Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Jun 26, 2011; 2(6): 161-166
Published online Jun 26, 2011. doi: 10.4331/wjbc.v2.i6.161
Published online Jun 26, 2011. doi: 10.4331/wjbc.v2.i6.161
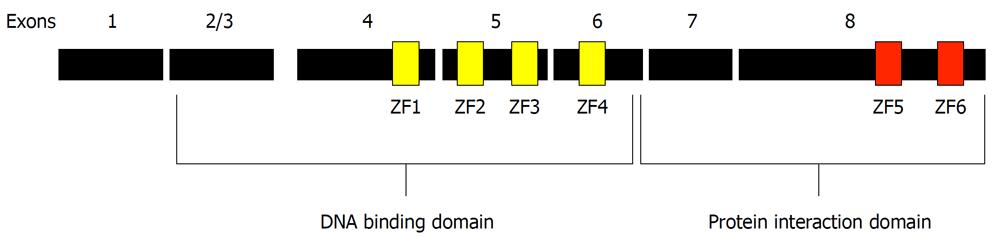
Figure 1 Architecture of the Ikaros protein.
The full-length Ikaros is shown in context of the exons present in the longest form of Ikaros. The positions of the six zinc fingers are shown in their approximate locations. Fingers 1-4 are contiguous as well as fingers 5 and 6.
Figure 2 Primary sequence of the six zinc fingers of Ikaros.
The sequences of the six zinc fingers of human Ikaros are shown along with their respective linkers (UniProt: gi|3913926). The letters in red are the consensus Cys and His residues that chelate zinc in the fingers. The blue highlighted letters represent the -1, 2, 3, and 6 positions of the finger helices read from left to right.
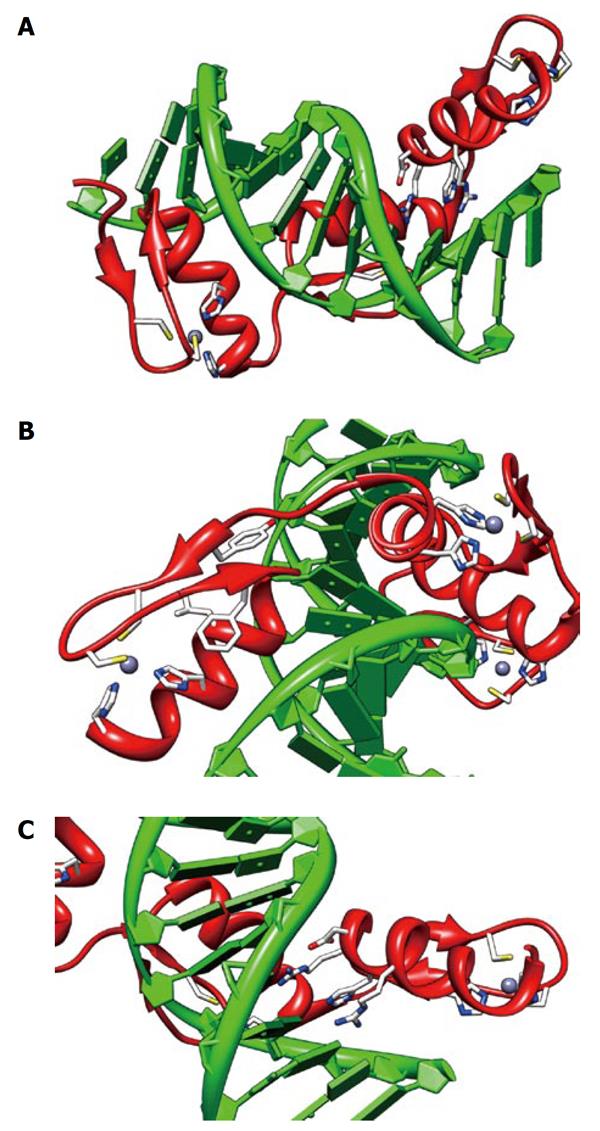
Figure 3 Structure of an engineered peptide with three tandem zinc fingers similar to Ikaros ZF2-3.
The views shown are from 1MEY (pdb), an engineered three tandem zinc finger peptide (shown in red) in complex with cognate DNA[4] shown in green. All three fingers show the zinc (grey sphere) complexed to two sulfurs of cysteine (yellow) and two imidazole nitrogen (blue). A: An overview of the zinc fingers nestled in the major groove of the DNA. The N terminus is on the left. The C-terminal finger has the DNA-interacting side chains shown; B: A view of the N-terminal finger showing the seven essential residues for zinc finger structural integrity; C: A view of the DNA-interacting residues on the C-terminal finger, -1:arg, 2:asp, 3:his, 6:arg. The views were produced using CHIMERA[35].
- Citation: Payne MA, Biochemistry DOCA, University LS, Parkway 4R, Riverside, 92515 C, States U. Zinc finger structure-function in Ikaros Marvin A Payne. World J Biol Chem 2011; 2(6): 161-166
- URL: https://www.wjgnet.com/1949-8454/full/v2/i6/161.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i6.161