Published online Jan 15, 2017. doi: 10.4239/wjd.v8.i1.18
Peer-review started: July 12, 2016
First decision: September 12, 2016
Revised: September 30, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 15, 2017
To determine the clinical and dietary predictors of common carotid artery intima media thickness (CCA IMT) in a cohort of subjects with type 1 and type 2 diabetes.
Participants with type 1 (n = 23) and type 2 diabetes (n = 127) had mean and mean maximum CCA IMT measured using B mode ultrasound. Dietary intake was measured using a food frequency questionnaire. Clinical and dietary predictors of mean and mean maximum CCA IMT were determined using linear regression analysis adjusted for potential confounders.
The main predictors of mean and mean maximum CCA IMT were age and weight. After multivariate adjustment there were no dietary predictors of CCA IMT. However, in subjects that were not prescribed a lipid lowering medication alcohol consumption was positively associated with CCA IMT after multivariate adjustment. No difference existed in CCA IMT between subjects with type 1 or type 2 diabetes once age was adjusted for.
CCA IMT was predominantly predicted by age and weight in these subjects with diabetes. The finding that CCA IMT was not different between people with type 1 and type 2 diabetes warrants further investigation in a larger cohort.
Core tip: This paper examines clinical, dietary and biochemical predictors of common carotid artery intima media thickness (CCA IMT) in a population of participants with type 1 and type 2 diabetes. The only predictors of CCA IMT in this group were age and body weight. After age adjustment CCA IMT was not different in subjects with type 1 or type 2 diabetes.
- Citation: Petersen KS, Keogh JB, Meikle PJ, Garg ML, Clifton PM. Clinical and dietary predictors of common carotid artery intima media thickness in a population with type 1 and type 2 diabetes: A cross-sectional study. World J Diabetes 2017; 8(1): 18-27
- URL: https://www.wjgnet.com/1948-9358/full/v8/i1/18.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i1.18
In 2013, 8.3% of the world’s population had type 1 or type 2 diabetes and the incidence is projected to increase by 55% to 8.8% by 2035[1]. Individuals with type 1 and type 2 diabetes have two to three times the risk of developing cardiovascular disease (CVD) compared to the general population[2-4]. Dietary intake is a modifiable risk factor for CVD with epidemiological studies showing that better diet quality is associated with a reduced risk of CVD in people with diabetes[5,6].
Carotid intima media thickness (IMT) measured using B mode ultrasound, is an early marker of atherosclerosis[7] and predictor of CVD[8]. Type 1 and type 2 diabetes is associated with greater carotid IMT when compared to non-diabetic subjects[9,10]. In individuals with type 1 or type 2 diabetes, per 0.1 mm increase in common carotid artery intima media thickness (CCA IMT), the hazard ratio for a cardiovascular event is 1.12 (95%CI: 1.07-1.16)[11].
Lifestyle factors play a role in the aetiology of carotid IMT progression; an improvement in dietary (saturated fat, fibre, potassium, calcium intake) and lifestyle (smoking and physical activity) factors was associated with lower CCA IMT after 20 years, in a cohort of young adults, independent of demographic factors, medication use and baseline dietary and lifestyle factors[12]. In addition, epidemiological studies show that a higher intake of fruit, olive oil, wholegrains and soluble fibre and lower consumption of saturated fat in favour of polyunsaturated fat is associated with lower carotid IMT[13]. The aim of this study is to determine the clinical and dietary predictors of CCA IMT in a population of adults with type 1 and type 2 diabetes.
This is a cross-sectional study investigating the predictors of CCA IMT in subjects with type 1 and type 2 diabetes. One hundred and fifty subjects were recruited by public advertisement between August 2012 and December 2013. Subjects eligible for inclusion were adults (age > 18 years) with diagnosed type 1 or type 2 diabetes for any duration, managed with diet, oral hypoglycaemic agents and/or insulin. Subjects were excluded if they reported having cancer, unstable CVD requiring intervention, heart failure, significant renal impairment (eGFR < 30 mL/min) or liver disease. Ethics approval was obtained from the University of South Australia Human Research Ethics Committee and all participants provided written informed consent. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12612001052820).
Subjects attended the clinic after an overnight fast on one occasion. Anthropometric measurements and blood pressure were taken by one operator. A blood sample was taken and a random spot urine sample was provided. Ultrasound was used to measure CCA IMT. Participants completed the online version of the Dietary Questionnaire for Epidemiological Studies Version 2 Food Frequency Questionnaire (DQES v2 FFQ) to determine habitual dietary intake.
Height was measured using a stadiometer (SECA, Hamburg, Germany) to the nearest 0.1 cm while barefoot/flat footwear. Weight was measured to the nearest 0.05 kg using calibrated electronic scales (SECA, Hamburg, Germany) while the participants were barefoot/light footwear and wearing light clothing.
Clinic brachial blood pressure was measured using an automated sphygmomanometer (SureSigns VS3; Philips, North Ryde, Australia) once the participant had been seated for 5 min. A normal sleeve (16 cm × 52 cm) was used for an arm circumference of 24-32 cm and a large sleeve (16 cm × 70 cm) for an arm circumference of 32-42 cm. A minimum of four consecutive readings were taken at 1 min intervals. The first reading was discarded and the following three consistent measurements, i.e., systolic blood pressure within a range of 10 mmHg, were used.
Measurements of the carotid artery were taken using B mode ultra-sound by one operator, with an intra-observer coefficient of variation (CV) of 4.4% (n = 34). Participants were supine with their head positioned at 45 degrees away from the side of the neck being measured. A high resolution ultrasound machine with a 12 MHz transducer was used (Samsung Medison MySono U6, South Korea). A 1 cm region of the IMT on the far wall of the common carotid artery was measured using automatic edge detection software (Samsung Medison MySono U6 Auto IMT, South Korea) as recommended in the Mannheim Carotid Intima-Media Thickness and Plaque Consensus Paper (2004-2006-2011)[14]. Three clips (3 s each) were captured and the mean of 10 measurements taken from each of these clips was averaged for a mean and mean maximum CCA IMT value.
A fasting blood sample was taken and serum total cholesterol, HDL cholesterol, triglycerides, C reactive protein and glucose were measured using a Konelab 20XTi automatic analyser (Thermo Electron Corporation, Louisville, CO, United States) with reagents from Thermo Fisher Scientific (Melbourne, Australia). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula [total cholesterol - high-density lipoprotein (HDL) cholesterol - (triglycerides × 0.45)][15]. Three subjects had a triglyceride level > 4.5 mmol/L which precluded LDL measurement. Serum carotenoids were measured by high performance liquid chromatography according to a previously published protocol[16]. Lipid analysis was performed by liquid chromatography, electrospray ionization-tandem mass spectrometry as previously published[17]. Briefly, 333 individual lipid species from 25 classes were measured. The median intra assay CV was 8%.
A random spot urine sample was provided by participants when they attended the clinic. Aliquots of the urine were taken and frozen at -20 °C until analysis of sodium, potassium, creatinine and albumin was done by SA Pathology (Frome Rd, Adelaide, Australia), an accredited commercial laboratory. The albumin to creatinine ratio was calculated from one spot urine sample to determine the presence of micro-albuminuria. Micro-albuminuria was defined as an albumin to creatinine ratio > 2.5 for men and > 3.5 for women[18].
The participants were asked to provide the pathology report from their most recent haemoglobin A1c (HbA1c) measurement or the result was sourced from their general practitioner or the pathology company.
Habitual dietary intake was measured using the electronic version of the DQES v2 FFQ as previously described[19]. This FFQ has been validated in a population with type 1 and type 2 diabetes[20].
Data are presented as mean ± standard deviation (SD) or median (interquartile range) depending on the distribution. Data were checked for normality using Shapiro-Wilk and Kolmogorov-Smirnov values. Independent samples t tests were used to determine differences between subjects with type 1 and type 2 diabetes for continuous variables and χ2 tests were used for categorical variables. Pearson’s correlation was used to determine clinical and dietary predictors of mean and mean maximum CCA IMT. Clinical variables that were correlated (P < 0.1) with CCA IMT were entered into stepwise linear regression (includes both forwards and backwards selection) to determine predictors. For inclusion in the model P < 0.05, bivariate correlations adjusted for age, sex and weight were used to determine the dietary predictors of CCA IMT. Serum carotenoids and serum lipid concentrations were normalised to their respective interquartile ranges to account for the variation in relative abundance in serum. P values for the serum lipid species were corrected for multiple comparisons using the Benjamini Hochberg approach[21]. Analysis was performed using SPSS (version 19, 2010, SPSS Inc, Chicago, IL). Statistical significance was set at P < 0.05.
Subjects were 150 free-living people with diagnosed type 1 or type 2 diabetes (Table 1). After adjustment for age and weight there was no statistically significant difference between the participants with type 1 or type 2 diabetes with regards to blood pressure, total cholesterol, LDL cholesterol, HbA1c or CCA IMT. The subjects with type 2 diabetes did have lower HDL cholesterol (1.1 ± 0.3 mmol/L vs 1.5 ± 0.3 mmol/L; P < 0.001) and higher triglycerides (1.4 ± 1.2 vs 0.6 ± 0.4; P < 0.05) when compared to the type 1 subjects. The type 1 subjects had been diagnosed with diabetes for a longer duration compared to the participants with type 2 diabetes (19 ± 14 years vs 8 ± 7 years; P = 0.001). Fifty-seven percent of the cohort was men.
| Characteristic | Whole cohort (n = 150) | Type 1 diabetes (n = 23) | Type 2 diabetes (n = 127) | P value1 |
| Age (yr) | 56 ± 14 | 36 ± 14 | 60 ± 11 | 0.0001 |
| Weight (kg) | 96.9 ± 21.4 | 85.6 ± 25.2 | 98.9 ± 20.0 | 0.006 |
| Height (m) | 1.7 ± 0.1 | 1.71 ± 0.1 | 1.71 ± 0.1 | 0.90 |
| BMI (kg/m2) | 33.1 ± 7.0 | 29.4 ± 9.3 | 33.8 ± 6.3 | 0.005 |
| Sex, male n (%) | 86 (57) | 9 (39) | 77 (61) | 0.06 |
| Diagnosed with diabetes (yr) | 9.7 ± 9.3 | 19.1 ± 14.2 | 8.1 ± 6.9 | 0.001 |
| Smoking status n (%) | 0.25 | |||
| Never smoked | 74 (49) | 15 (65) | 59 (47) | |
| Past smoker | 68 (46) | 7 (31) | 61 (48) | |
| Current smoker | 8 (5) | 1 (4) | 7 (5) | |
| Smoking pack years2 (yr) | 9.6 ± 15.6 | 1.7 ± 4.4 | 11.0 ± 16.5 | 0.008 |
| Systolic blood pressure (mmHg) | 127 ± 14 | 124 ± 14 | 128 ± 14 | 0.15 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 69 ± 9 | 72 ± 10 | 0.18 |
| Mean arterial pressure (mmHg) | 90 ± 10 | 87 ± 8 | 91 ± 10 | 0.10 |
| Heart rate (bpm) | 74 ± 13 | 77 ± 10 | 73 ± 13 | 0.21 |
| Pulse pressure (mmHg) | 56 ± 13 | 54 ± 15 | 56 ± 13 | 0.60 |
| Mean CCA IMT (mm) | 0.71 ± 0.13 | 0.58 ± 0.12 | 0.73 ± 0.11 | < 0.001 |
| Mean maximum CCA IMT (mm) | 0.79 ± 0.14 | 0.66 ± 0.13 | 0.81 ± 0.12 | < 0.001 |
| Prescribed anti-hypertensive medication n (%) | 85 (57) | 7 (30) | 78 (61) | 0.006 |
| Prescribed lipid lowering medication n (%) | 82 (55) | 4 (17) | 78 (61) | < 0.001 |
| Diabetes treatment n (%) | < 0.001 | |||
| None | 30 (20.0) | 0 (0) | 30 (24) | |
| OHA | 65 (43.3) | 0 (0) | 65 (51) | |
| Insulin | 26 (17.3) | 23 (100) | 3 (2) | |
| OHA + insulin | 29 (19.3) | 0 (0) | 29 (23) | |
| Total cholesterol (mmol/L) | 4.1 ± 1.0 | 4.1 ± 1.0 | 4.1 ± 1.0 | 0.99 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.4 | 1.5 ± 0.3 | 1.1 ± 0.3 | < 0.001 |
| LDL cholesterol (mmol/L) | 2.4 ± 0.9 | 2.4 ± 0.9 | 2.4 ± 0.9 | 0.97 |
| Triglycerides (mmol/L) | 1.3 ± 1.2 | 0.6 ± 0.4 | 1.4 ± 1.2 | 0.003 |
| Glucose (mmol/L) | 8.1 ± 3.0 | 10.1 ± 4.0 | 7.7 ± 2.6 | < 0.001 |
| HbA1c (%) (mmol/mol) | 7.3 ± 1.4 (57 ± 16) | 7.6 ± 1.1 (60 ± 12) | 7.3 ± 1.5 (56 ± 16) | 0.27 |
| CRP (mg/L) | 2.5 ± 2.2 | 1.6 ± 1.9 | 2.6 ± 2.3 | 0.15 |
| Presence of Microalbuminuria n (%)3 | ||||
| Yes | 23 (15) | 3 (13) | 20 (16) | 0.74 |
| No | 127 (85) | 20 (87) | 107 (84) | |
| Spot urine Na:K | 1.3 ± 0.9 | 1.4 ± 0.9 | 1.3 ± 0.9 | 0.62 |
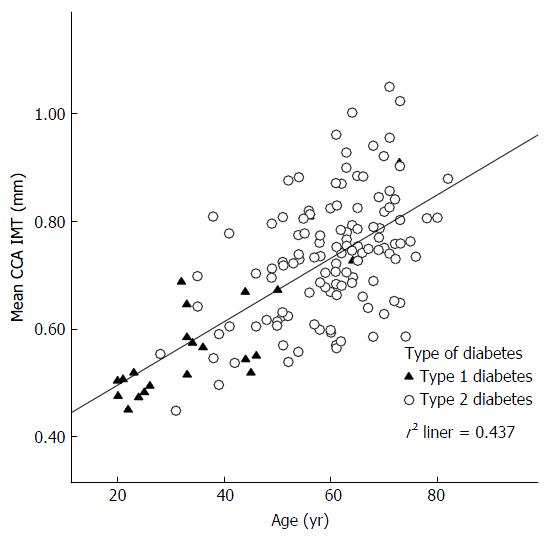
Age was the strongest predictor of mean CCA IMT (β = 0.006; t = 10.8; P < 0.001) and mean maximum CCA IMT (β = 0.006; t = 10.5; P < 0.001). Figure 1 shows the correlation between age and mean CCA IMT by diabetes type. After age adjustment there was no statistically significant difference between mean (type 1: 0.69 mm; type 2: 0.71 mm; P > 0.05) or mean maximum CCA IMT (type 1: 0.78 mm; type 2: 0.79 mm; P > 0.05) by diabetes type.
In univariate analysis CCA IMT was strongly correlated with age and weakly correlated with smoking pack years, body mass index (BMI), systolic blood pressure, HDL cholesterol and prescription of anti-hypertension and lipid lowering medication (Table 2). After adjustment for age, weight was the only other correlate of mean (β = 0.001; t = 2.4; P = 0.017) and mean maximum CCA IMT (β = 0.001; t = 2.3; P = 0.02). The correlation between weight and CCA IMT was independent of sex. The summarised r2 values for the mean and mean maximum CCA IMT models (including age and weight) were 0.46 and 0.44, respectively.
| Predictor | Mean CCA IMT | Mean maximum CCA IMT | Mean CCA IMT | Mean maximum CCA IMT | ||||
| r | r | β | t | P | β | t | P | |
| Age | 0.661 | 0.651 | 0.006 | 10.8 | 0.0001 | 0.006 | 10.5 | 0.001 |
| Sex | -0.13 | -0.143 | - | - | ||||
| Type of diabetes | 0.431 | 0.411 | - | - | ||||
| Years since diabetes diagnosis | 0.08 | 0.09 | - | - | ||||
| Smoking pack years | 0.192 | 0.192 | - | - | ||||
| Weight | 0.163 | 0.163 | 0.001 | 2.4 | 0.017 | 0.001 | 2.3 | 0.02 |
| BMI | 0.182 | 0.182 | - | - | ||||
| Systolic blood pressure | 0.261 | 0.271 | - | - | ||||
| Diastolic blood pressure | -0.01 | -0.006 | - | - | ||||
| Heart rate | -0.241 | -0.231 | - | - | ||||
| Pulse pressure | 0.291 | 0.291 | - | - | ||||
| Anti-hypertension medication prescription | -0.341 | -0.321 | - | - | ||||
| Lipid lowering medication prescription | -0.301 | -0.301 | - | - | ||||
| Total cholesterol | -0.001 | -0.001 | - | - | ||||
| LDL cholesterol | -0.007 | -0.02 | - | - | ||||
| HDL cholesterol | -0.182 | -0.172 | - | - | ||||
| Triglycerides | 0.13 | 0.143 | - | - | ||||
| Fasting glucose | 0.03 | 0.03 | - | - | ||||
| HbA1c | -0.04 | -0.04 | - | - | ||||
| Microalbuminuria | 0.153 | 0.153 | - | - | ||||
| Summarised r2 model | 0.46 | 0.44 | ||||||
When participants prescribed an anti-hypertensive medication were excluded from the analysis, age and weight remained independent predictors of mean and mean maximum CCA IMT and the summarised r2 values were 0.55 and 0.53, respectively (n = 65). When participants that were prescribed a lipid lowering medication were removed from the analysis, univariate analysis (n = 68) showed that age (r = 0.70; P = 0.001), diabetes type (r = 0.44; P = 0.001), smoking pack years (r = 0.25; P = 0.04), systolic blood pressure (r = 0.43; P = 0.001), pulse pressure (r = 0.33; P = 0.007) and prescription of anti-hypertensive medication (r = -0.39; P = 0.001) were correlated with mean CCA IMT. In a stepwise model age (β = 0.005; t = 6.8; P = 0.001) and systolic blood pressure (β = 0.002; t = 2.4; P = 0.02) were the only predictors of mean CCA IMT. The summarised r2 value for the model was 0.51.
Median macronutrient intake for the cohort was 21%, 37%, 14% and 40%, respectively for protein, total fat, saturated fat and carbohydrate (Table 3). Dietary intake by food group is presented in Table 4. After adjustment for age, sex and weight there were no dietary predictors of mean or mean maximum CCA IMT, see Tables 3 and 4. When weight was removed from the model there were still no dietary predictors.
| Nutrient intake | Median (IQR) | Mean CCA IMT | Mean maximum CCA IMT | ||
| Univariate | Adjusted3 | Univariate | Adjusted3 | ||
| r | r | r | r | ||
| Energy (kcal) | 1652 (1343, 2165) | -0.162 | -0.042 | -0.152 | -0.052 |
| Protein (g/d) | 88 (66,112) | -0.181 | -0.022 | -0.171 | -0.022 |
| % E protein | 21 (18, 23) | 0.022 | 0.102 | 0.032 | 0.112 |
| Total fat (g/d) | 66 (52, 92) | -0.181 | -0.072 | -0.181 | -0.082 |
| % E total fat | 37 (33, 40) | -0.142 | -0.132 | -0.162 | -0.152 |
| Saturated fat (g/d) | 25 (19, 35) | -0.161 | -0.072 | -0.171 | -0.092 |
| % E saturated fat | 14 (12, 16) | -0.152 | -0.142 | -0.161 | -0.162 |
| Monounsaturated fat (g/d) | 24 (18, 33) | -0.181 | -0.072 | -0.181 | -0.082 |
| % E monounsaturated fat | 13 (12, 15) | 0.192 | -0.092 | -0.132 | -0.112 |
| Polyunsaturated fat (g/d) | 11 (8, 14) | -0.142 | -0.062 | -0.132 | -0.052 |
| % E polyunsaturated fat | 6 (5, 7) | 0.032 | -0.022 | 0.032 | -0.022 |
| Carbohydrate (g/d) | 160 (134, 216) | -0.132 | -0.062 | -0.132 | -0.062 |
| % E carbohydrate | 40 (36, 44) | 0.042 | -0.062 | 0.042 | -0.062 |
| Sugar (g/d) | 74 (56, 94) | -0.012 | 0.032 | -0.012 | -0.032 |
| Fibre (g/d) | 20 (16, 26) | -0.122 | -0.042 | -0.122 | -0.052 |
| Sodium (mg/d) | 2177 (1696, 2957) | -0.162 | -0.062 | -0.152 | -0.062 |
| Potassium (mg/d) | 2771 (2128, 3403) | -0.072 | 0.032 | -0.062 | 0.042 |
| Alcohol (g/d) | 2 (0, 8) | 0.062 | 0.112 | 0.072 | 0.112 |
| Dietary intake | g/d median (IQR) | Mean CCA IMT | Mean maximum CCA IMT | ||
| Univariate | Adjusted4 | Univariate | Adjusted4 | ||
| r | r | r | r | ||
| Total breads and cereals | 204 (141, 320) | -0.202 | -0.073 | -0.182 | -0.053 |
| Breakfast cereal | 18 (3, 45) | -0.182 | -0.183 | -0.192 | -0.203 |
| Bread | 60 (30, 90) | 0.063 | -0.083 | 0.063 | -0.073 |
| Pasta/rice | 59 (32, 109) | -0.281 | 0.013 | -0.271 | 0.013 |
| Total vegetables/legumes | 151 (112, 217) | 0.063 | 0.103 | 0.063 | 0.093 |
| Vegetables | 140 (103, 206) | 0.063 | 0.113 | 0.063 | 0.103 |
| Legumes | 7 (3, 13) | -0.023 | -0.073 | -0.023 | -0.063 |
| Total fruit | 239 (120, 340) | 0.133 | 0.113 | 0.133 | 0.103 |
| Fresh/canned fruit | 187 (99, 282) | 0.192 | 0.163 | 0.182 | 0.153 |
| Juice | 6 (0, 41) | -0.083 | -0.073 | -0.093 | -0.073 |
| Total dairy | 381 (232, 501) | 0.033 | 0.063 | 0.043 | 0.073 |
| Reduced fat dairy | 277 (44, 446) | 0.083 | 0.083 | 0.103 | 0.113 |
| Full fat dairy | 9 (2, 22) | -0.063 | -0.033 | -0.073 | -0.053 |
| Total meats and alternatives | 169 (105, 217) | -0.153 | -0.013 | -0.153 | -0.013 |
| Red meat | 56 (31, 106) | -0.212 | -0.043 | -0.202 | -0.043 |
| Processed meat | 19 (8, 32) | 0.013 | 0.023 | 0.013 | 0.013 |
| Chicken | 34 (19, 51) | -0.163 | 0.033 | -0.153 | 0.043 |
| Fish | 29 (15, 49) | -0.013 | 0.043 | 0.013 | 0.043 |
| Tofu | 0 (0, 1) | -0.103 | -0.063 | -0.093 | -0.043 |
| Nuts | 4 (1, 10) | -0.172 | -0.013 | -0.172 | -0.023 |
| Eggs | 13 (10, 34) | 0.073 | -0.043 | 0.063 | -0.053 |
| Total extra foods5 | 126 (66, 236) | 0.033 | 0.073 | 0.043 | 0.083 |
Subgroup analysis showed that in those subjects that were not prescribed a lipid lowering medication (n = 68) alcohol consumption was associated with mean (β = 0002; t = 2.5; P = 0.02) and mean maximum (β = 0.002; t = 2.4; P = 0.02) CCA IMT after multivariate adjustment for predictors (age, sex and systolic blood pressure). There were no other dietary predictors of CCA IMT identified in the subjects not prescribed a lipid lowering medication. Subgroup analysis including only subjects that were not prescribed an anti-hypertensive medication (n = 65) showed no dietary predictors of CCA IMT after multivariate adjustment.
The spot urine sodium to potassium ratio was not associated with mean or mean maximum CCA IMT. No association existed between mean or mean maximum CCA IMT and total serum carotenoids, β-cryptoxanthin, lutein, zeaxanthin, lycopene, α-carotene or β-carotene. There was no correlation between lipid species, expressed as concentration normalised to the interquartile range and mean or mean maximum CCA IMT, after adjustment for predictors and multiple comparisons. Subgroup analysis by anti-hypertensive and lipid lowering prescription showed no differential effect for the association between CCA IMT and serum carotenoids, lipid species and the spot urine sodium to potassium ratio.
In this cohort of well-controlled subjects with type 1 and type 2 diabetes the main predictors of mean CCA IMT were age and weight explaining 46% of the variance in the model. After adjustment for age, sex and weight there were no dietary predictors of mean or mean maximum CCA IMT. However, in subjects that were not prescribed a lipid lowering medication alcohol consumption was positively associated with CCA IMT after multivariate adjustment. There was no correlation between serum lipid species or carotenoids and CCA IMT. It was found that after adjustment for age CCA IMT was not different between people with type 1 and type 2 diabetes.
In this population, age was the strongest predictor of CCA IMT. Age is well established as a predictor of CCA IMT in subjects with type 1[10,22] and type 2 diabetes[23]. In addition, weight was a positive predictor of CCA IMT in this study, independent of sex, and has been previously associated with CCA IMT in cohorts with and without diabetes[24,25]. Univariate analysis showed male sex was non-significantly associated with greater mean (P = 0.12) and mean maximum (P = 0.089) CCA IMT. Previously it has been shown that male sex is associated with greater CCA IMT in people with diabetes[23,24]. Univariate analysis showed that smoking pack years was weakly positively associated with mean and mean maximum CCA IMT but this correlation did not persist after adjustment for age and weight. This is likely to be because less than half of the cohort had ever smoked and only 5% were current smokers.
Blood pressure, lipids, presence of micro-albuminuria and glycaemic control were not correlated with CCA IMT after age was adjusted for. When subjects that were prescribed a lipid lowering medication were removed from the analysis systolic blood pressure did independently predict CCA IMT. Therefore we may not have detected associations between CCA IMT and blood pressure, lipids, presence of micro-albuminuria and glycaemic control because over 50% of the cohort were prescribed lipid lowering and anti-hypertensive medications and the participants were well controlled in terms of blood pressure, lipid and glucose levels. In this cohort recommendations for blood pressure (< 140/85 mmHg), LDL cholesterol (< 2.5 mmol/L), triglycerides (< 2.2 mmol/L) and HDL cholesterol (> 1 mmol/L) as defined by the 2013 European Society of Cardiology/European Association for the Study of Diabetes Guidelines on Diabetes, Pre-diabetes and Cardiovascular Disease were met[26]. In our cohort mean CCA IMT was lower than what has been observed in other populations with diabetes of a similar age. In a meta-analysis involving 4420 individuals with type 1 or type 2 diabetes the mean CCA IMT was 0.79 ± 0.19 mm (mean age 61; IQR = 36-76 years) compared to 0.71 ± 0.13 mm in our cohort (mean age 56 ± 14 years)[11].
The lack of an association between HbA1c and CCA IMT in the present study may be explained by the mean HbA1c (7.3% ± 1.4%) which is lower than what has been reported in studies that have shown a relationship between carotid IMT and HbA1c. Kinouchi et al[27] found HbA1c was associated with maximum carotid IMT in a Japanese cohort with type 2 diabetes (n = 167) with a mean HbA1c of 8.3% ± 2.3%. Similarly, Shah et al[28] showed a positive relationship between HbA1c and CCA IMT in a cohort of youth with type 2 diabetes (n = 129) with a baseline mean HbA1c of 8.6% ± 3.3%. In the SEARCH CVD study in youth with type 1 diabetes BMI z score was the only modifiable risk factor related to carotid IMT[29].
This study shows that CCA IMT values measured at one time-point are not different between subjects with type 1 and type 2 diabetes, once age is adjusted for, despite the subjects with type 2 diabetes being more metabolically at risk due to higher weight, BMI and triglycerides and lower HDL cholesterol. In contrast to our finding, in a cohort of subjects with newly diagnosed type 1 or type 2 diabetes (type 1: 33 ± 24 d since diagnosis; type 2: 36 ± 21 d since diagnosis) aged 14 to 30 years (mean age type 1: 21 ± 5 years; type 2: 22 ± 5 years) CCA IMT was significantly greater in those with type 2 diabetes[30]. It is likely that the subjects with type 2 diabetes in the study by Gu et al[30] had lived with metabolic abnormalities for many years in contrast with the type 1 subjects. In the context of the current study, the finding of the study by Gu et al[30] suggests that the duration of type 1 diabetes contributes to carotid IMT. This is supported by a study showing that in subjects with type 1 diabetes, for a mean of 5.5 years, carotid IMT was comparable to healthy subjects 10 years older[31]. Previous research shows carotid IMT progression is similar in people with type 1 (0.036 mm/year)[32] and type 2 diabetes (0.04 mm/year)[33] and significantly greater than the rate of progression observed in non-diabetic people (0.0147 mm/year)[34].
Type 1 and type 2 diabetes are perceived to be different diseases but the risk factors for CVD in both types of diabetes are similar and include insulin resistance, obesity, inflammation, and renal disease[35]. In addition, the burden of CVD is comparable in people with type 1 and type 2 diabetes[3]. Lind et al[4] showed that even when people with type 1 diabetes achieve a HbA1c of less than 6.9% (52 mmol/mol) the hazard ratio for death from a cardiovascular cause is 2.92 (95%CI: 2.07-4.13) compared to age and sex matched people without diabetes. In summary, it was found that CCA IMT was not different between people with type 1 and type 2 diabetes once age was accounted for, but it must be acknowledged that a small number (n = 23) of people with type 1 diabetes were included in the study. In the future this finding needs to be investigated in a larger cohort of age matched people with type 1 and type 2 diabetes; ideally a healthy control group would be included to determine whether age has a differential effect in people with diabetes.
In this cohort we observed no association between dietary intake and CCA IMT once age, weight and sex were adjusted for. Subgroup analysis showed that in those that were not prescribed a lipid lowering medication alcohol consumption was positively associated with CCA IMT after multivariate adjustment. Alcohol consumption has been previously shown to correlate with CCA IMT in a healthy Korean population such that in men an inverse relationship was observed but this was attenuated to non-significance after adjustment for lipids; in women a positive association between alcohol consumption and CCA IMT was observed[36]. The Cardiovascular Risk in Young Finns study also showed a positive association between alcohol intake and CCA IMT[37].
Previous observational studies, with a similar sample size to the present study, conducted in populations with type 2 diabetes[38,39] have shown that greater fruit consumption is associated with lower carotid IMT (n = 255) and an inverse association between plasma vitamin C concentration and CCA IMT has been shown in individuals with type 1 diabetes (n = 59)[40]. Curtis et al[41] conducted a 1 year randomised controlled trial in post-menopausal women (n = 93) with type 2 diabetes and found that supplementation with 27 g/d of flavonoid-enriched chocolate did not change CCA IMT progression compared to the placebo. A recent meta-analysis of randomised controlled trials showed that lifestyle modification can slow carotid IMT progression[42].
Studies investigating the relationship between carotid IMT and carotenoids including α-carotene, β-carotene, lutein, zeathanin and β-cryptoxanthin have yielded mixed results[43], although there is evidence suggesting an inverse association exists between lycopene and carotid IMT[44]. A recent trial showed that supplementation with lutein (20 mg/d) and lycopene (20 mg/d) reduced CCA IMT progression after 12 mo compared with the placebo treatment in a healthy Chinese population (n = 144)[45]. The authors are not aware of any studies reporting on the relationship between carotenoids and carotid IMT in people with diabetes.
In this analysis there was no association between serum lipid species and CCA IMT. A previous study showed that there was an inverse association between CCA IMT and lysophosphatidylcholine (LPC) 16:0 after adjustment for age and sex (r = -0.13; P = 0.01)[46]. This study also showed that LPC 16:0 and LPC 20:4 were negatively associated with the development of CVD after 12 years of follow-up (LPC 16:0: OR = 0.79; P = 0.028; LPC 20:4; OR = 0.77; P = 0.024 per standard deviation increase)[46]. Lipid profiling has been shown to discriminate between unstable and stable coronary artery disease (CAD) and it has been suggested that changes in plasma lipids precede the development of plaque instability[17]. Lipidomic analysis can predict the burden of non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of CAD[47]. In the study by Ellims et al[47] CCA IMT was not associated with coronary artery plaque burden, although CCA IMT is only weakly correlated with CAD assessed by quantitative coronary angiography[48], despite carotid IMT being correlated with coronary IMT, measured using intravascular ultrasound[49]. Therefore we may not have observed a relationship between CCA IMT and serum lipid species because lipid species are associated with more advanced disease progression.
Limitations of this analysis include the cross-sectional design, small sample size and the use of a FFQ to measure dietary intake, although serum carotenoids were measured as biological markers of vegetable intake. In addition, only CCA IMT was measured and a different result may have been observed if internal carotid artery IMT or bifurcation IMT had been measured as these components are more strongly associated with CAD[50]. Another limitation of this study is that dietary habits were only measured at one point in time and may not be reflective of lifetime exposure, whereas CCA IMT is determined by lifetime exposure, and it is possible that dietary habits or reporting of dietary habits was altered by a range of factors including diabetes diagnosis. The dietary predictors of carotid IMT need to be investigated in a larger cohort as this study may have lacked the required statistical power to detect an effect. Especially since more than half of the cohort was prescribed a lipid lowering or anti-hypertensive medication and this may obscure the relationship between dietary intake and CCA IMT. Power analysis shows that with 120 participants an r value of 0.178 (P < 0.05) or 3% of the variance can be detected so major effects should have been found.
In conclusion, in this cohort of well-controlled individuals with type 1 and type 2 diabetes the strongest predictors of CCA IMT were age and weight, which accounted for 46% of the variance in the model. After multivariate adjustment there were no dietary predictors of mean or mean maximum CCA IMT. However, in subjects that were not prescribed a lipid lowering medication alcohol consumption was positively associated with CCA IMT after multivariate adjustment. There was no correlation between serum lipid species or carotenoids and CCA IMT. There was no difference in mean or mean maximum CCA IMT between subjects with type 1 and type 2 diabetes once age was accounted for but this finding needs to be investigated further.
The authors of this study would like to acknowledge the contributions of study participants and the staff of the University of South Australia. Peter J Meikle is supported by a NHMRC senior research fellowship (1042095). This work was supported by the OIS Program of the Victorian Government, Australia. Jennifer B Keogh is a Fellow of the South Australian Cardiovascular Research Development Program funded by the Heart Foundation and the Government of South Australia. Peter M Clifton is supported by a NHMRC Principal Research Fellowship. Kristina S Petersen is funded by an Australian Postgraduate Award + UniSA Rural and Isolated Top-up Scholarship. This research was jointly funded through these fellowships and the University of South Australia.
Common carotid artery intima media thickness (CCA IMT) is an early marker of atherosclerosis and predictor of cardiovascular disease (CVD). The dietary predictors of CCA IMT in individuals with diabetes are not well defined. Likewise, there is a lack of data from comparative investigations of CCA IMT in individuals with long duration type 1 and type 2 diabetes.
Diabetes diagnosis is an important risk factor for CVD, approximately doubling the risk of a cardiovascular event, and this is independent of conventional risk factors including sex, age, smoking status, body mass index and systolic blood pressure. Due to the increasing incidence of diabetes defining strategies to reduce the burden of CVD in people with diabetes is a priority.
After adjustment for age, sex and weight there were no dietary predictors of CCA IMT identified. For the first time is was shown that there was no difference in mean or mean maximum CCA IMT between subjects with long duration type 1 and type 2 diabetes once age was accounted for. This finding needs to be investigated further in a larger cohort of age matched individuals with type 1 and type 2 diabetes.
The etiology of type 1 and type 2 diabetes are different but the two type of diabetes confer similar CVD risk, which cannot be entirely explained by traditional cardiovascular risk factors. In this study the authors showed that after adjustment for age, CCA IMT was not different in subjects with type 1 and type 2 diabetes. This finding should be explored further.
CCA IMT is visualized using B mode ultrasound. The intima-media complex is the area of tissue starting at the luminal edge of the artery and ending at the boundary between the media and the adventitia. CCA IMT is a measure of early atherosclerosis.
This is an interesting study evaluating the predictors of carotid intima-media thickness in patients with diabetes mellitus. The study is well-designed and the results are clearly presented.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Grilo EC, Liang QL, Tziomalos K S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2812] [Article Influence: 281.2] [Reference Citation Analysis (1)] |
| 2. | Carson AP, Tanner RM, Yun H, Glasser SP, Woolley JM, Thacker EL, Levitan EB, Farkouh ME, Rosenson RS, Brown TM. Declines in coronary heart disease incidence and mortality among middle-aged adults with and without diabetes. Ann Epidemiol. 2014;24:581-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care. 2008;31:714-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 570] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 5. | Liese AD, Bortsov A, Günther AL, Dabelea D, Reynolds K, Standiford DA, Liu L, Williams DE, Mayer-Davis EJ, D’Agostino RB. Association of DASH diet with cardiovascular risk factors in youth with diabetes mellitus: the SEARCH for Diabetes in Youth study. Circulation. 2011;123:1410-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Huffman FG, Zarini GG, McNamara E, Nagarajan A. The Healthy Eating Index and the Alternate Healthy Eating Index as predictors of 10-year CHD risk in Cuban Americans with and without type 2 diabetes. Public Health Nutr. 2011;14:2006-2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 596] [Cited by in F6Publishing: 639] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379:2053-2062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 9. | Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. 2006;23:609-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | de Andrade Junior CR, Silva EL, da Matta Mde F, Castier MB, Rosa ML, Gomes MB. Influence of a family history of type 2 diabetes, demographic and clinical data on carotid intima-media thickness in patients with type 1 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2014;13:87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | den Ruijter HM, Peters SA, Groenewegen KA, Anderson TJ, Britton AR, Dekker JM, Engström G, Eijkemans MJ, Evans GW, de Graaf J. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative. Diabetologia. 2013;56:1494-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Petersen KS, Clifton PM, Keogh JB. The association between carotid intima media thickness and individual dietary components and patterns. Nutr Metab Cardiovasc Dis. 2014;24:495-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 1087] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 15. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] [Cited in This Article: ] |
| 16. | Barua AB, Kostic D, Olson JA. New simplified procedures for the extraction and simultaneous high-performance liquid chromatographic analysis of retinol, tocopherols and carotenoids in human serum. J Chromatogr. 1993;617:257-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Meikle PJ, Wong G, Tsorotes D, Barlow CK, Weir JM, Christopher MJ, MacIntosh GL, Goudey B, Stern L, Kowalczyk A. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:2723-2732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Karalliedde J, Viberti G. Microalbuminuria and cardiovascular risk. Am J Hypertens. 2004;17:986-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Hodge A, Patterson AJ, Brown WJ, Ireland P, Giles G. The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust N Z J Public Health. 2000;24:576-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 503] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Petersen KS, Smith JM, Clifton PM, Keogh JB. Dietary intake in adults with type 1 and type 2 diabetes: validation of the Dietary Questionnaire for Epidemiological Studies version 2 FFQ against a 3-d weighed food record and 24-h urinalysis. Br J Nutr. 2015;114:2056-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Statist Soc Ser B. 1995;57:289-300. [DOI] [Cited in This Article: ] |
| 22. | Distiller LA, Joffe BI, Melville V, Welman T, Distiller GB. Carotid artery intima-media complex thickening in patients with relatively long-surviving type 1 diabetes mellitus. J Diabetes Complications. 2006;20:280-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Cardoso CR, Marques CE, Leite NC, Salles GF. Factors associated with carotid intima-media thickness and carotid plaques in type 2 diabetic patients. J Hypertens. 2012;30:940-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Pinto CS, Lana JM, Gabbay MA, de Sa JR, Dib SA. HDL cholesterol levels and weight are the main determinants of subclinical atherosclerosis in the young with type 1 diabetes and suitable glycaemic control. Diab Vasc Dis Res. 2014;11:125-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Koskinen J, Magnussen CG, Sabin MA, Kähönen M, Hutri-Kähönen N, Laitinen T, Taittonen L, Jokinen E, Lehtimäki T, Viikari JS. Youth overweight and metabolic disturbances in predicting carotid intima-media thickness, type 2 diabetes, and metabolic syndrome in adulthood: the Cardiovascular Risk in Young Finns study. Diabetes Care. 2014;37:1870-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1378] [Cited by in F6Publishing: 1394] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 27. | Kinouchi M, Aihara K, Fujinaka Y, Yoshida S, Ooguro Y, Kurahashi K, Kondo T, Aki N, Kuroda A, Endo I. Diabetic conditions differentially affect the endothelial function, arterial stiffness and carotid atherosclerosis. J Atheroscler Thromb. 2014;21:486-500. [PubMed] [Cited in This Article: ] |
| 28. | Shah AS, Dolan LM, Kimball TR, Gao Z, Khoury PR, Daniels SR, Urbina EM. Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and young adults with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:3740-3745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Shah AS, Dabelea D, Fino NF, Dolan LM, Wadwa RP, D’Agostino R, Hamman R, Marcovina S, Daniels SR, Urbina EM. Predictors of Increased Carotid Intima-Media Thickness in Youth With Type 1 Diabetes: The SEARCH CVD Study. Diabetes Care. 2016;39:418-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Gu W, Huang Y, Zhang Y, Hong J, Liu Y, Zhan W, Ning G, Wang W. Adolescents and young adults with newly diagnosed Type 2 diabetes demonstrate greater carotid intima-media thickness than those with Type 1 diabetes. Diabet Med. 2014;31:84-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Yamasaki Y, Kawamori R, Matsushima H, Nishizawa H, Kodama M, Kajimoto Y, Morishima T, Kamada T. Atherosclerosis in carotid artery of young IDDM patients monitored by ultrasound high-resolution B-mode imaging. Diabetes. 1994;43:634-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Frost D, Beischer W. Progression of the carotid artery intima-media thickness in young patients with type 1 diabetes. Diabetes Care. 2003;26:545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Yamasaki Y, Kodama M, Nishizawa H, Sakamoto K, Matsuhisa M, Kajimoto Y, Kosugi K, Shimizu Y, Kawamori R, Hori M. Carotid intima-media thickness in Japanese type 2 diabetic subjects: predictors of progression and relationship with incident coronary heart disease. Diabetes Care. 2000;23:1310-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985-2994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 255] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 35. | Duca L, Sippl R, Snell-Bergeon JK. Is the risk and nature of CVD the same in type 1 and type 2 diabetes? Curr Diab Rep. 2013;13:350-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Kim MK, Shin J, Kweon SS, Shin DH, Lee YH, Chun BY, Choi BY. Harmful and beneficial relationships between alcohol consumption and subclinical atherosclerosis. Nutr Metab Cardiovasc Dis. 2014;24:767-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Juonala M, Viikari JS, Kähönen M, Laitinen T, Taittonen L, Loo BM, Jula A, Marniemi J, Räsänen L, Rönnemaa T. Alcohol consumption is directly associated with carotid intima-media thickness in Finnish young adults: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2009;204:e93-e98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Chan HT, Yiu KH, Wong CY, Li SW, Tam S, Tse HF. Increased dietary fruit intake was associated with lower burden of carotid atherosclerosis in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 2013;30:100-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Zhu Y, Zhang Y, Ling W, Feng D, Wei X, Yang C, Ma J. Fruit consumption is associated with lower carotid intima-media thickness and C-reactive protein levels in patients with type 2 diabetes mellitus. J Am Diet Assoc. 2011;111:1536-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Odermarsky M, Lykkesfeldt J, Liuba P. Poor vitamin C status is associated with increased carotid intima-media thickness, decreased microvascular function, and delayed myocardial repolarization in young patients with type 1 diabetes. Am J Clin Nutr. 2009;90:447-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Curtis PJ, Potter J, Kroon PA, Wilson P, Dhatariya K, Sampson M, Cassidy A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: a double-blind randomized controlled trial. Am J Clin Nutr. 2013;97:936-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Jhamnani S, Patel D, Heimlich L, King F, Walitt B, Lindsay J. Meta-analysis of the effects of lifestyle modifications on coronary and carotid atherosclerotic burden. Am J Cardiol. 2015;115:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Riccioni G, Bazzano LA. Antioxidant plasma concentration and supplementation in carotid intima media thickness. Expert Rev Cardiovasc Ther. 2008;6:723-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Rissanen TH, Voutilainen S, Nyyssönen K, Salonen R, Kaplan GA, Salonen JT. Serum lycopene concentrations and carotid atherosclerosis: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2003;77:133-138. [PubMed] [Cited in This Article: ] |
| 45. | Zou ZY, Xu XR, Lin XM, Zhang HB, Xiao X, Ouyang L, Huang YM, Wang X, Liu YQ. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2014;111:474-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Fernandez C, Sandin M, Sampaio JL, Almgren P, Narkiewicz K, Hoffmann M, Hedner T, Wahlstrand B, Simons K, Shevchenko A. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One. 2013;8:e71846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Ellims AH, Wong G, Weir JM, Lew P, Meikle PJ, Taylor AJ. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014;15:908-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Adams MR, Nakagomi A, Keech A, Robinson J, McCredie R, Bailey BP, Freedman SB, Celermajer DS. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995;92:2127-2134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 263] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Amato M, Montorsi P, Ravani A, Oldani E, Galli S, Ravagnani PM, Tremoli E, Baldassarre D. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J. 2007;28:2094-2101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |