Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 180-203
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.180
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.180
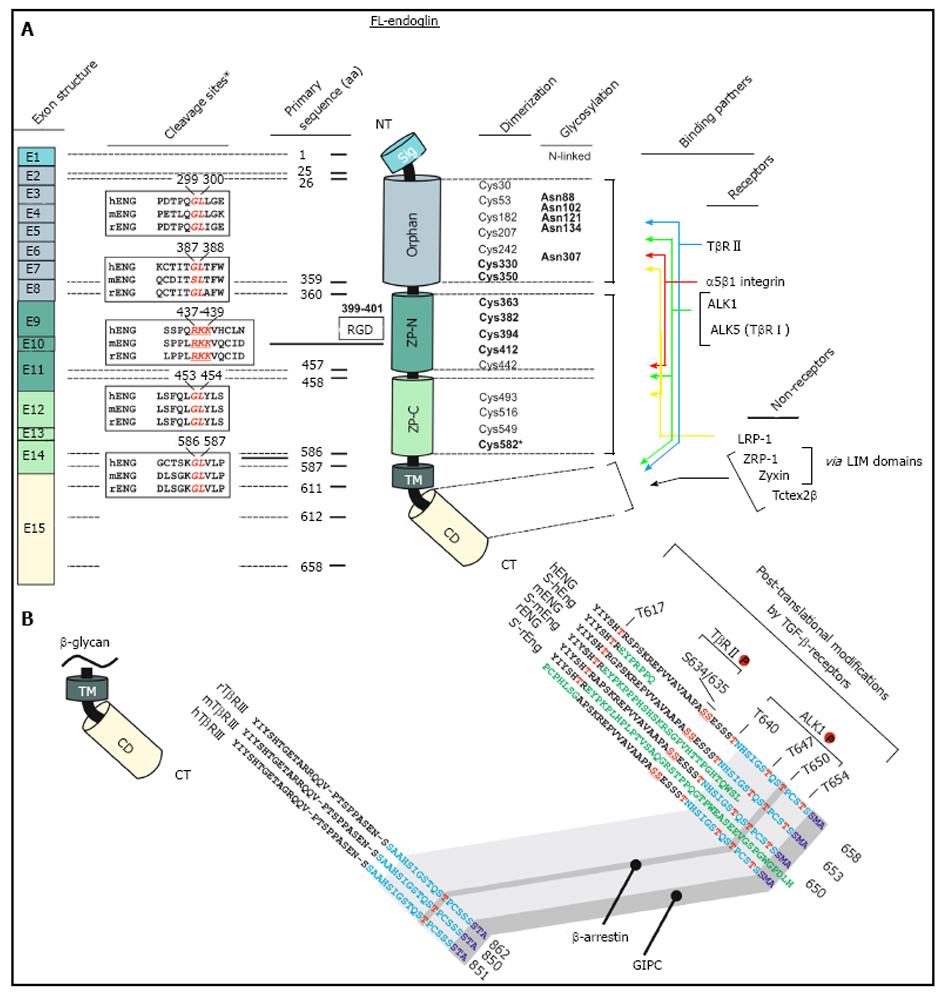
Figure 1 Schematic representation of the structural and functional modules of human endoglin.
A: Left, first panel, Exon structure: Structure of the endoglin gene and assignment of respective protein modules. Left, second panel, cleavage sites: Predicted consensus proteolytical cleavage sites deduced from the primary sequence and experimentally confirmed matrix metalloproteinases-14 cleavage site between aa positions 586 and 587[59]. Middle panel, primary sequence positions: aa boundaries of the structural domains of human endoglin and location of the Arginine-Glycin-Aspartic acid (RGD) sequence that is only present in human endoglin. Right, first panel, dimerization: The cysteine residues of the extracellular domain of endoglin are depicted including the 8 highly conserved residues within the ZP-domain[47]. Cysteines that are involved in dimerization are shown in bold. Cysteine 582 that is involved in human endoglin dimerization is not present in the mouse and rat homologues[56]. Right, second panel, glycosylation: This figure part displays verified N-linked glycosylation sites within human endoglin. There is experimental evidence for O-linked glycosylation, but respective sites are not shown[1]. Right, third panel, binding partners: Depicted are interaction partners of endoglin as either receptor proteins (upper) or cytosolic non receptors proteins (lower). Colored arrows indicate interacting domains of endoglin with its binding partners. Zyxin and ZRP-1 bind to endoglin via their LIM-domains; B: Displayed is an aa alignment of betaglycan (left) and endoglin (right) of human, mouse and rat. Receptor kinase substrates (serine and threonines) are shown in red. Threonine 650 is essential for binding to β-arrestin2[66]. The C-termini of betaglycan and endoglin that are highly conserved are indicated in light blue. The PDZ-I domain which binds to GIPC is depicted in dark blue[67]. The alternative C-termini which results from differential splicing are shown in green.
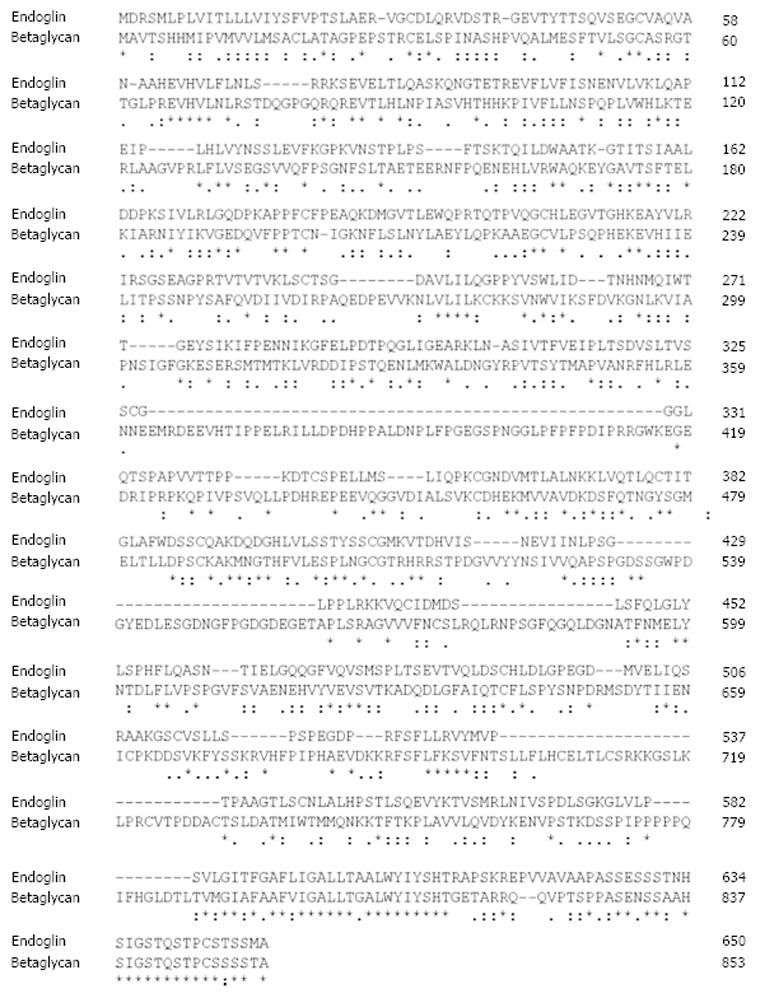
Figure 2 Sequence alignment of rat endoglin and betaglycan.
The protein sequences of rat endoglin and betaglycan were aligned using the ClustalW2 algorithm. Respective sequences of rat endoglin (AAS67893) and betalycan (AAA42236.1) were taken from the GenBank. Please note the high degree of similarity of both proteins at their C-termini. Fully conserved aa in endoglin are marked by asterisk (*), positions that carry aa with strongly similar properties by a colon (:) and positions with weakly similar properties by a period (.), respectively. Please note that the highest degree of homology is found at the C-terminal regions that encompass the cytosolic part of endoglin
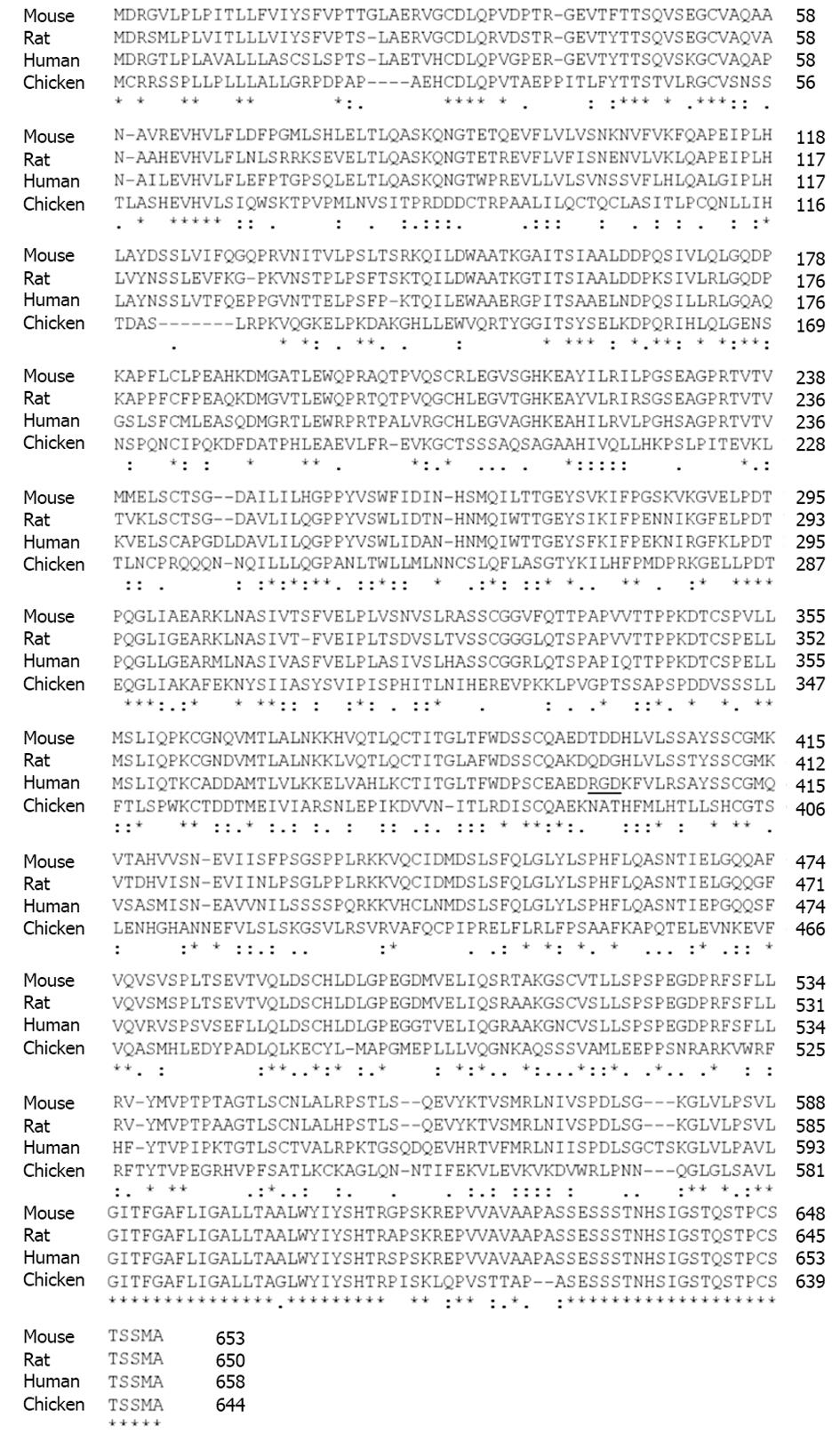
Figure 3 Sequence alignment of endoglin from different species.
The protein sequences of rat, mouse, human, and chicken endoglin were aligned using the ClustalW2 tool (http://www.expasy.org/genomics/sequence_alignment). Sequences of mouse (NP_031958), rat (AAS67893), human (NP_001108225) and chicken (AAT84715) were taken from the GenBank (http://www.ncbi.nlm.nih.gov/). The Arginine-Glycin-Aspartic acid sequence in human endoglin (aa 399-aa 401) is underlined. Fully conserved aa in endoglin are marked by asterisk (*), positions that carry aa with strongly similar properties by a colon (:) and positions with weakly similar properties by a period (.), respectively. Please note that the highest degree of homology is found at the C-terminal regions that encompass the cytosolic part of endoglin.
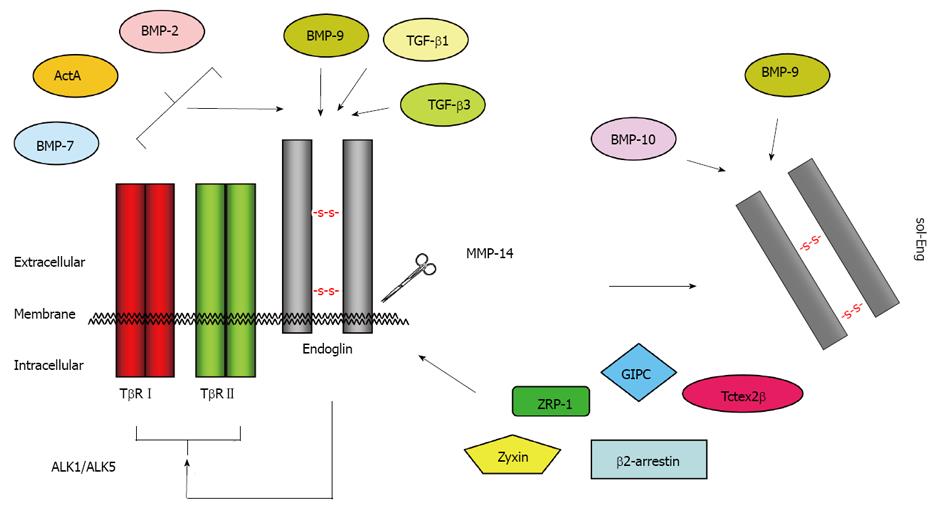
Figure 4 Binding partners of endoglin.
Endoglin physically interacts via its extracellular domain with TGF-β1, TGF-β3 and BMP-9[110]. The short cytoplasmic domain has affinity for ZRP-1[63], Zyxin[64], GIPC[67], β-arrestin-2[66], and Tctex2β[65]. In conjunction with TβRI and TβRII, the binding spectrum is extended to BMP-2, BMP-7 and ActA[111]. After proteolytic cleavage (shedding) by MMP-14 (also known as membrane-type matrix metalloproteinase MT1-MMP), the soluble form of endoglin (sol-Eng) is released[59]. This form has capacity to bind BMP-9 and BMP-10[113]. TGF: Transforming growth factor; BMP: Bone morphogenetic protein.
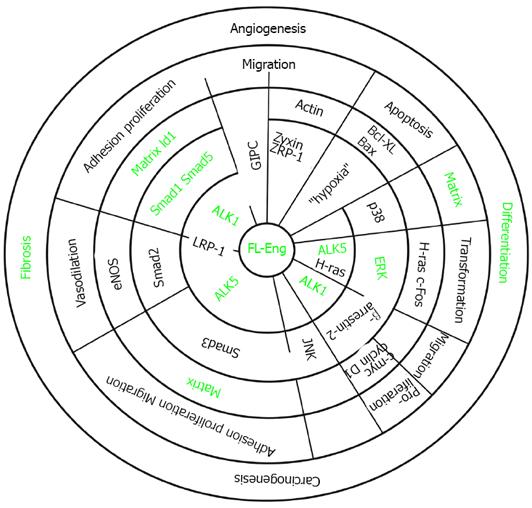
Figure 5 Association of endoglin with different signaling cascades.
The concentrical circles display the hierarchy of signaling. Signaling starts at the membrane with receptors and adaptors (inner two circles). The next circle represents activated intermediates and adaptors. Thereafter, target genes are indicated. These are involved in shaping a cellular response which is part of a complex process (last circle). Partially open radial lines indicate that the corresponding molecules interact or interaction of molecules is mediated by the protein displayed on the radial line (LRP-1). The green font indicates items addressed in liver cells which are modulated by endoglin.
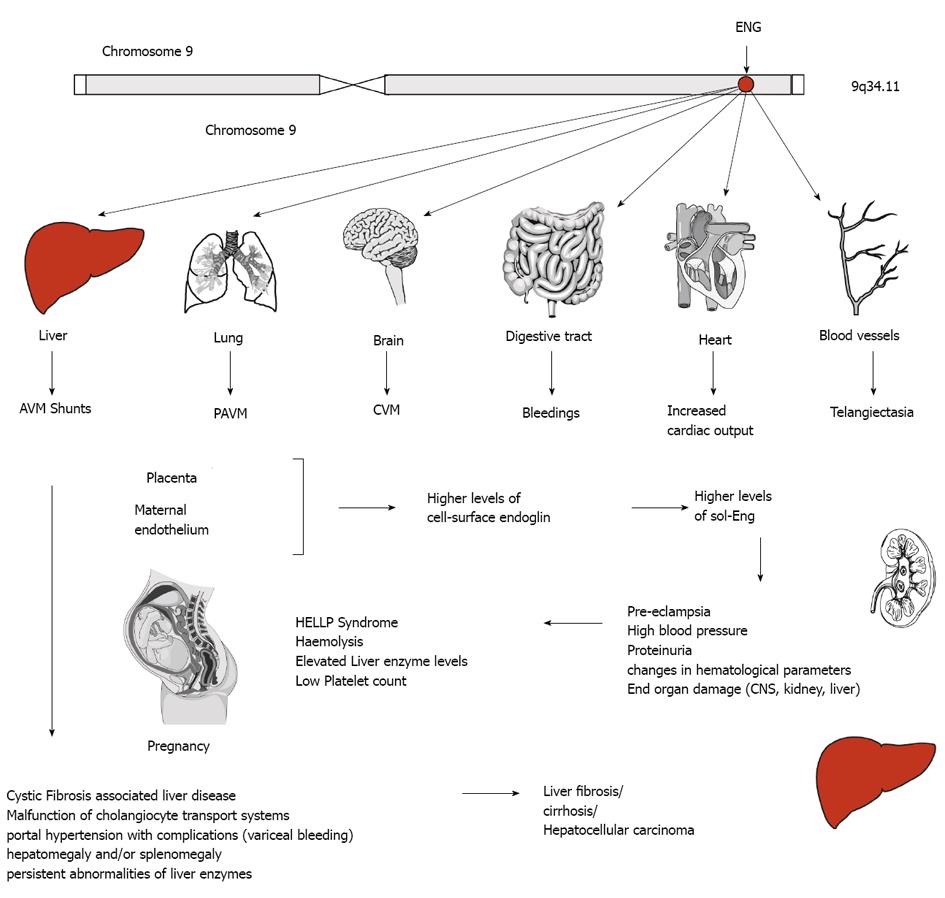
Figure 6 Endoglin and disease.
The human endoglin gene (ENG) is located on the long arm of human chromosome 9. Mutations are inherited in an autosomal dominant manner and affect several organs. In liver, abnormal connection formed between blood vessels, arteriovenous malformations (AVM), malfunction of the cholangiocyte transport system gives rise to liver damage indicated by portal hypertension, persistent abnormalities of liver enzymes, hepatomegaly and/or splenomegaly, fibrosis, cirrhosis or even hepatocellular carcinoma. Intrahepatic connection between arteries and veins results in a large amount of blood bypasses for which the heart compensates by increasing the cardiac output resulting on long term in heart insufficiency. Similar arteriovenous (pulmonary AVM, cerebral AVM) are found in lung and brain. In the digestive tract bleedings occur and telangiectasias of blood vessels are found on the skin of the hands, face and mouth. During pregnancy, the placenta and the maternal endothelium produce higher levels of cell-surface endoglin that is shedded and leads to higher systemic concentration of soluble endoglin (sol-Eng) that leads to an imbalance of the antiangiogenic factors resulting in life-threatening obstetric complication (e.g., pre-eclampsia, HELLP syndrome).
Figure 7 Endoglin in diagnostics.
Several distinct mutations in the endoglin gene (ENG) give rise to hereditary hemorrhagic telangiectasia (HHT) that is mainly characterized by epistaxes (nosebleed), various visceral lesions, telangiectasia (spider veins) and arteriovenous malformations. Patients often show an appropriate family history. The clinical diagnosis “HHT” is made if three of the four classical signs (i.e., epistaxes, visceral lesions, telangiectasia and family history) occur. Elevated levels of soluble endoglin have been reported in patients suffering from hemolysis, elevated liver enzymes and low platelets syndrome (HELLP), pre-eclampsia, type 2 diabetes, atherosclerosis, tumorgenesis in several organs, and fibrogenesis.
- Citation: Meurer SK, Alsamman M, Scholten D, Weiskirchen R. Endoglin in liver fibrogenesis: Bridging basic science and clinical practice. World J Biol Chem 2014; 5(2): 180-203
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/180.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.180