may make clinicians choose one particular test. Current literature either suggest that CCTA and functional tests can serve equally well as first-line tests for patients with suspected CAD[8,9] or that CCTA may have a benefit over more standard testing[10].
An elevated cardiac troponin (cTn) has long been a pre-requisite for the diagnosis of AMI and has become a diagnostic gold standard for AMI[11]. The value of cTnI in stable angina patients, in contrast to those with ACS and myocardial infarction, is not well understood. The high sensitive troponin assays were able to measure troponin in approximately 50% of normal, healthy individuals. In the most very highly sensitive troponin assays (uscTnI) the cTnI is measurable in almost all healthy individuals[12]. Studies have investigated the use of uscTnI in the early diagnosis of CAD in selected groups of patients without known clinically relevant heart disease. In stable symptomatic patients undergoing coronary computed tomography angiography (CCTA) elevated concentrations of uscTnI was associated with CAD[7,13]. The studies suggest that though uscTnI was not sufficient to use the test as a single standalone test, the test may improve the selection of patients for further investigation and treatment[14,15]. The clinical utility of uscTnI is not fully defined in a RACPC either in (1) differentiating non acute chest pain of uncertain origin; or (2) detecting patients with functionally relevant CAD at risk of progression to AMI. Further, the role of uscTnI in the management of patient with established CHD presenting to a RACPC is yet to be evaluated.
In the United Kingdom, QRISK3 calculations are used in the assessment of 10 year risk of cardiac events. In Worcester, RACPC are staffed by specialist cardiac nurses, in a service led by a specialist cardiologist. Currently they use QRISK3 as part of their initial clinical evaluation of patients, prior to stratifying patients into non-invasive techniques or to identify patients requiring further invasive testing or immediate angiography. In this report we describe the use of uscTnI in the investigation of patients, presenting to the RACPC with probable stable angina. Cardiac troponin assays are not currently used in the investigation of patients with suspected stable CAD in the RACPC. The current study, to our knowledge for the first time, describes the use of uscTnI in the investigation of stable CAD in a RACPC in a non-teaching “district general” hospital in the United Kingdom. Unlike previous studies, this study has been carried out in a non-selected group of patients, and included those with previously known cardiac disease, who presented with suspected stable angina to the RACPC.
We investigated 172 patients referred to the RACPC at the Worcester Royal Hospital between the period September 2018 and March 2019. Blood samples were taken at the community physician surgery prior to review by RACPC, which was within 2 wk of referral by the community physician. The patients were outpatients whose physicians believed that non urgent cardiovascular testing was necessary for suspected CAD. Patients with stable chest pain or chest pain equivalent and those with previous history of CAD who presented with chest pain were included in the study. Seven patients with previous cardiac pathology were referred to RACPC by their physicians. A sixty two year old male with a prior history of CAD and cardiac stent placement who presented with increasing chest pain was assigned to coronary angiography. Three patients with previous coronary artery bypass graft or stent insertion were classified as non-cardiac chest pain or were already under the care of cardiologists. The mean age of all patients was 62.1 years (range 23-88 years) of which 51.1% were women. The proportion of patients (given as percentage of the study patients) with cardiovascular risk factors were, with hypertension (51%), current or ex-tobacco users (44%), dyslipidemia (48%), diabetes (27%), family history of CAD (68%); increased BMI was recorded in 29% of the patients. Calculated QRISK3 ranged from 1-88%, mean 16%. Patient electronic medical notes both inpatient and outpatient notes were reviewed and followed for major adverse cardiac events for a period of six months following the end of the study. Receiver operating characteristic curves (ROC), statistical analysis were performed using GraphPad Prism (United Kingdom) and graphs were constructed using the same software.
In addition, the patients had routine tests (renal function, liver enzymes, serum calcium, HbA1c) to which uscTnI was added as part of the performance assessment of pathway that included uscTnI in the clinical evaluation of the patient. The study was carried out according to the Declaration of Helsinki and the NHS data protection policy. Patients were excluded if they required urgent evaluation for suspected ACS.
Following clinical assessment the patients with suspected stable CAD were assigned to 24 h monitoring ECG, exercise tolerance test (ETT), echocardiogram (echo), exercise or pharmacological stress echo, CCTA, coronary angiogram and percutaneous coronary intervention (PCI), medical treatment for angina, or were discharged as clinical assessment suggested non-cardiac chest pain. More complex patients were designated for review by specialist cardiologists.
The results of the uscTnI test were not provided to the RACPC staff and subsequent care of the patient was dependent on his or her clinical assessment of the subject, which was independent of uscTnI value.
The patient sera were collected within 24 h of phlebotomy, stored and transported frozen in dry ice (quality controlled by the courier) to Barcelona prior to measurement of uscTnI. uscTnI was measured by the Singulex Clarity (United States) assay. The detection limit of the assay was 0.12 ng/L. The assay CV was 10% at 0.53 ng/L. Imprecision was 3.16% to 12% for the assay. uscTnI was detectable in 96.8% of healthy individuals. The 99th percentile for healthy individuals was 8.15 ng/L. The assay was stated as outperforming other high sensitivity cTnI assays in terms of analytical sensitivity[12,16].
For the exercise tolerance test the Bruce protocol was followed with ECG recording. A semi-supine bike was used for exercise stress echocardiogram (increasing the workload by 25 Watts every 2 min until the 85% or more of target (heart rate) HR is achieved. Target heart rate was calculated by 220 - age). For dobutamine stress echocardiogram with ischaemia the protocol used was: 10 µg/kg/min and infused up to a maximum 40 µg/kg/min in 10/µg/kg/min increments at every 3-5 min according to HR response (to achieve a minimum. of 85% of the target HR for the test to be diagnostic). If target HR was not achieved at 40 µg/kg/min, iv atropine was given. CCTA was done on a 64-slice CT scanner (Toshiba Acquilion Cx or Acquilion Prime, Toshiba). All scans are reported by specialist cardiologists or radiologists. The stenosis was graded as mild (< 50%), moderate (50%-70%) or severe (> 70%).
Following coronary angiogram degree of stenoses was graded as normal, mild (< 50% stenosis), moderate (50%–74% stenosis), severe (> 75%-99% stenosis), and critical (> 99% stenosis). The angiogram was reported by specialist cardiologists.
Patient demographics following clinical assessment by RACPC is shown in Table 1. The flow chart of patients according clinical assessment is given in Figure 1 and the uscTnI values according to further testing carried out for functional CAD is summarised in Table 2. The investigations offered to patients were ETT 7.6%, 24 h ECG 1.2%, Echo 14.5%, stress echo 8.1%, CCTA 12.8%, coronary angiogram 13.4%, 17.4% were discharged with the diagnosis of non-cardiac chest pain, 3.5% were diagnosed and treated as stable angina, 4.1% were further referred to specialist cardiologists, 4.1% were known cardiology patients, electronic medical records were not available in 10.4%, 2.9% were admitted with ACS during the study. The diagnostic conundrum presented by individual patients is summarised according to outcome measures, prior to analysis of the data by ROC curves.
| Non-cardiac chest pain | ETT | ECG | Angina | Review | Lost to follow-up | Previous cardiac problems | Stress Echo (negative) | Stress Echo (positive) | Echo (negative) | Echo (pos) | CCTA (negative) | CCTA (positive) | Angiogram (negative) | Angiogram (positive) | ACS | |
| Age (yr) | 57 (23-88) | 54 (36-66) | 66 (62-69) | 73 (66-79) | 66 (43-84) | 64 (41-84) | 71 (67-87) | 63 (49-82) | 68 | 67 (43-84) | 72 (55-83) | 53 (34-63) | 62 (51-77) | 59 (50-65) | 58 (46-81) | 69 (62-86) |
| Sex (M/F) | 15/15 | 11/2 | 0/2 | 1/5 | 5/2 | 4/14 | 3/4 | 4/9 | 1/0 | 10/9 | 5/1 | 4/8 | 5/5 | 3/3 | 12/5 | 2/3 |
| BMI (> 25 kg/m2) (n) | 9 | 4 | 1 | 2 | 4 | 8 | 0 | 2 | 1 | 4 | 0 | 4 | 5 | 1 | 6 | 2 |
| Diabetes (n) | 2 | 4 | 0 | 1 | 2 | 1 | 0 | 2 | 0 | 2 | 2 | 2 | 1 | 0 | 4 | 3 |
| Hyperlipidmia (n) | 11 | 8 | 1 | 2 | 4 | 6 | 4 | 6 | 1 | 11 | 5 | 5 | 7 | 2 | 11 | 3 |
| Hypertension (n) | 10 | 7 | 2 | 4 | 4 | 7 | 3 | 7 | 1 | 13 | 5 | 4 | 8 | 3 | 11 | 2 |
| Family History of CAD (n) | 6 | 5 | 1 | 0 | 3 | 7 | 0 | 3 | 0 | 5 | 2 | 8 | 6 | 3 | 10 | 0 |
| Smoker (n) | 11 | 5 | 0 | 4 | 5 | 9 | 1 | 6 | 0 | 10 | 4 | 3 | 9 | 1 | 8 | 0 |
| Past History MI/stent/CABG (n) | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aspirin (n) | 7 | 2 | 0 | 2 | 1 | 6 | 3 | 4 | 0 | 4 | 2 | 4 | 5 | 2 | 6 | 2 |
| Statin (n) | 5 | 3 | 0 | 2 | 1 | 3 | 5 | 3 | 1 | 7 | 4 | 1 | 2 | 0 | 10 | 1 |
| ISMN/Nitroglycerin (n) | 9 | 1 | 1 | 5 | 3 | 6 | 4 | 7 | 1 | 7 | 3 | 4 | 5 | 3 | 8 | 2 |
| Anti-hypertensive (n) | 7 | 7 | 1 | 2 | 3 | 2 | 5 | 4 | 1 | 8 | 4 | 2 | 1 | 1 | 14 | 2 |
| Creatinine (µmol/L) (n) | 83 (52-122) | 79 (54-100) | 77 (71-82) | 83 (70-103) | 82 (51-98) | 75 (47-109) | 71 (36-95) | 72 (56-88) | 84 | 78 (52-102) | 83 (63-103) | 71 (52-88) | 72 (52-99) | 78 (56-98) | 81 (46-134) | 76 (65-86) |
| QRISK3% | 2-> 88 | 12 (7-40) | 9 (5-13) | 22 (6-34) | 22-41 | 19 (2-58) | NA | 13 (3-36) | 32 | 1 to > 40 | 12 to 45 | 9.4 (1-35) | 22 (6-37) | 8 (2-16) | 8 to > 40 | |
| n | 30 | 13 | 2 | 6 | 7 | 18 | 7 | 13 | 1 | 19 | 6 | 12 | 10 | 6 | 17 | 5 |
| Subgroup | uscTnI ng/L (minimum value) | uscTnI ng/L (average value) | uscTnI ng/L (maximum value) | n | P value |
| Referred back to the community practitioner (non-cardiac chest pain) | 0.71 | 2.9 | 17 | 30 | |
| Exercise test | 0.97 | 2.6 | 9.7 | 13 | |
| 24 h ECG | 1.9 | 2.3 | 2.6 | 2 | |
| Diagnosed angina, treated with medication | 1.8 | 5.0 | 9.2 | 6 | |
| Advised further review by specialist cardiologist | 1.0 | 12 | 70 | 7 | |
| Lost to follow up | 0.8 | 2.6 | 7.5 | 18 | |
| Previously diagnosed with cardiac problems and under the care of a cardiologist | 1.5 | 7.2 | 33.2 | 7 | |
| Echo (normal) | 0.46 | 3.0 | 8.6 | 19 | |
| Echo (mild abnormalities) | 2.47 | 6.44 | 17.0 | 6 | |
| Stress echo (negative) | 0.98 | 2.1 | 3.9 | 13 | |
| Stress echo(positive) | 3.1 | 3.1 | 3.1 | 1 | |
| CCTA (negative) | 0.58 | 2.8 | 9.3 | 12 | NS |
| CCTA (positive) | 1.1 | 2.7 | 8.7 | 10 | |
| Angiogram(negative) | 1.1 | 1.8 | 2.2 | 6 | < 0.05 |
| Angiogram(positive) | 0.94 | 7.3 | 49 | 17 |
The patients are divided into follow-up testing subgroups. Clinical details of patients presenting with uscTnI > upper quartile of the reference range (6 ng/L) are described in each group.
Patients classified as non-cardiac chest pain: Thirty patients were classified as non-cardiac chest pain and not investigated further by the RACPC. Seventy one year old female patient with a past history of AMI, coronary artery bypass surgery and uscTnI = 16.6 ng/L, was referred to the heart failure clinic with severe left ventricular systolic dysfunction. Two patients with uscTnI at the upper quartile of the reference range: 71year old female with persistent gastric reflux and uscTnI = 8.2 ng/L, QRISK3 score = 12%, and 89 year man with a history of pulmonary embolism, idiopathic pulmonary fibrosis and osteoarthritis with a uscTnI=7.7 ng/L, QRISK3 score rated as high, were categorised as non-cardiac chest pain.
Exercise tolerance test: Thirteen patients had a negative exercise tolerance test. A 55 year old male with constant chest pain and an uscTnI value of 9.7 ng/L and QRISK3 score of 9% assigned to ETT developed a non-limiting chest pain during exercise and was described as low risk.
Three patients with ECG changes suggestive of ischemia and a further patient with a normal exercise tolerance test and typical angina pain underwent cardiac catheterisation and coronary angiogram. These patients are included in the section with the group assigned to coronary angiography.
24 h ECG monitoring: Twenty four hour ECG monitoring detected ectopic activity with ventricular and supraventricular ectopics in two patients.
Echocardiography: Twenty five patients were designated for investigation with echocardiography. One 78 year old female with an uscTnI = 8.6 and QRISK3 = 20% was diagnosed with atrial fibrillation. A further 78 year old male with uscTnI at the upper quartile of the reference range, 6.2 ng/L, QRISK3 = 54% and normal systolic and ventricular function was diagnosed with angina and his medication was changed to a beta-blocker. He was diagnosed with adenocarcinoma of the prostate 12 years previously and treated with androgen blockade and radiotherapy. He was referred for further review by cardiology specialists.
Echocardiography diagnosed mild abnormalities in six patients. Two patients were diagnosed with recent onset atrial fibrillation (71 year old male with uscTnI = 9.3 ng/L) and pre-existing left bundle branch block (79 year old male with uscTnI = 17.0 ng/L).
Angina: Six patients were diagnosed with and treated as stable angina. Two patients had measured uscTnI values of 7.9 and 9.2 ng/L.
Review by a specialist in cardiology: Seven patients were further reviewed by a medical specialist in cardiology. A 63 y old male patient with a prior history of cardiovascular accident, subarachnoid haemorrhage and alcohol dependence and uscTnI value of 70 ng/L was treated with beta-blockers and placed under surveillance.
Lost to follow up: Medical notes following RACPC evaluation were not available for eighteen patients. A 74 year old female with an uscTnI = 7.5 ng/L and QRISK3 of 46% was diagnosed with stable angina and referred by RACPC staff for review by specialist cardiologists. Further follow-up of this patient was not recorded in the medical electronic notes.
Patients with previous cardiac pathology: Seven patients with previous cardiology pathology were reviewed by cardiologist. A 72 year old male with prior history of coronary artery bypass graft 9 years previously presented with a uscTnI of 32.2 ng/L. His medication was changed to include statins and ISMN (Isosorbide mononitrate). A further 76 year old female with known AF presented with an uscTnI=6.1 ng/L.
Stress echocardiography: Thirteen patients categorised to stress echocardiography, were classified as normal. A single patient showed mild inducible ischemia.
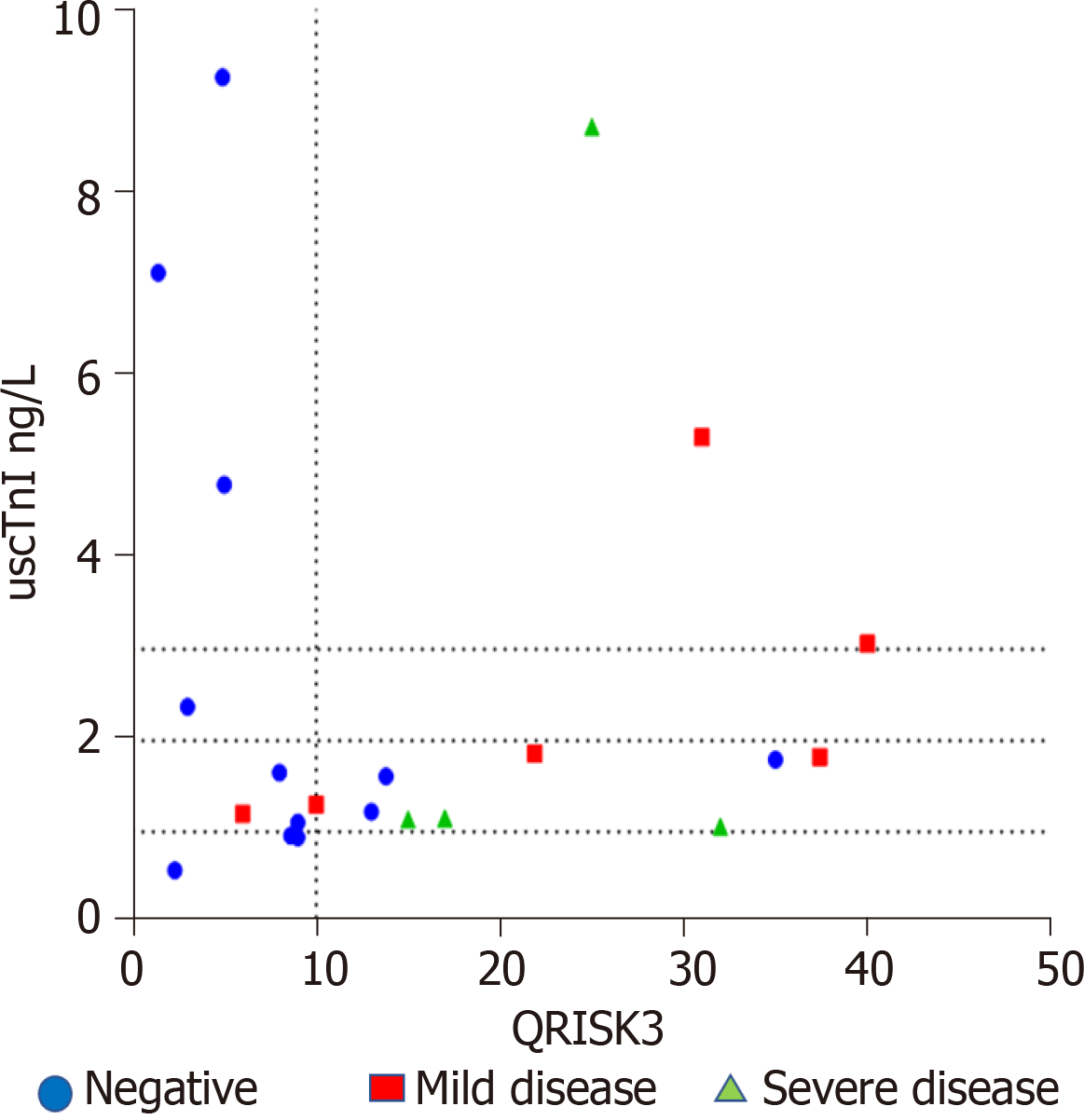
CCTA: Of the twenty two patients allocated to CCTA 12 were classified as normal, and 6 with mild CAD and 4 with severe disease. The mean uscTnI concentration in patients with obstructive CAD was 2.7 (range 1.1-8.7) ng/L and without CAD was 2.8 (range 0.6-9.3) ng/L. There was considerable overlap between the values. Fifty nine year old female with no relevant previous history and uscTnI = 9.3 ng/L and QRISK3 score 5%, and a 42 year old female with a history of gastroesophageal reflux and uscTnI = 7.1 ng/L and QRISK3 < 2% had normal CCTA. Sixty one year old male with uscTnI = 8.7 ng/L and QRISK3 score of 25% demonstrated severe obstructive disease. A further 62 year old female with a QRISK3 of 17% and uscTnI = 1.2 ng/L demonstrated severe CAD following CCTA and presented with ACS and Non-ST segment elevation myocardial infarction (NSTEMI) prior to completion of investigation. A plot of QRISK3 scores and uscTnI (Figure 2) suggested that 3/22 patients with an uscTnI < 1 ng/L and QRISK3 score < 10% showed a normal CCTA.
Coronary angiography: Twenty three patients underwent coronary angiography. Six patients showed normal coronary arteries. Fifty nine year old male, with definite angina pain, uscTnI = 2.23 ng/L and QRISK3 = 8%, underwent ETT which showed ventricular bigeminy. Investigative echo and coronary angiogram were classified as normal.
Coronary angiography detected severe disease in two patients: 58 year old male with uscTnI = 3.2 ng/L, QRISK3 13%, 53 year old male with uscTnI = 48.5 ng/L and QRISK3 10%. ETT detected ischemic changes in both patients. A further patient, 49 year old male with uscTnI = 1.4 ng/L and QRISK 23%, and normal ETT, and referred for further investigation for ‘typical’ angina pain, developed NSTEMI prior to completion of investigation. A 60 year old female uscTnI = 2.9 ng/L and QRISK3 score 8% presented with crescendo angina and a troponin change of 26 ng/L, Coronary angiogram showed severe CAD in both patients. uscTnI increased in patients with the increasing severity of coronary artery occlusion (Table 3). Higher uscTnI was more likely to be associated with severe occlusion of CAD. A plot of QRISK3 and uscTnI in this group showed that patients were not classified within lower quartile (< 1 ng/L uscTnI and QRISK3 score < 10%).
| Group | Normal | Mild disease | Moderate disease | Severe disease |
| Mean uscTnI ng/L | 1.8 | 3.3 | 7.3 | 10 |
| Min uscTnI ng/L | 1.1 | 2.0 | 1.0 | 0.94 |
| Max uscTnI ng/L | 2.2 | 3.9 | 22 | 49 |
| n | 6 | 5 | 5 | 7 |
ACS: Two patients presented with ACS and troponin changes prior to assessment by RACPC. A fifty eight year old male with a baseline uscTnI = 1.9 ng/L presented to the emergency department with a cardiac troponin change of 42 ng/L. Coronary angiogram showed mild occlusion. A 63 year old female who was admitted, prior to RACPC assessment, to the hospital with pyelonephritis showed a cardiac troponin change of 23.6 ng/L; coronary angiogram showed severe disease. Baseline uscTnI taken prior to hospital admission was not available in this patient. One 73 year old male and 86 year old female were classified as recent acute cardiac events, a review suggested uscTnI levels of 2290 and 221 ng/L, respectively, prior to review by RACPC. A further 62 year old female with a past history of breast cancer and uscTnI = 3.6 ng/L died of cardiac arrest prior to review by RACPC staff. Patient follow-up over a period of 6 mo after RACPC review did not result in further admissions with ACS.
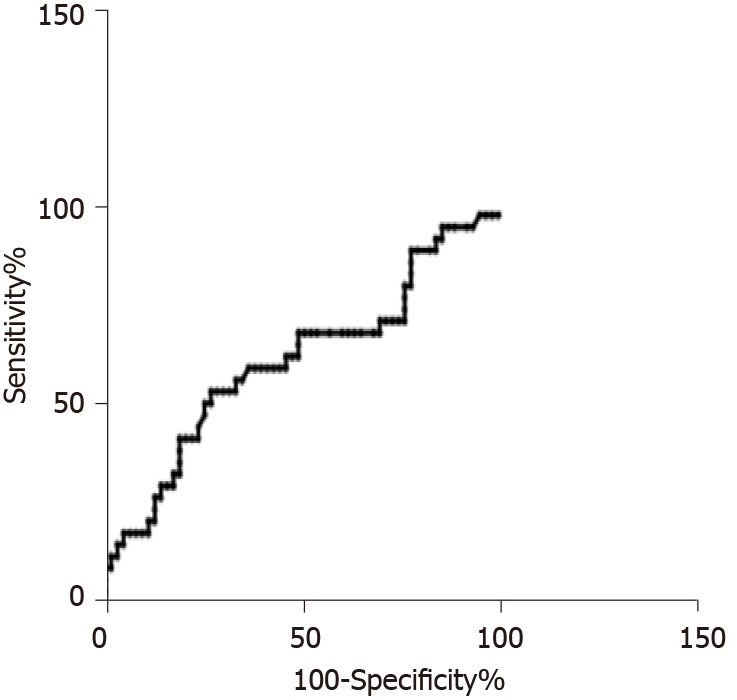
We examined whether uscTnI can predict the presence or exclude the presence of coronary artery stenosis in patients assigned to ETT, Echo, Stress Echo, CCTA and coronary angiogram using ROC, Figure 3. The analysis included 63 patients who tested negative and 34 patients who tested positive. ROC analysis showed an AUC of 0.63 (95%CI: 0.51-0.73). At a value of uscTnI value > 0.52 ng/L, sensitivity was 100% and specificity 1.6%. At a value of uscTnI > 11.6 ng/L, sensitivity was 10.7% and specificity 100%. We found a single patient (84 year old male) with an uscTnI = 0.46 ng/L with a normal echo. Ten patients with uscTnI > 11.6 ng/L were detected within the study. Three with severe CAD disease following coronary angiogram (uscTnI = 13.4 ng/L, 18.3 ng/L, 48.5 ng/L), one with moderate disease (uscTnI = 22.3 ng/L), left bundle branch block (uscTnI = 17.0 ng/L), known ischemic heart disease (uscTnI = 33.2 ng/L), chronic morbidity for monitoring by cardiologist (70.4 ng/L), a further patient diagnosed with non-cardiac chest pain (uscTnI = 16.6 ng/L), and two with recent previous ACS events (uscTnI = 221 and 2290 ng/L).
Early detection of CAD remains an important task as effective treatments are available to relieve symptoms and reduce mortality[5]. Suspected stable angina remains a common presenting complaint. The challenge remains to identify those with CAD and those with intermediate pre-test probability who need further functional testing. The pre-test probability of stable CHD is determined by the presence of cardiovascular risk factors, age, sex and the nature of the presenting chest pain[5]. NICE guidelines, United Kingdom suggest that the diagnosis of stable angina is based on clinical assessment alone or clinical assessment and diagnostic testing[6]. In the United Kingdom, RACPC is the first place of referral in the investigation of suspected stable angina. In Worcester specialist nursing staff triage patients using clinical assessment which include among others, detailed clinical history, physical examination, QRISK3 as well as typicality of chest pain. In this study we investigated the use of a very highly sensitive troponin assay, an assay that can detect cTnI in 98% of healthy individuals, in the diagnosis and management of stable angina in the RACPC. In previous studies, uscTnI to rule out functionally relevant CAD was investigated in patients without known previous CAD; in this study we aimed to investigate the application of the assay in an unselected group of patients who presented to the RACPC.
Previous reports have stated that levels of uscTnI were significantly higher in patient with exercise induced ischemia, Using a combination of pre-ETT clinical assessment and uscTnI, the authors suggest a cut-off value of 1.54 ng/L to rule out 15% of patients from further testing, but acknowledge that further refinement is required because even when used with clinical judgement uscTnI provided only moderate diagnostic accuracy[17,18]. This value may be influenced by other structural cardiac abnormalities. In our patient series, the patient co-morbidity included congestive cardiac failure, history of breast cancer and sarcoidosis. In this study, investigation on two patients with uscTnI 3.2, and 48.5 ng/, and abnormal ETT which showed ischemic changes, showed severe CAD. A further patient with a normal ETT, uscTnI = 1.4 ng/L and classified for a coronary angiogram because of “typical angina pain” developed NSTEMI prior to further investigation. Coronary angiogram showed severe CAD. This study suggests overlap in uscTnI values in patients assigned to ETT and classified as with and without CAD. Strategies used to stratify patients into different investigation methods, as well as patient selection for RACPC assessment may influence cut-off levels used for uscTnI.
An additional study, included patients referred for investigation of functionally relevant CAD by rest/stress myocardial perfusion single-photon emission tomography/computer tomography. The patients with clinically relevant cardiac disease were excluded. An uscTnI value of < 0.5 ng/L ruled out functionally relevant CAD in 10% of patients[15]. The SCOT-HEART trial investigated patients with suspected stable angina assigned to CCTA for obstructive CAD. The median concentration of uscTnI assay in patients without CAD was 1.2 ng/L and with CAD was 1.9 ng/L. Addition of uscTnI to the CADC risk model reduced the number of patients determined to be at intermediate or high risk by the CADC model by 10%[14]. The authors suggest that the study demonstrates that the use of uscTnI as a biomarker can improve the diagnosis of stable obstructive CAD. In the current study there was considerable overlap in uscTnI values between patients with and without obstructive CAD. However a plot of QRISK3, a risk evaluation of cardiac events at 10 years and uscTnI showed that at QRISK3 < 10% and uscTnI < 1 ng/L, 14% of patients with normal CCTA scan were within this quartile. This study confirms previous studies that the assessment of stable angina in patients referred for CCTA may be improved by the addition of uscTnI. Cardiac imaging remains a valuable tool for the assessment of CAD and the early detection of CAD remains an important task, CCTA has recently come under scrutiny due to increased cost, limitations such as increased radiation or user dependent interpretation or used inappropriately in patients with very low pre-test probability of CAD[4,5]. Additional studies are mandatory to elucidate if the rule out of 14% of patients is applicable to a larger cohort of patients pre-selected for CCTA.
Increase in uscTnI occurred in patients referred for coronary angiogram and increased with degree of severity of CAD. Given that previous studies suggest an association between high sensitive cTnI and the presence of CAD without ACS[19], this study confirmed an association between uscTnI and severity of CAD in the patient group referred for coronary angiogram. Further, the use of troponin assays in an RACPC setting may identify patients with recent ACS events and result in earlier assessment and treatment of these patients.
The question remains how these findings and uscTnI can be applied in clinical practice in the RACPC in the United Kingdom. A comparison of ROC curves for patients who underwent both functional testing, CCTA and coronary angiogram did not identify a single uscTnI value with a high enough sensitivity and specificity (and negative and positive predictive value) for stable CAD. In the population referred to the RACPC for further evaluations a low level of uscTnI ≤ 0.52 ng/L may decrease the number of patients required for further investigation and high levels > 11.6 ng/L may identify those who require further evaluation. It is unlikely that uscTnI within the range > 0.52-11.6 ng/L will be a standalone test for suspected stable angina. The cohort we studied included patients with several comorbidities and previous history of CHD that often pose a diagnostic and prognostic challenge as to the cause and significance of the presenting chest pain. This study suggests uscTnI as a sensitive marker of cardiac damage would perform best when combined with clinical assessment of the patient. It must be emphasized that the findings of this study would not mean that in the RACPC clinical judgment can be substituted with uscTnI. Should a single uscTnI be performed in either primary care or at presentation to RACPC, it needs to be interpreted with clinical evaluation.
These findings complement previous data on the use of uscTnI in the diagnosis of CAD in suspected stable angina. To our knowledge this is the first study to investigate the use of a “highly” sensitive cardiac troponin assay in a RACPC. The limitations of this study are that this is a single-centre study and bias may have been introduced due to referral characteristics. We do not believe that lack of access to patient medical records, which was not based on patient demographics led to sample bias. We also need to emphasise that in this institution myocardial ischemia was investigated by several methods in patients with a wide range of clinical probability of CAD based on clinical evaluation.
The study suggests that in the presence of clinical comorbidities and previous CHD the uscTnI needs to be used in conjunction with clinical assessment. Diagnostic cut off values of uscTnI in an RACPC setting depend on patient population and comor
The study suggests that in the presence of clinical comorbidities and previous CHD the uscTnI needs to be used in conjunction with clinical assessment. Diagnostic cut off values of uscTnI in an RACPC setting depend on patient population and comorbidities. Further work is required to investigate the use of “highly sensitive” cardiac troponin values in the selection of patients suspected with CAD for further investigation and treatment.
In the United Kingdom rapid access chest pain clinics (RACPC) have been set up in hospital centers to address the problem of non-acute chest pain of uncertain origin. The early detection of coronary artery disease (CAD) in these patients can lead to effective treatment. In this study we looked at the value of a highly sensitive troponin I assay in the rule out of functionally relevant CAD in patients referred for further assessment and investigation in a RACPC. To our knowledge this is the first study to be carried out in a non-teaching hospital in patients who presented with several clinical co-morbidities.
While functional studies and imaging techniques are valuable in the evaluation of patients with suspected CAD, highly sensitive troponin I which detects even minute concentrations of troponin I in the serum may provide further information on cardiac injury that may be associated with CAD.
The aim was to assess the role of troponin in assisting clinical decision making in the setting of a RACPC in a non-teaching hospital. This has not been explored previously.
One hundred and seventy two patients admitted to the rapid access clinic were studied. Unlike previous studies, patients with a previous history of CAD were included in the study. Following clinical assessment the patients with suspected stable CAD were assigned to 24 h monitoring electrocardiogram, exercise tolerance test, echocardiogram, exercise or pharmacological stress echo, coronary computed tomography angiography, coronary angiogram and percutaneous coronary intervention, medical treatment for angina, or were discharged as clinical assessment suggested non-cardiac chest pain. More complex patients were designated for review by specialist cardiologists.
Receiver operating characteristic curves suggest that patients with troponin I ≤ 0.52 ng/L were less likely to present with CAD and values > 11.6 ng/L required further evaluation. In the range > 0.52-11.6 ng/L troponin I was not a standalone test. In all cases troponin was best used in conjunction with clinical assessment. In patients assigned and preselected for coronary computed tomography angiography and coronary angiogram troponin I was an indicator of the severity of CAD. Cut-off levels of troponin I were determined by the patient population cohort.
The study suggests that in unselected patients presenting with suspected stable angina to a rapid access clinic troponin I is best used in conjunction with clinical evaluation of the patient. Diagnostic cut-off levels are dependent on patient population.
What is now required is further work with different population groups.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karavaş E S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Mant J, McManus RJ, Oakes RA, Delaney BC, Barton PM, Deeks JJ, Hammersley L, Davies RC, Davies MK, Hobbs FD. Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care. Health Technol Assess. 2004;8:iii, 1-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Department of Health and Social Care. National Service Framework for Coronary Heart Disease. Available from: https://www.gov.uk/government/publications/quality-standards-for-coronary-heart-disease-care. [Cited in This Article: ] |
| 3. | Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 551] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 4. | Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Pressler SJ, Sellke FW, Shen WK; American College of Cardiology/Americal Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Preventive Cardiovascular Nurses Association; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2015;149:e5-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2772] [Cited by in F6Publishing: 2878] [Article Influence: 261.6] [Reference Citation Analysis (0)] |
| 6. | NICE guidance. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Clinical guideline [CG95]. 2010. Available from: https://www.nice.org.uk/guidance/cg95. [Cited in This Article: ] |
| 7. | Januzzi JL Jr, Suchindran S, Hoffmann U, Patel MR, Ferencik M, Coles A, Tardif JC, Ginsburg GS, Douglas PS; PROMISE Investigators. Single-Molecule hsTnI and Short-Term Risk in Stable Patients With Chest Pain. J Am Coll Cardiol. 2019;73:251-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators. Outcomes of anatomical vs functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 973] [Cited by in F6Publishing: 1004] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 9. | Papachristidis A, Vaughan GF, Denny SJ, Akbari T, Avornyo E, Griffiths T, Saunders E, Byrne J, Monaghan MJ, Al Fakih K. Comparison of NICE and ESC proposed strategies on new onset chest pain and the contemporary clinical utility of pretest probability risk score. Open Heart. 2020;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | SCOT-HEART Investigators, Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 686] [Cited by in F6Publishing: 794] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 11. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72:2231-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1366] [Cited by in F6Publishing: 1944] [Article Influence: 324.0] [Reference Citation Analysis (1)] |
| 12. | Garcia-Osuna A, Gaze D, Grau-Agramunt M, Morris T, Telha C, Bartolome A, Bishop JJ, Monsalve L, Livingston R, Estis J, Nolan N, Sandlund J, Ordonez-Llanos J. Ultrasensitive quantification of cardiac troponin I by a Single Molecule Counting method: analytical validation and biological features. Clin Chim Acta. 2018;486:224-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Januzzi JL Jr, Suchindran S, Coles A, Ferencik M, Patel MR, Hoffmann U, Ginsburg GS, Douglas PS; PROMISE Investigators. High-Sensitivity Troponin I and Coronary Computed Tomography in Symptomatic Outpatients With Suspected CAD: Insights From the PROMISE Trial. JACC Cardiovasc Imaging. 2019;12:1047-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Adamson PD, Hunter A, Madsen DM, Shah ASV, McAllister DA, Pawade TA, Williams MC, Berry C, Boon NA, Flather M, Forbes J, McLean S, Roditi G, Timmis AD, van Beek EJR, Dweck MR, Mickley H, Mills NL, Newby DE. High-Sensitivity Cardiac Troponin I and the Diagnosis of Coronary Artery Disease in Patients With Suspected Angina Pectoris. Circ Cardiovasc Qual Outcomes. 2018;11:e004227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Walter JE, Honegger U, Puelacher C, Mueller D, Wagener M, Schaerli N, Strebel I, Twerenbold R, Boeddinghaus J, Nestelberger T, Sazgary L, Marbot S, du Fay de Lavallaz J, Kaiser C, Osswald S, Wild D, Rentsch K, Zellweger M, Reichlin T, Mueller C. Prospective Validation of a Biomarker-Based Rule Out Strategy for Functionally Relevant Coronary Artery Disease. Clin Chem. 2018;64:386-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Chapman AR, Lee KK, McAllister DA, Cullen L, Greenslade JH, Parsonage W, Worster A, Kavsak PA, Blankenberg S, Neumann J, Sörensen NA, Westermann D, Buijs MM, Verdel GJE, Pickering JW, Than MP, Twerenbold R, Badertscher P, Sabti Z, Mueller C, Anand A, Adamson P, Strachan FE, Ferry A, Sandeman D, Gray A, Body R, Keevil B, Carlton E, Greaves K, Korley FK, Metkus TS, Sandoval Y, Apple FS, Newby DE, Shah ASV, Mills NL. Association of High-Sensitivity Cardiac Troponin I Concentration With Cardiac Outcomes in Patients With Suspected Acute Coronary Syndrome. JAMA. 2017;318:1913-1924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Tanglay Y, Twerenbold R, Lee G, Wagener M, Honegger U, Puelacher C, Reichlin T, Mann S, Druey S, Hochgruber T, Zürcher S, Radosavac M, Kreutzinger P, Pretre G, Stallone F, Hillinger P, Jaeger C, Rubini Gimenez M, Freese M, Wild D, Rentsch K, Osswald S, Zellweger MJ, Mueller C. Incremental value of a single high-sensitivity cardiac troponin I measurement to rule out myocardial ischemia. Am J Med. 2015;128:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Lee G, Twerenbold R, Tanglay Y, Reichlin T, Honegger U, Wagener M, Jaeger C, Rubini Gimenez M, Hochgruber T, Puelacher C, Radosavac M, Kreutzinger P, Stallone F, Hillinger P, Krivoshei L, Herrmann T, Mayr R, Freese M, Wild D, Rentsch KM, Todd J, Osswald S, Zellweger MJ, Mueller C. Clinical benefit of high-sensitivity cardiac troponin I in the detection of exercise-induced myocardial ischemia. Am Heart J. 2016;173:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Mueller D, Puelacher C, Honegger U, Walter JE, Badertscher P, Schaerli N, Strebel I, Twerenbold R, Boeddinghaus J, Nestelberger T, Hollenstein C, du Fay de Lavallaz J, Jeger R, Kaiser C, Wild D, Rentsch K, Buser A, Zellweger M, Reichlin T, Mueller C. Direct Comparison of Cardiac Troponin T and I Using a Uniform and a Sex-Specific Approach in the Detection of Functionally Relevant Coronary Artery Disease. Clin Chem. 2018;64:1596-1606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |