Copyright
©The Author(s) 2016.
World J Diabetes. Nov 15, 2016; 7(19): 483-514
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
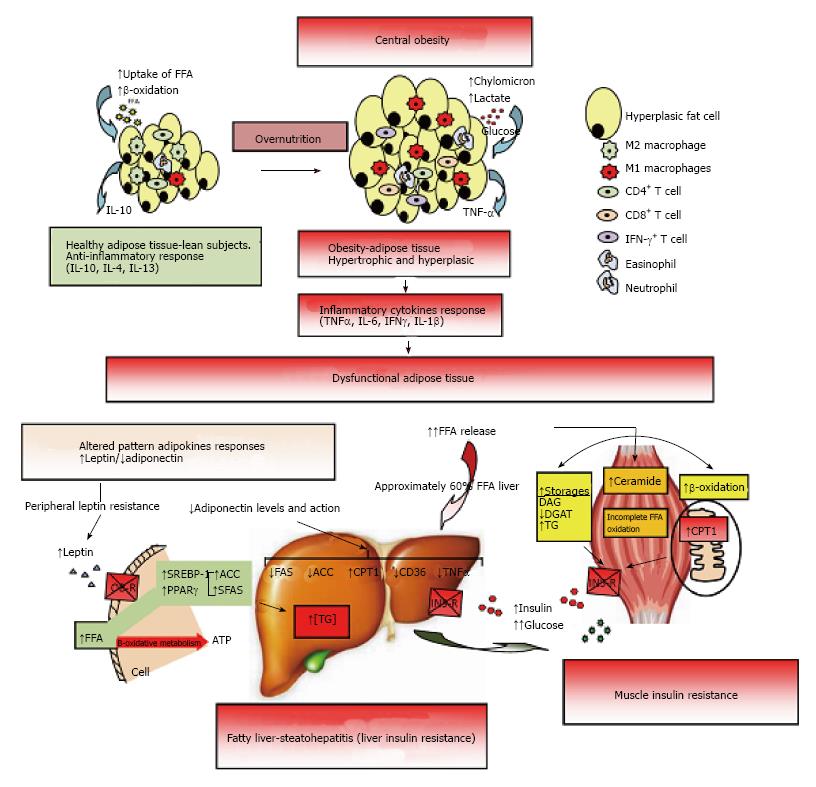
Figure 2 Dysfunctional adipose tissue.
Early central obesity is associated with a low-grade chronic inflammatory state characterized by slow infiltration of macrophages which are an important source of inflammation of this adipose tissue[275,276]. Several macrophage subtypes can be found, and simply put, are divided in pro-inflammatory M1 or alternatively activated M2, although in vivo studies reveal a spectrum of macrophage phenotypes[277]. Adipocytes and immune cells such as T cells and macrophages participate in the activation and production of inflammatory cytokines[170,275,278,279]. The M1 macrophages mainly found in obesity, are induced from precursor M0 macrophages by stimulation of components of bacteria (lipopolysaccharide) and type 1 T-helper (Th1) inflammatory cytokines like IFN-γ and TNF-α. The M2 macrophages are activated by type 2 (Th2) cytokines such as IL-4 and IL-13. The M2 macrophages are abundant in adipose tissue of lean subjects and appear to be involved in remodeling, tissue repair, and maintenance of insulin sensitivity through the production and expression of IL-10, IL-1 receptor antagonist, and arginase-1. Whereas M1 macrophages use glucose for energy, M2 macrophages activate the β-oxidation of fatty acids[277,280]. Finally, M1 macrophages are the major source of inflammatory cytokines including TNF-α which inhibits adipose cell differentiation by activating Wnt signaling and suppressing expression of PPAR-γ transcription factor essential for the development and function of adipocyte, and reducing the effect on stored triglycerides[281,282]. The subcutaneous adipose tissue will continue to expand to an equilibrium point. When this capacity is exceeded, glucose and lipid uptake begins to decline and insulin levels are raised to maintain serum glucose in the normal range[215]. In addition, when WAT is unable to expand (inflexibility), associated with insulin resistance state, a continuous release of FFA to interstice begins, generating a systemic lipotoxic effect in muscle, liver, etc., (lipotoxicity). The adipose tissue itself begins a slow process of low-level chronic inflammation (macrophages, lymphocytes, etc.) which increases local release of TNF-α and IL-6[166]. TNF-α and IL-6 levels are inversely related with peripheral and hepatic glucose-uptake which is insulin-mediated[283]. The liver keeps excess uptake of FFA in serum to capacity by joining with glycerol (TAG) and slowly fatty liver is developed (NAFLD). It has been shown that peripheral fatty acids contribute approximately 60% of total TAG stored in the liver, whereas the novo lipogenesis in the liver is approximately 26% and approximately 15% is from the diet[284]. On the other hand, leptin levels respond directly to adipose expansion, while adiponectin levels tend to decrease when metabolic syndrome is developed. The elevated leptin levels should increase lipolysis in non-adipose tissues, decreasing excess fatty acids in these cells. However, this action of leptin may be partially blocked by the anabolic effect established by hyperinsulinemia, settling down leptin system dysfunction (peripheral leptin resistance)[115]. In addition, the decreased adiponectin levels are inversely related to peripheral glucose uptake and directly related with progressive development of chronic liver disease by fat infiltration. Adiponectin exerts a protective action on liver fat accumulation, favoring lipolysis by promoting the action of CPT-1, while interfering with the action of FAS, ACO and TNF-α, and decreasing the expression and action of CD-36 protein that promotes the transport of fatty acids[129]. Finally, both leptin and adiponectin seem to regulate the deposition of fat in insulin-sensitive tissues by increasing fat oxidation. IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; IL: Interleukin; PPAR-γ: Peroxisome proliferator-activated receptor-γ; WAT: White adipose tissue; FFA: Free fatty acids; NAFLD: Non alcoholic fatty liver disease; CPT-1: Carnitine palmitoyltransferase-1; FAS: Fatty acid synthase; ACO: Acyl CoA carboxylase.
- Citation: Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes 2016; 7(19): 483-514
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/483.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.483