Published online Jan 15, 2017. doi: 10.4239/wjd.v8.i1.11
Peer-review started: September 12, 2016
First decision: September 30, 2016
Revised: October 21, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: January 15, 2017
Processing time: 117 Days and 4.2 Hours
To determine a potential relationship between serum undercarboxylated (ucOC) concentration and cardiovascular risk factors in type 2 diabetes (T2D) patients and healthy subjects (HS).
A cross-sectional study was conducted on 140 subjects classified into two groups, 70 with T2D and 70 HS. Medical history and physical examination with anthropometric measurements were obtained from all subjects. Body fat percentage was determined by bioelectrical impendency analysis. Serum ucOC concentration was determined by enzyme immunoassay, while serum levels of insulin and hsCRP were obtained using high sensitivity enzyme-linked immunosorbent assay. Insulin resistance was determined using the homeostasis model assessment-IR. Lipid profile [triglycerides, total cholesterol (TC), high-density lipoproteins (HDL-c), low density lipoproteins (LDL-c), very low-density lipoproteins] was determined by spectrophotometry and standard formulas when applicable.
The T2D patient group showed significantly higher values of waist circumference, waist-to-hip ratio, systolic blood pressure (SBP), diastolic blood pressure (DBP), current smoking, and alcohol use when compared to the HS group (P < 0.05). We observed a significantly lower serum ucOC concentration in T2D than in HS (1.5 ± 1.4 vs 2.3 ± 1.8, P < 0.05). In the whole study population, ucOC concentration was inversely correlated with body mass index (BMI) (r = -0.236, P < 0.05), fasting plasma glucose (r = -0.283, P < 0.01) and HDL-c (r = -0.255, P < 0.05); and positively correlated with LDL-c/HDL-c ratio (r = 0.306, P < 0.05) and TC/HDL-c ratio (r = 0.284, P < 0.05). In the T2D group, serum ucOC concentration was inversely correlated with BMI (r = -0.310, P < 0.05) and body-fat percentage (r = -0.311, P < 0.05), and positively correlated with DBP (r = 0.450, P < 0.01). In HS group a positive correlation between serum levels of ucOC and SBP (r = 0.277, P < 0.05) was observed.
Serum ucOC is a potential marker for cardiovascular risk in Mexicans because it is related to adiposity parameters, blood pressure and lipid profile.
Core tip: Lower levels of undercarboxylated osteocalcin (OC) are found in diabetic patients as this hormone is involved in various glucorregulatory mechanisms; however evidence regarding its role in cardiovascular disease development is still pending. Here we show the correlation between levels of undercarboxylated OC and markers of cardiovascular risk.
- Citation: Sanchez-Enriquez S, Ballesteros-Gonzalez IT, Villafán-Bernal JR, Pascoe-Gonzalez S, Rivera-Leon EA, Bastidas-Ramirez BE, Rivas-Carrillo JD, Alcala-Zermeno JL, Armendariz-Borunda J, Llamas-Covarrubias IM, Zepeda-Moreno A. Serum levels of undercarboxylated osteocalcin are related to cardiovascular risk factors in patients with type 2 diabetes mellitus and healthy subjects. World J Diabetes 2017; 8(1): 11-17
- URL: https://www.wjgnet.com/1948-9358/full/v8/i1/11.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i1.11
Osteocalcin (OC) is a non-collagenous peptide composed of 49 aminoacids and produced by osteoblasts. Circulating OC has two variants, carboxylated (cOC) and undercarboxylated (ucOC)[1-3]. While cOC has high affinity for calcium ions contained in hydroxyapatite, is actively involved in osteogenesis[4] and enhances osteoclast maturation[5], ucOC functions as a regulatory hormone in glucose metabolism improving insulin sensitivity and secretion, β-cell mass and glucose tolerance[6,7]. Low serum OC levels and the index ucOC/cOC are associated with increased fasting plasma glucose (FPG) and IR in several cross-sectional and prospective studies[8-12] and with risk for developing type 2 diabetes (T2D)[10,13]. On the other hand, bone metabolism, T2D and cardiovascular diseases (CVD) interact with one another through complex mechanisms[14-21]. For example, bone-related proteins like OC, osteopontin (OPN), osteoprotegerin (OPG) and receptor-activated nuclear factor-k B ligand have been correlated with atherosclerotic arteries, suggesting a potential role of bone molecules in the pathophysiology of vascular disease[22,23]. Furthermore, a previous study showed that OC was inversely correlated with peripheral atherosclerosis markers (intima-media thickness and ankle-brachial pulse-wave velocity) in T2D patients[23]. This data suggests that OC might be involved in the development of CVD in T2D patients. However, direct evidence regarding ucOC concentrations and cardiovascular risk factors (CVRF) in humans is limited. The present study was designed to determine the correlation between ucOC levels and CVRF in T2D and healthy subjects (HS).
We performed a cross-sectional analysis of 140 subjects (70 T2D patients and 70 HS) aged 54.0 ± 5.0 and 51.8 ± 7.4, respectively attending the Program for Detection and Treatment of Congenital and Acquired Metabolic Diseases at a university center (Universidad de Guadalajara) in western Mexico. We obtained the medical history and physical examination from all subjects. Blood for biochemical determinations was drawn after an overnight fasting period. Subjects with hepatic, renal, parathyroid and thyroid dysfunction or taking medications known to influence bone or calcium metabolism, such as vitamin D, bisphosphonates, calcitonin, estrogen, tamoxifen or corticosteroids, were excluded.
This study was done according to the Ethical Principles for Medical Research Involving Human Subjects (Declaration of Helsinki) and was approved by the University of Guadalajara Ethics Committee. Informed consent was obtained from all participants before enrollment in the study.
Anthropometric measurements were performed in all subjects. Height was measured using a stadiometer with ± 0.001 m accuracy (Seca, Hamburg, Germany). Weight and body fat percentage (BFP) were determined using a fixed frequency (50 kHz) bioimpendance analyzer scale (TBF-300 Tanita, Tokyo, Japan) with an applied current of 0.8 mA. Body mass index (BMI) was calculated by Quetelet index (kg/m2). Waist circumference (WC) was measured using a flexible measuring tape midway between the lowest rib and the superior iliac crest border in the mid axillary line. Hip circumference (HC) was measured around the buttocks at the greater trochanter level. Waist-to-hip ratio (WHR) was calculated by dividing WC/HC. After 10 min in the seated position, blood pressure was measured 3 times using an electronic aneroid sphygmomanometer (Omron, model HEM-7220 LA; Kyoto, Japan); the last 2 measures were averaged for analysis.
Blood samples were collected after a 12-h overnight fast period. Samples were centrifuged at 4000 rpm for 10 min to obtain serum, which was stored at -70 °C for later processing. FPG was quantified by the glucose oxidase method (Biosystems, Barcelona, Spain). Serum triglycerides (TG), total cholesterol (TC), cholesterol bound to high-density lipoproteins (HDL-c) and cholesterol bound to low density lipoproteins (LDL-c) were determined by enzymatic colorimetric procedures (Biosystems, Barcelona, Spain). Cholesterol bound to very low-density lipoproteins (VLDLc) was calculated using Friedewald’s equation (TG divided by 5)[24]. Fasting serum insulin (FINS) levels were measured by enzyme-linked immunosorbent assay (ELISA) (GenWay Biotech, Inc. San Diego CA, United States). Serum hsCRP concentration was quantified by high sensitivity ELISA (Abcam, CA, United States). Serum ucOC was determined by enzyme immunoassay (Takara Bio, Inc. Otsu, Japan).
The IR was estimated by homeostasis model assessment-IR (HOMA-IR) calculated according to the following formula: HOMA-IR = FINS (mU/L) × FPG (mmol/L)/22.5[25].
Data was analyzed using the Statistical Package for the Social Sciences v17.0 (SPSS, Inc., Chicago, Illinois). Every analysis was performed by a biomedical statistician. The Kolmogorov-Smirnov one-sample test was performed for assessing the sample cumulative distribution. Quantitative continuous variables were presented as mean ± SD. Normally distributed variables were analyzed using the two independent samples t-test. To analyze non-normally distributed data the Mann-Whitney U test was performed. Pearson’s χ2 test was used for categorical variable analysis (i.e., gender, alcohol use, and current smoking). Pearson correlation coefficient was used to assess the strength of the association between ucOC concentration levels and CVRF. A P value < 0.05 was considered statistically significant.
Demographic and clinical characteristics of all subjects are shown in Table 1. Significant differences in WC, WHR, systolic blood pressure (SBP), diastolic blood pressure (DBP), current smoking, and alcohol use were observed between groups. Biochemical parameters according to study groups are shown in Table 2. TC, TG, HDL-c, LDL-c, VLDL-c, LDLc/HDL-c ratio, TC/HDL-c ratio, FPG, HOMA-IR, and ucOC were significantly different between groups (P < 0.05).
| Variable | T2D (n = 70) | HS( n = 70) | P value |
| Mean ± SD | Mean ± SD | ||
| Age (yr) | 54.0 ± 5.0 | 51.8 ± 7.4 | NS |
| Gender M/F | 29/41 | 30/40 | NS |
| Weight (kg) | 75 ± 18.1 | 72.1 ± 14.8 | NS |
| Height (cm) | 159.6 ± 10.1 | 161.1 ± 7.5 | NS |
| BMI (kg/m2) | 29.3 ± 6.5 | 27.6 ± 5.2 | NS |
| WC (cm) | 99.2 ± 18.1 | 90.7 ± 12.3 | < 0.01 |
| WHR | 0.93 ± 0.14 | 0.87 ± 0.08 | < 0.01 |
| Fat percentage | 34.5 ± 8.5 | 33.2 ± 8.7 | NS |
| SBP (mmHg) | 143.3 ± 21.5 | 113.3 ± 11 | < 0.001 |
| DBP (mmHg) | 86 ± 12.3 | 74.9 ± 7.5 | < 0.001 |
| Current smoking n (%) | 24 (38.1) | 9 (27.3) | < 0.05 |
| Alcohol use n (%) | 29 (46) | 2 (3.9) | < 0.001 |
| Physical inactivity n (%) | 32 (50.8) | 31 (60.8) | NS |
| Variable | T2D (n = 70) | HS (n = 70) | P value |
| Mean ± SD | Mean ± SD | ||
| TC (mg/dL) | 220.7 ± 84.7 | 181 ± 35.5 | < 0.01 |
| TG (mg/dL)1 | 140.9 (87.6-200.5) | 108.0 (84.0-145.0) | < 0.05 |
| HDLc (mg/dL) | 43.1 ± 15.5 | 68.3 ± 18.0 | < 0.001 |
| LDLc (mg/dL) | 129.8 ± 37.1 | 108.9 ± 33.1 | < 0.05 |
| VLDLc (mg/dL) | 31.1 ± 18.6 | 25.8 ± 20.0 | < 0.05 |
| LDLc/HDLc | 3 ± 1.7 | 2.2 ± 0.1 | < 0.05 |
| TC/HDLc | 4.7 ± 1.9 | 3.4 ± 1.5 | < 0.01 |
| FPG (mg/dL) | 161.9 ± 69.5 | 88.3 ± 9.0 | < 0.001 |
| FINS (mcUI/mL) | 15.8 ± 7.0 | 13.8 ± 11.6 | NS |
| HOMA-IR | 6.8 ± 4.1 | 3 ± 2.6 | < 0.001 |
| hs-CRP (ng/L) | 3 ± 3.1 | 2.1 ± 0.8 | NS |
| ucOC (ng/mL) | 1.5 ± 1.4 | 2.3 ± 1.8 | < 0.05 |
In the whole study population the serum ucOC concentration was inversely correlated with BMI, FPG and HDL-c, while it was positively correlated with both LDL-c/HDL-c ratio and TC/HDL-c ratio. In the T2D group and inverse correlation between serum levels of ucOC, body fat percentage, and BMI were observed. Conversely, serum levels of ucOC were positively correlated with DBP in this group. In the group of HS, a positive correlation between serum levels of ucOC and systolic pressure was observed (Table 3).
| ucOC concentration (ng/mL) | ||||||
| Variable | Whole population (n = 140) | T2D (n = 70) | HS (n = 70) | |||
| r | P value | r | P value | r | P value | |
| BMI (kg/m2) | -0.236 | 0.023 | -0.310 | 0.046 | -0.166 | 0.244 |
| Body fat (%) | -0.201 | 0.054 | -0.311 | 0.048 | -0.126 | 0.379 |
| SBP (mmHg) | -0.083 | 0.431 | 0.018 | 0.908 | 0.277 | 0.049 |
| DBP (mmHg) | 0.155 | 0.137 | 0.450 | 0.003 | 0.209 | 0.141 |
| FPG (mg/dL) | -0.283 | 0.006 | -0.286 | 0.070 | -0.110 | 0.443 |
| HDL-c (mg/dL) | -0.255 | 0.036 | 0.117 | 0.655 | -0.096 | 0.503 |
| LDL-c/HDL-c | 0.306 | 0.015 | -0.102 | 0.697 | 0.286 | 0.054 |
| TC/HDL-c | 0.284 | 0.019 | -0.158 | 0.544 | 0.221 | 0.120 |
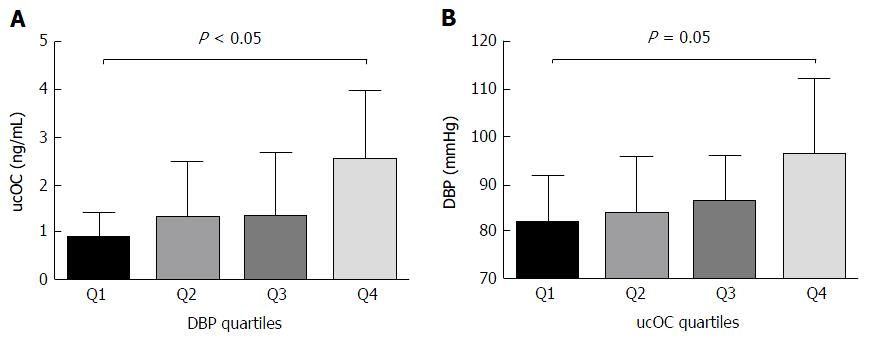
Because of the strong correlation found between DBP and ucOC in T2D patients, participants were classified by DBP quartiles, and then ucOC serum levels were compared between quartiles. Also patients were classified according to ucOC quartiles and DBP was analyzed in each ucOC quartile. The ucOC serum levels were higher in T2D patients with DBP in Q4 than in Q1 (P < 0.05, Figure 1A). Additionally, DBP was higher in patients with ucOC in Q4 than those with ucOC in Q1 (P = 0.05, Figure 1B).
In this study lower levels of ucOC were found in T2D when compared to HS. Although low levels of total OC and ucOC in T2D patients have been previously reported[11,26-28], there is little evidence describing the relationship between serum levels of ucOC and CVRF.
The association between ucOC concentration and T2D is consistent with in vitro and in vivo studies that link OC to energetic equilibrium[3,4]. According to previous reports, there is an inverse correlation between serum ucOC levels and; IR, BMI, BFP, FPG and HOMA-IR[7,22,23,29-31].
In our study, multiple associations between ucOC levels and CVRF in T2D patients were found. In T2D patients, ucOC concentration was inversely correlated with BMI and BFP and a positively correlated with DBP. In HS, a positive correlation between ucOc and SBP was also established. Additionally, those T2D patients with highest ucOC levels (Q4) had higher DBP than those with the lowest ucOC (Q1).
The inverse relationship between ucOC and BMI was previously reported by Chen et al[32] and Tan et al[33] in Chinese men, which might be explained by the role of OC in energy metabolism and by the observation that the OC knockout mice model has abnormal levels of visceral fat[6].
This might be the first report indicating a positive relationship between ucOC levels and DBP in T2D patients and a positive correlation of SBP with ucOC in HS. In non-diabetic young adults, Polgreen et al[34] found lower SBP in those having cOC and total OC in Q4 than in Q1, but did not report anything regarding ucOC and DBP. Although Chen, Tan and colleagues found a weak inverse correlation between DBP and total OC they did not did not determined ucOC levels[32,33] which would’ve been desirable since various biological effects outside-the-bone have been endorsed to ucOC[6].
We also observed an inverse association between ucOC levels, BMI, FPG and HDLc, as well as a positive correlation with LDLc/HDLc and TC/HDLc ratios in the whole study population. These data suggest that serum levels of ucOC could be related to CVRF in both HS and T2D patients. These findings agree with the higher ucOC serum levels found by Okura et al[35] in hypertensive patients with carotid calcification than in those without carotid calcification, suggesting that ucOC might be a potential biomarker for carotid artery calcification. Our work also agrees with the results published by Kanazawa et al[23] who found that total serum OC concentration was negatively associated with atherosclerotic parameters (intima-media thickness and ankle-brachial pulse-wave velocity) independent of other CVRF in diabetic men. Other studies had reported that bone proteins such as matrix Gla protein, OPN and OPG are markers of vascular calcification and are expressed in arteries presenting atherosclerosis[22,23]. Moreover, T2D patients are particularly prone to develop CVD due to the role that diabetes plays in endothelial dysfunction, atherogenesis and vascular calcification[36-39].
Recent studies on animals suggest that OC may have beneficial effects on serum TG levels, but the clinical relevance of this remains elusive[29,31]. Although a significant correlation between serum OC concentration and TG was not found in our study, ucOC levels were inversely correlated with BMI and BFP in the T2D group and with BMI and HDL-c in whole population. This suggests a possible role of ucOC in lipid metabolism regulation. The discrepancy between our study and those conducted in animals could reside in the differences between human and animal lipid metabolism. Numerous studies regarding this issue suggest that TG are positively correlated with bone density while HDL-c is negatively correlated. These observations imply that a common mechanism of lipid and bone metabolism exists. However, more studies need to be performed in order to achieve proper understanding of the pathophysiological relationship between the OC levels and cardiovascular disease[40-49].
Virmani et al[41] have defined atherosclerosis as a chronic inflammatory process that can be accelerated by high blood pressure secondary to vasoactive peptides such as angiotensin and endothelin-1. Proinflammatory and prothrombotic risk markers play a very important role in the atheroma formation process, which contributes to the progression of vascular disease in T2D patients by activating inflammatory signaling and oxidative stress, both triggers of the endothelium injury process. Schurgers et al[39], found that matrix Gla protein was not carboxylated in atherosclerotic arteries, which could explain the positive correlation between uOC levels and SBP, DBP, LDL-c/HDL-c and TC/HDL-c, well known markers of cardiovascular disease[46,47].
To our knowledge, this is the first study proposing a link between ucOC serum levels and CVRF in a Mexican population. The main limitation of our study is that it cannot identify causal relationship due to its design. Additional studies are needed to determine specifically whether serum ucOC concentration could be considered an independent CVRF. Our findings will generate new hypotheses regarding the role of this protein, not only as a hormone in energy and bone metabolism and its well-studied role in regulation of glucose metabolism, insulin secretion and sensitivity; but also in endothelial dysfunction and as a marker of cardiovascular risk in patients with T2D.
Most reports that studied the relationship between OC and glucose metabolism include only total OC levels determination and data dealing with ucOC in T2D patients in Mexicans is scarce. Our study investigated serum ucOC levels in this population and thus provides information that helps further explore the role of OC in glucose metabolism and CVRF related to T2D.
Serum ucOC levels are related to CVRF in both T2D and HS. Specifically, ucOC is related to adiposity markers and blood pressure, as well as lipid profile. Thus, serum ucOC might be a cardiovascular risk marker in the Mexican population.
The authors thank Rogelio Troyo Sanroman for statistical support.
Undercarboxylated (ucOC) is a non-collagenous peptide involved in various biological processes, including glucose metabolism. The relationship between low levels of ucOC and type 2 diabetes (T2D) is well established and some have proposed ucOC as a marker for metabolic risk. However, the role of ucOC and cardiovascular diseases (a common comorbidity in T2D) has not been well defined.
Osteocalcin (OC) research now involves fields in energy metabolism, male fertility and brain development. Also a potential receptor to mediate its functions has been proposed, the GPRC6A receptor. However, in cardiovascular disease investigation, there is continuous interest in analyzing the role of OC in atherosclerosis indexes and vascular calcification.
The authors have described various correlations between ucOC levels and markers of cardiovascular risk such as blood pressure, high-density lipoproteins, body mass index and body fat percentage in a Mexican population. Other reports have analyzed the role of ucOC role in arterial calcification in hypertensive patients such as Okura et al, however no other group has studied its relationship with cardiovascular risk factors (CVRF) in T2D Mexican patients like in the present report.
The analysis postulates the possible emergence of ucOC as an independent CVRF in T2D patients.
Osteocalcin: Non-collagenous protein that is found in the bone extracellular matrix and in the serum of circulating blood, it is produced by osteoblasts especially in the presence of vitamin D. This hormone exists in two forms: Carboxylated and undercarboxylated; Carboxylated osteocalcin: A form of osteocalcin in which its 3 residues of glutamic acid that reside at the 17, 21 and 24th positions are gamma carboxylated through a vitamin K depend process; Undercarboxylated osteocalcin: Osteocalcin that has less than 3 residues of carboxyglutamic acid.
This is a useful manuscript.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Papanas N, Tarantino G S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990-1047. [PubMed] |
| 2. | Price PA. Gla-containing proteins of bone. Connect Tissue Res. 1989;21:51-57; discussion 57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Razzaque MS. Osteocalcin: a pivotal mediator or an innocent bystander in energy metabolism? Nephrol Dial Transplant. 2011;26:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Villafán-Bernal JR, Sánchez-Enríquez S, Muñoz-Valle JF. Molecular modulation of osteocalcin and its relevance in diabetes (Review). Int J Mol Med. 2011;28:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ishida M, Amano S. Osteocalcin fragment in bone matrix enhances osteoclast maturation at a late stage of osteoclast differentiation. J Bone Miner Metab. 2004;22:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2030] [Cited by in RCA: 1804] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 7. | Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266-5270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 8. | Saleem U, Mosley TH, Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2010;30:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Villafán-Bernal JR, Llamas-Covarrubias MA, Muñoz-Valle JF, Rivera-León EA, González-Hita ME, Bastidas-Ramírez BE, Gurrola-Díaz CM, Armendáriz-Borunda JS, Sánchez-Enríquez S. A cut-point value of uncarboxylated to carboxylated index is associated with glycemic status markers in type 2 diabetes. J Investig Med. 2014;62:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Sarkar PD, Choudhury AB. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 2013;17:1631-1635. [PubMed] |
| 13. | Hwang YC, Jeong IK, Ahn KJ, Chung HY. Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos Int. 2012;23:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Lieben L, Callewaert F, Bouillon R. Bone and metabolism: a complex crosstalk. Horm Res. 2009;71 Suppl 1:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 301] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 18. | McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 279] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 370] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 21. | Srivatsa SS, Harrity PJ, Maercklein PB, Kleppe L, Veinot J, Edwards WD, Johnson CM, Fitzpatrick LA. Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J Clin Invest. 1997;99:996-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Gössl M, Mödder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, Lerman LO, Khosla S, Lerman A. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] |
| 25. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24517] [Article Influence: 612.9] [Reference Citation Analysis (1)] |
| 26. | Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta. 2008;396:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellström D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 28. | Díaz-López A, Bulló M, Juanola-Falgarona M, Martínez-González MA, Estruch R, Covas MI, Arós F, Salas-Salvadó J. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metab. 2013;98:4524-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Stępień E, Wypasek E, Stopyra K, Konieczyńska M, Przybyło M, Pasowicz M. Increased levels of bone remodeling biomarkers (osteoprotegerin and osteopontin) in hypertensive individuals. Clin Biochem. 2011;44:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999;33:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Gössl M, Mödder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 32. | Chen L, Li Q, Yang Z, Ye Z, Huang Y, He M, Wen J, Wang X, Lu B, Hu J. Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese. Diabet Med. 2013;30:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, Peng T, Xia N, Mo Z. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism. 2011;60:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Polgreen LE, Jacobs DR, Nathan BM, Steinberger J, Moran A, Sinaiko AR. Association of osteocalcin with obesity, insulin resistance, and cardiovascular risk factors in young adults. Obesity (Silver Spring). 2012;20:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Okura T, Kurata M, Enomoto D, Jotoku M, Nagao T, Desilva VR, Irita J, Miyoshi K, Higaki J. Undercarboxylated osteocalcin is a biomarker of carotid calcification in patients with essential hypertension. Kidney Blood Press Res. 2010;33:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007;3:667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 808] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 38. | Bao YQ, Zhou M, Zhou J, Lu W, Gao YC, Pan XP, Tang JL, Lu HJ, Jia WP. Relationship between serum osteocalcin and glycaemic variability in Type 2 diabetes. Clin Exp Pharmacol Physiol. 2011;38:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 40. | Li JJ, Chen JL. Inflammation may be a bridge connecting hypertension and atherosclerosis. Med Hypotheses. 2005;64:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2860] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 42. | Tang YJ, Sheu WH, Liu PH, Lee WJ, Chen YT. Positive associations of bone mineral density with body mass index, physical activity, and blood triglyceride level in men over 70 years old: a TCVGHAGE study. J Bone Miner Metab. 2007;25:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tankó LB. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Adami S, Braga V, Gatti D. Association between bone mineral density and serum lipids in men. JAMA. 2001;286:791-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Dennison EM, Syddall HE, Aihie Sayer A, Martin HJ, Cooper C. Lipid profile, obesity and bone mineral density: the Hertfordshire Cohort Study. QJM. 2007;100:297-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, Battaglia E. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Kha HT, Basseri B, Shouhed D, Richardson J, Tetradis S, Hahn TJ, Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Miner Res. 2004;19:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Li J, Zhang H, Yang C, Li Y, Dai Z. An overview of osteocalcin progress. J Bone Miner Metab. 2016;34:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |