Copyright
©The Author(s) 2017.
World J Diabetes. Apr 15, 2017; 8(4): 154-164
Published online Apr 15, 2017. doi: 10.4239/wjd.v8.i4.154
Published online Apr 15, 2017. doi: 10.4239/wjd.v8.i4.154
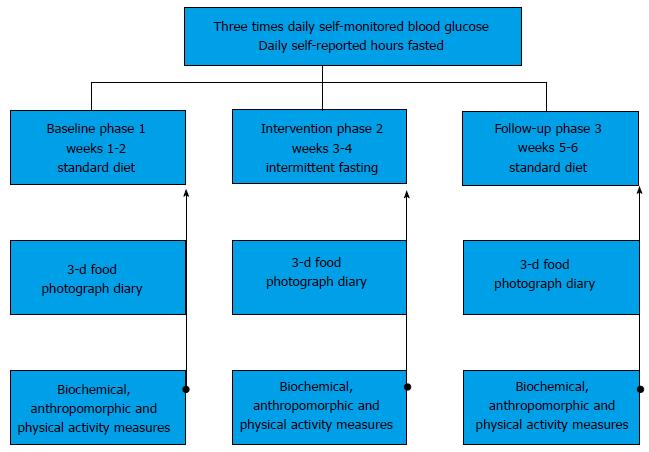
Figure 1 Study design.
During the three-phase, 6 wk study, participants engaged in normal dietary patterns (breakfast, lunch and dinner) during weeks 1-2 (Phase 1, baseline) and 5-6 (Phase 3, follow-up). For weeks 3-4 (Phase 2, intervention) participants followed the IF meal timing pattern with a goal of daily fasts for 18-20 h per day, and a 4-6 h feeding period. Ad libidum zero-calorie intake, including coffee and tea during fasting hours, was permitted. Hours fasted and self-monitored blood glucose (fasted a.m., random afternoon pre-meal, postprandial evening) was reported daily throughout the study. On the last day (day 14, 28 and 42) of each phase, biochemical (fasting bloodwork), anthropomorphic (clinical assessment), and YPAS physical activity was collected. A 3-d consecutive photographic food diary was collected during each of the three phases. YPAS: Yale Physical Activity Survey; IF: Intermittent fasting.
- Citation: Arnason TG, Bowen MW, Mansell KD. Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World J Diabetes 2017; 8(4): 154-164
- URL: https://www.wjgnet.com/1948-9358/full/v8/i4/154.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i4.154