Copyright
©The Author(s) 2016.
World J Diabetes. Nov 15, 2016; 7(19): 483-514
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
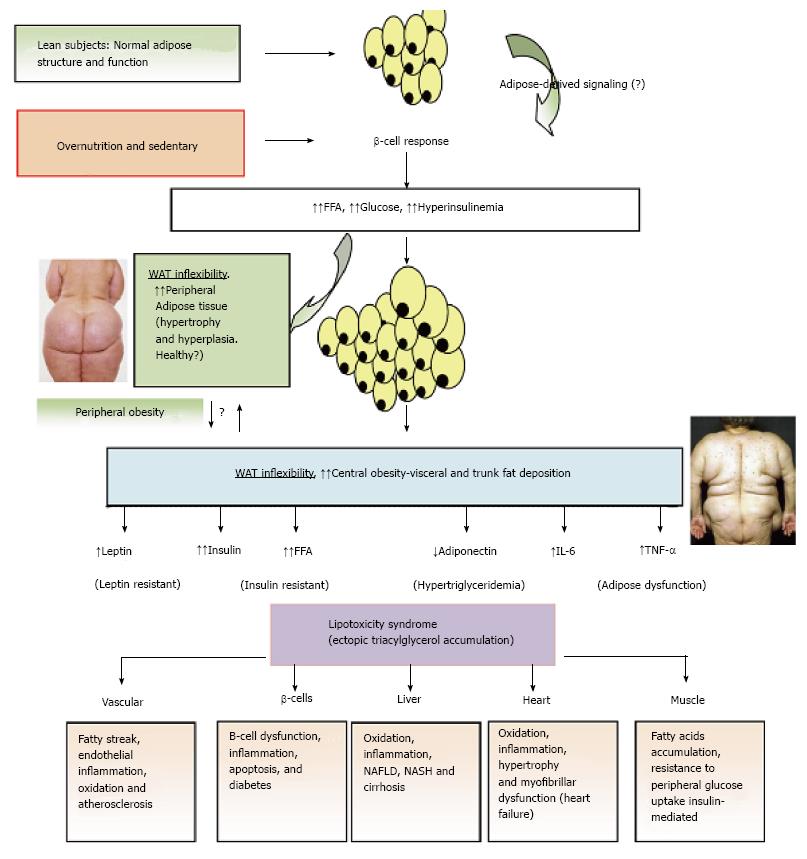
Figure 3 Adipose tissue expandability and metabolic syndrome.
After a long period of overeating with positive energy balance, associated with increased hormones such as insulin, adipose tissue responds by increasing its storage capacity, which is determined by a number of factors. Individuals with a higher capacity for storing fat, mainly when peripheral WAT is expanded (WAT flexibility), most subjects will remain metabolically normal for a longer period, despite obesity developing. These subjects are observed to be metabolically healthy (MHO). Chronic inflammatory response leads to dysfunctional adipose tissue with increased local and endocrine secretion of acute phase reactants and inflammatory signaling pathways[285]. Abnormal cytokine and adipokines production is related to insulin resistance, hyperglycemia, altered lipid profile and cardiovascular diseases[115,286,287]. Insulin resistance slowly results from increased accumulation of lipids in other nonadipose tissues such as muscle (lipotoxicity) due to enhanced release of fatty acids from hypertrophic and hyperplasic adipocyte cells. In addition, when adipocytes achieve their maximal storage capacity, they begin to alter their adipokynes secretion profile. Therefore, a proinflammatory milieu with elevation in IL-6 and TNF-α and altered adipokines profile, with decreased adiponectin and increased leptin levels, with peripheral leptin resistance, in a dysfunctional adipose system is observed. This suggests that the limitation in storage capacity could be necessary and even precedes the development of metabolic factors. Ectopic lipid accumulation in non-adipocyte cells causes lipotoxicity in these organs and tissues, including inflammation and finally apoptosis. Thus, lipotoxicity in β-cell could decrease beta cell mass (dysfunction of β-cell secretion) and would cause diabetes. Increased fat in liver leads to hepatic steatosis (NAFLD) and steatohepatitis (NASH) and would cause hepatic dysfunction, in the heart would cause myocardiac dysfunction, in the endothelial fatty streak would be precursor of generalized arteriosclerosis, etc. At what point the adipose tissue begins to fail is likely to be determined by genetic and epigenetic factors. However, the question is: Can storage capacity in WAT be enhanced to meet an increased demand[288]? So far, in human trials, the PPAR-γ agonists (TZDs), that remove fat from central deposits toward more favorable peripheral deposits, have been shown to improve lipid profile, insulin-sensitivity, and reduce diabetes and NAFLD[269]. WAT: White adipose tissue; MHO: Metabolically healthy obese; IL: Interleukin; TNF-α: Tumor necrosis factor-α; NAFLD: Non alcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; PPAR-γ: Peroxisome proliferator-activated receptor-γ; TZD: Thiazolidinedione.
- Citation: Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes 2016; 7(19): 483-514
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/483.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.483