Published online Feb 15, 2014. doi: 10.4239/wjd.v5.i1.59
Revised: November 27, 2013
Accepted: December 13, 2013
Published online: February 15, 2014
AIM: To minimize the expansion of pancreatic mesenchymal cells in vitro and confirm that β-cell progenitors reside within the pancreatic epithelium.
METHODS: Due to mesenchymal stem cell (MSC) expansion and overgrowth, progenitor cells within the pancreatic epithelium cannot be characterized in vitro, though β-cell dedifferentiation and expansion of MSC intermediates via epithelial-mesenchymal transition (EMT) may generate β-cell progenitors. Pancreatic epithelial cells from endocrine and non-endocrine tissue were expanded and differentiated in a novel pancreatic epithelial expansion medium supplemented with growth factors known to support epithelial cell growth (dexamethasone, epidermal growth factor, 3,5,3’-triiodo-l-thyronine, bovine brain extract). Cells were also infected with a single and dual lentiviral reporter prior to cell differentiation. Enhanced green fluorescent protein was controlled by the rat Insulin 1 promoter and the monomeric red fluorescent protein was controlled by the mouse PDX1 promoter. In combination with lentiviral tracing, cells expanded and differentiated in the pancreatic medium were characterized by flow cytometry (BD fluorescence activated cell sorting), immunostaining and real-time polymerase chain reaction (PCR) (7900HT Fast Realtime PCR System).
RESULTS: In the presence of 10% serum MSCs rapidly expand in vitro while the epithelial cell population declines. The percentage of vimentin+ cells increased from 22% ± 5.83% to 80.43% ± 3.24% (14 d) and 99.00% ± 0.0% (21 d), and the percentage of epithelial cells decreased from 74.71% ± 8.34% to 26.57% ± 9.75% (14 d) and 4.00% ± 1.53% (21 d), P < 0.01 for all time points. Our novel pancreatic epithelial expansion medium preserved the epithelial cell phenotype and minimized epithelial cell dedifferentiation and EMT. Cells expanded in our epithelial medium contained significantly less mesenchymal cells (vimentin+) compared to controls (44.87% ± 4.93% vs 95.67% ± 1.36%; P < 0.01). During cell differentiation lentiviral reporting demonstrated that, PDX1+ and insulin+ cells were localized within adherent epithelial cell aggregates compared to controls. Compared to starting islets differentiated cells had at least two fold higher gene expression of PDX1, insulin, PAX4 and RFX (P < 0.05).
CONCLUSION: PDX1+ cells were confined to adherent epithelial cell aggregates and not vimentin+ cells (mesenchymal), suggesting that EMT is not a mechanism for generating pancreatic progenitor cells.
Core tip: Previously, we demonstrated that mesenchymal stem cells could be expanded from human endocrine and non-endocrine pancreas cell fractions in vitro. However, we were unable to complete cell differentiation of mesenchymal cell intermediates to functional endocrine cells. In this study we utilized a novel cell culture medium to prevent epithelial cell de-differentiation and mesenchymal cell expansion. After epithelial cell expansion in this medium cells were differentiated via our previously described protocol and we confirmed by lineage tracing, flow cytometry, immunostaining and real-time polymerase chain reaction that islet progenitors reside in the pancreatic epithelium and are not derived via a mesenchymal cell intermediate.
- Citation: Seeberger KL, Anderson SJ, Ellis CE, Yeung TY, Korbutt GS. Identification and differentiation of PDX1 β-cell progenitors within the human pancreatic epithelium. World J Diabetes 2014; 5(1): 59-68
- URL: https://www.wjgnet.com/1948-9358/full/v5/i1/59.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i1.59
Islet transplantation is an attractive alternative to daily insulin injections to achieve a more physiological means for restoring glucose homeostasis[1-3]. Identifying and understanding the origin of a potential human β-cell progenitor could alleviate the current shortage of donor islets and contribute to the overall knowledge of β-cell regeneration. However, the study of β-cell progenitors is fraught with controversy, as several conflicting models and mechanisms describing the origin and existence of these progenitor cells have been proposed. Despite lineage tracing experiments utilizing transgenic mouse models[4-6] the exact origin of β-cell progenitors residing within the pancreas is yet to be elucidated. For example β-cell progenitors have been proposed to originate from: β-cell replication[4], acinar cell transdifferentiation[7,8], ductal cell transdifferentiation[9-12], pancreas derived multipotent precursors[13], pluripotent islet survivor cells[14] and β-cell dedifferentiation with expansion of mesenchymal stem cell (MSC) intermediates via epithelial mesenchymal transition (EMT)[15-20].
Previously we reported[21] that MSCs, also referred to as multi-potent stromal cells[22], could be expanded 12-fold from human islet depleted pancreatic tissue (IDPT) that remains following islet isolation. We demonstrated that these pancreatic MSCs could be partially differentiated into islet-like cells. However, in a follow up study[23] we could not restore an epithelial phenotype during tissue culture or generate functional endocrine cells. We hypothesized that this was due in part to our experimental culture conditions, which favored pancreatic MSC expansion and negatively selected pancreatic epithelial cells.
In this study we report that, during in vitro pancreatic MSC expansion, epithelial cells also proliferate and when these epithelial cells are enriched and differentiated, this cell population expresses developmental transcription factors indicative of a β-cell progenitor such as PAX4 and RFX6[24-26]. Therefore, to maintain epithelial cell phenotype and allow long-term study of this cell population in vitro, we utilized a pancreatic epithelial expansion medium that minimized epithelial cell dedifferentiation and MSC overgrowth in combination with our differentiation protocol[21,23]. Furthermore, by utilizing single and dual lentiviral reporters where, enhanced green fluorescent protein (EGFP) is controlled by the rat Insulin 1 (Ins1) promoter and monomeric red fluorescent protein (mRFP) is controlled by the mouse PDX1 promoter[26] we determined that PDX1+ cells observed after 25 d post-differentiation were epithelial cells. Unlike the reversible (EMT) model first described by Gershengorn et al[15] and the dedifferentiation of β-cells then replication of β-cell-derived cells described by Russ et al[20], we propose that β-cell progenitors reside within the human pancreatic epithelium and that these cells have the potential to respond favorably to in vitro differentiation without dedifferentiation into a MSC intermediate via EMT. Overall, we report a novel cell culture media that promotes pancreatic epithelial cell survival and minimizes MSC overgrowth, and report that PDX1+ cells observed 25 d post-differentiation are epithelial cells.
Human islets (n = 9) and IDPT (n = 13) were obtained from the Edmonton Clinical Islet Transplant Program (University of Alberta and Alberta Health Services). Written, informed, consent was provided by donor relatives and all protocols were approved by the UofA Research Ethics Office. Average donor age was 54 (30-71 years) and islet purity assessed by dithizone staining ranged between 10%-40%. IDPT (< 5% insulin positive cells) was obtained following removal of islets by density gradient purification[21,23,27]. Upon receipt, IDPT was cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Burlington, ON Canada) supplemented with 0.5% w/v fraction V bovine serum albumin (Sigma-Aldrich, Oakville, ON Canada), 1% insulin-transferrin-selenium (Sigma-Aldrich) and 100 U penicillin/1000 U streptomycin (Invitrogen). Islets were cultured in Connaught Medical Research Laboratories-1066 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 2 mmol/L L-glutamine (Invitrogen), 10 mmol/L hydroxyethyl piperazineethanesulfonic acid (HEPES) and 100 U penicillin/1000 U streptomycin (Invitrogen). Both IDPT and islets were cultured in 150 mm non-tissue culture treated plates (Corning, NY, United States) and maintained for 24-48 h at 37 °C in 5% CO2 and 95% air. Following culture, single cell suspensions were derived by, dissociating islets or cellular aggregates derived from the cultured IDPT with 0.05% trypsin, 0.5 mmol/L ethylenediaminetetraacetic acid (Invitrogen) in 1X PBS.
Single cell preparations were cultured and expanded in pancreatic MSC medium or pancreatic epithelial expansion medium. MSC medium is composed of RPMI-1640 supplemented with 10% FBS, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate (Invitrogen), 71.5 μmol/L β-mercaptoethanol (Sigma-Aldrich), 20 ng/mL epidermal growth factor (EGF, R&D, Minneapolis, MN United States), 20 ng/mL fibroblast growth factor (Invitrogen) and 100 U pencillin/1000 U streptomycin[21,23,27]. Cell confluence was achieved in 10-14 d and cells required passaging every 7 d after that. From both islets and IDPT at the 2nd and 3rd passage we routinely generate a cell population with MSC characteristics as previously described[21,23,27]. To preserve epithelial cell phenotype, single cells derived from islets or the IDPT were also cultured and expanded in a pancreatic epithelial expansion medium either on 150 mm tissue culture treated plates (Corning) or 12 mm poly-l-lysine coated cover slips (BD Biosciences) placed in 24 well tissue culture treated plates (Corning). Pancreatic epithelial expansion medium is composed of Dulbecco's Modified Eagle's Medium/F12 (Invitrogen) supplemented with 0.5% FBS, 0.1 μg/mL EGF, 0.4 μg/mL dexamethasone (Sigma-Aldrich), 14 mg/mL bovine brain extract (Lonza, Walkersville, MD United States), 0.05 μmol/L triiodo-l-thryonine sodium salt (Sigma-Aldrich), 0.1 mg/mL soybean trypsin inhibitor (US Biological, Swampscott, MA, United States), 0.5X ITS+premix (BD Biosciences) and 100 U penicillin/1000 U streptomycin. Expanded cell populations were subsequently differentiated using a multi-step protocol previously described[21,23,27,28] and characterized by flow cytometry, immunohistochemistry and real-time polymerase chain reaction (PCR). For differentiation, the cell monolayer was treated with 20 ng/mL OncostatinM (R&D) for 72 h. In steps 2 and 3 the medium was supplemented with 10 mmol/L nicotinamide (Sigma-Aldrich) for 72 h followed by 10 mmol/L nicotinamide and 10 nmol/L exendin4 (Sigma-Aldrich) for another 72 h. In step 4, 10 ng/mL of transforming growth factor-β1 (TGFβ-1; EMD Millipore, Billerica, MA, United States) was included with nicotinamide and exendin4 for 3-10 d with media changes every 72 h. Cell monolayers were detached with trypsin and aggregated by reconstituting cells at 125000 cells/mL in medium supplemented with nicotinamide, exendin4, TGFβ-1, 0.5X ITS+ premix (BD Biosciences, Beford, MA United States) and transferred to 100 mm ultra-low attachment non-tissue culture treated plates (Corning).
Double immunofluorescence (IF) analysis was performed on paraffin sections of single cells that had first been fixed with 1% formalin (Fisher Scientific, Nepean, ON Canada) and embedded in 2% low melting point agarose (Sigma-Aldrich) or cells which had been differentiated on 12 mm poly-l-lysine cover slips (BD Biosciences). Paraffin sections were processed and immunostained as previously described[23,28]. Cover slips were fixed in 1% formalin for 30 min in the dark at 4 °C, and then washed twice with 5% normal goat serum (NGS) in PBS. For antibodies requiring permeabilization, 0.3% saponin (Sigma) in PBS was applied for one minute, and another two washes of 5% NGS followed. All cover slips were then blocked with 20% NGS for 1 h in the dark. Primary antibodies were diluted in 5% NGS at the following concentrations: 1/200 anti-epithelial cell adhesion molecule (EpCAM, Stem Cell Technologies, Vancouver, BC Canada), 1/100 anti-vimentin (Dako, Mississauga, ON Canada), 1/25 anti-human proliferating cell nuclear antigen (PCNA, Invitrogen), 1/50 anti-CK19 (Dako), 1/5000 anti-glucagon (Sigma-Aldrich), 1/1000 anti-insulin (Dako), 1/1000 anti-pancreatic polypeptide (Dako), 1/1000 anti-somatostatin (Dako), 1/1000 anti-PDX1 (Abcam, Cambridge, MA, United States). All appropriate species-specific secondary antibodies were AlexaFluor 488 or 594 conjugates (Molecular Probes, Eugene, OR, United States) and diluted 1/200 in 5% NGS. Slides and or cover slips were cover-slipped with ProLong Gold anti-fade reagent with 4',6-diamidino-2-phenylindole (Invitrogen) to counter stain nuclei and preserve fluorescence. Negative controls were incubated without primary antibodies and positive controls were sections of normal human and infant pancreas. All slides were visualized with an Axioscope II equipped with AxioCam MRC and analyzed with Axiovision 4.6 (Carl Zeiss, Gottingen, Germany).
Single cells from islets and IDPT were stained and analyzed by fluorescence activated cell sorting (FACS) using the FACS Calibur (BD Biosciences) and Cell Quest Pro software and compared to matched isotype controls[23,27]. Cells were permeabilized with 0.3% saponin for 60 min and stained with 1/10 vimentin-FITC, 1/5 EpCAM-FITC and 1/15 PCNA-647 (BioLegend, San Diego, CA, United States). Isotype controls were; IgG1-FITC, IgG2a-FITC and IgG2a-647. Values are expressed as mean percent ± SE.
Islets and IDPT cells prior to cell culture, during expansion and post differentiation were preserved in Trizol reagent (Invitrogen) and stored at -80 °C. RNA was extracted in combination with the RNeasy Mini Kit (Qiagen, Mississauga, ON Canada) as per the manufacturer’s protocol. cDNA was synthesized as described[21]. Real-time PCR was performed using the “TaqMan gene expression assay” (Applied Biosystems, Invitrogen) and a 7900HT Fast Realtime PCR System (Applied Biosystems). Relative quantification was performed by utilizing the comparative Ct method and all results were compared to the control samples for each time point after normalizing to an endogenous control (beta 2-microglobulin) using the Relative Quantification Manager software (Applied Biosystems). Values are expressed as mean percent ± SE. cDNA negative controls contained water in place of RNA and RT-PCR negative controls contained water in place of cDNA, β2-microglobulin (β2m) ensured cDNA integrity.
Lentiviral vectors were kindly provided by Dr. James D. Johnson (University of British Columbia, Vancouver, BC, Canada) and described in detail by Szabat et al[26]. We received the following vectors: dual reporter mouse PDX1 promoter-mRFP/rat Ins1 promoter-EGFP, single reporter mouse PDX1 promoter-mRFP, single reporter rat Ins1 promoter-EGFP as well as the structural and envelope vectors. Virus was produced by transfection of 293T cells that were a gift from Dr. Patrick MacDonald (University of Alberta, Edmonton, AB, Canada) utilizing FuGENE6 Transfection Reagent (Roche Diagnostics, IN, United States) and the protocol first described by Dr. Garry Nolan Lab (http://www.stanford.edu/group/nolan/index.html). Virus was titred using the rat INS1 cell line (a gift from Dr. Patrick MacDonald) and titres were between 2-4 × 106 TU/mL with an infection efficiency of 40%-70%. Single cells from human islets or IDPT were plated at a density of 0.3 × 106 cells/well onto a 24 well plate that contained 12 mm poly-l-lysine cover slips and cultured in pancreatic epithelial expansion medium. Cells were allowed to adhere and infected at a multiplicity of infection of < 1. Protein expression (fluorescence) was monitored daily and peak fluorescence of human primary cells was routinely detected between 4-7 d post infection. Differentiation of infected primary cells was started at 4-7 d post-infection. Absolute counts of positive mRFP and EGFP cells were counted using ImageJ software[29].
Data is expressed as mean ± SE. Statistical comparisons were performed with STATA11 (StataCorp LP, College Station, TX) using one-way analysis of variance and Bonferroni post-hoc test. Acceptable level of significance was considered P < 0.05.
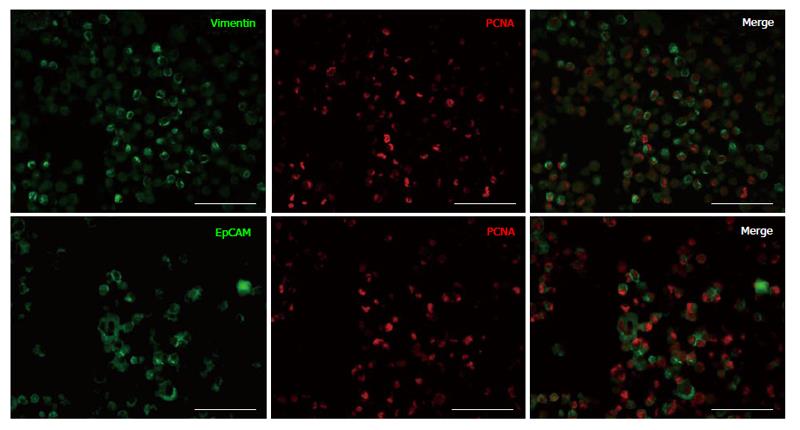
Previously, we demonstrated that during in vitro cell expansion of cells from IDPT, the proportion of epithelial cells decreased while vimentin positive cells (MSCs) significantly increased[21,23]. In this study, to determine if epithelial cells were still capable of proliferation, we assessed cell proliferation after 14 d in culture via dual IF staining and flow cytometry. Cells that were formalin fixed and embedded in agarose were stained with antibodies against EpCAM or vimentin (MSC) then co-stained for PCNA. After 14 d in culture we confirmed that vimentin+ cells were the predominant cell population and these cells were PCNA+ and proliferating (Figure 1). A small proportion of epithelial cells (EpCAM+) were still present and were also PCNA+ thus still proliferating (Figure 1). The proportion of vimentin and EpCAM cells that were positive for PCNA were quantified by FACS analysis at Time 0, 14 and 21 d in culture (Table 1). During cell expansion; the percentage of vimentin+ cells proliferating (PCNA+) increased from 22% ± 5.83% to 80.43% ± 3.24% (14 d) and 99.00% ± 0.0% (21 d), and the percentage of proliferating epithelial cells decreased from 74.71% ± 8.34% to 26.57% ± 9.75% (14 d) and 4.00% ± 1.53% (21 d), P < 0.01 for all time points. Therefore, MSC expansion culture conditions favor MSC expansion over epithelial cells
Our MSC expansion medium limits pancreatic epithelial cell growth in vitro[21,23,27]. We determined that sorted epithelial cells could expand and divide in MSC medium but vimentin+ MSCs were still the predominating cell population (not shown). By reducing the FBS content from 10% to 0.5% and including growth factors known to promote epithelial cell survival under reduced serum conditions such as EGF, 3,5,3’-triiodo-l-thyronine and bovine brain extract[30-32] we were able to minimize MSC overgrowth and preserve the epithelial cell phenotype (Table 2). IDPT cells cultured in pancreatic epithelial expansion medium contained significantly less vimentin+ cells (44.87% ± 4.93%) than cells expanded in MSC expansion medium (95.67% ± 1.36%; P < 0.01). Therefore, pancreatic epithelial expansion medium was used to trace cell fate and was supplemented for cell differentiation.
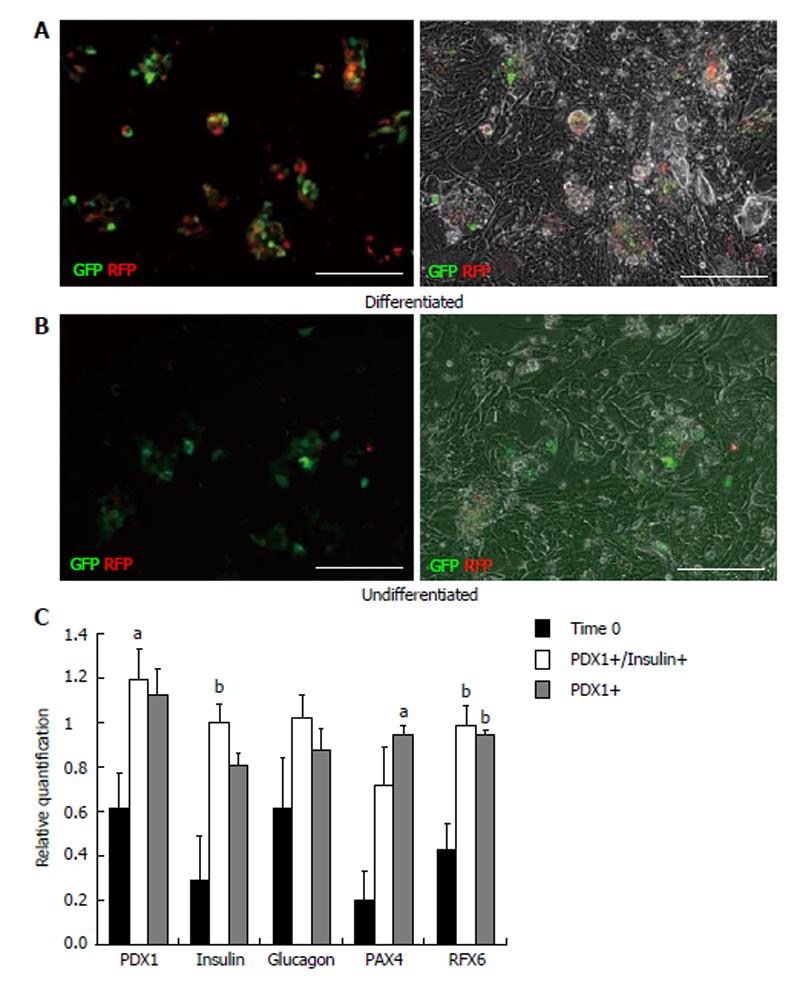
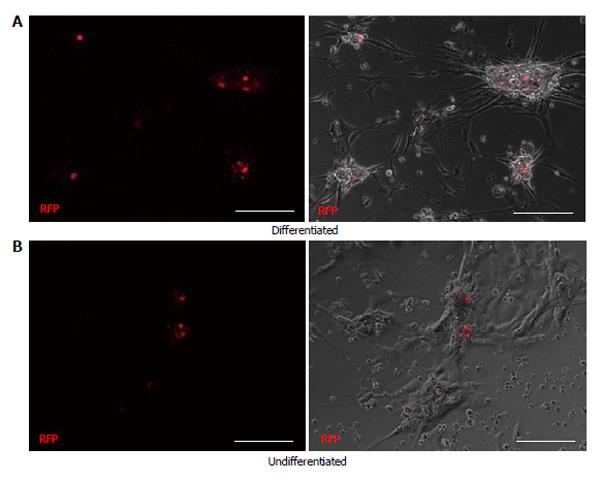
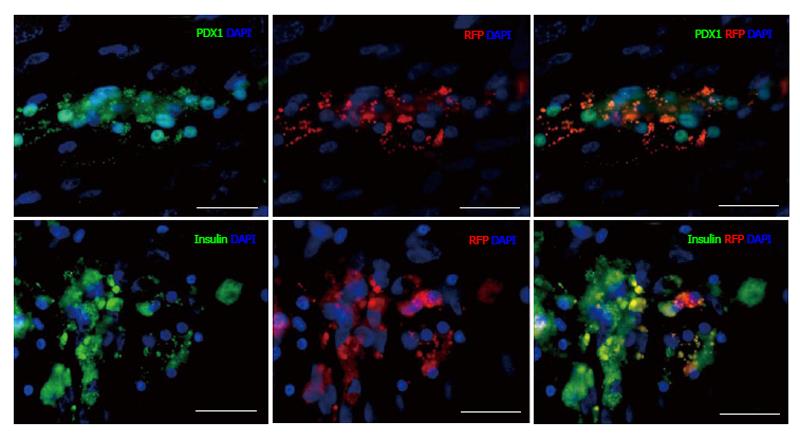
To determine the progenitor cell content within the pancreatic epithelium, cells from dissociated islets and IDPT were seeded onto poly-l-lysine coated cover slips placed in 24 well plates with pancreatic epithelial expansion medium. Prior to differentiation cells were infected with, the PDX1-mRFP-Ins1-EGFP dual reporter, PDX1-mRFP or Ins1-EGFP single reporter lentivirus then characterized via IF staining and real-time PCR. Fluorescence was detected between 4-7 d post infection in both islet and IDPT preparations, at which time differentiation was initiated. Controls were infected but not differentiated. During islet cell differentiation, cell aggregates formed throughout the cell monolayer. Within these adherent aggregates PDX1+ (RFP) and insulin+ (EGFP) expressing cells were observed (Figure 2A). In addition, both single positive (PDX1+ or INS+) and double positive (PDX1+ INS+) cells were observed (Figure 2A). In undifferentiated conditions fewer positive cells and cell aggregates were observed (Figure 2B). When analyzing image fields, in 4/4 cell preparations approximately twice as many PDX1+ and insulin+ cells were observed in differentiated conditions (51.25 ± 17.24 mRFP and 28.13 ± 9.34 EGFP) versus undifferentiated conditions (31.75 ± 10.83 mRFP and 14.50 ± 4.03 EGFP) as determined by absolute cell counts. Relative quantification of gene expression by real-time PCR (Figure 2C) confirmed this observation and demonstrated that differentiated islet cells compared to starting islets had increased expression of PDX1 (P < 0.05), insulin (P < 0.01) PAX4 (P < 0.05) and RFX6 (P < 0.01). A similar pattern was observed in differentiated (Figure 3A) and undifferentiated (Figure 3B) wells of cultured IDPT infected with PDX1-mRFP or PDX1-mRFP-Ins1-EGFP, although much less PDX1 and insulin expression (not shown) was observed compared to islet cell cultures.
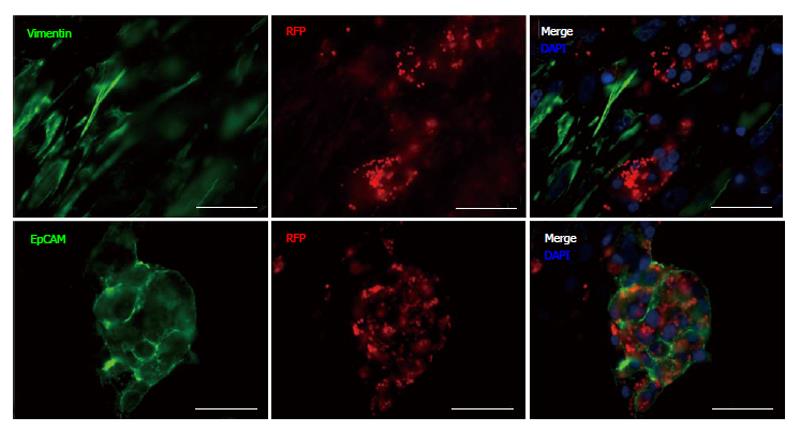
To identify cells within the differentiated cell aggregates that were PDX1+, and to confirm expression after lentiviral infection, cells were characterized by IF staining (Figures 4 and 5) utilizing the following antibodies: EpCAM, vimentin, CK19, PDX1, glucagon, insulin, pancreatic polypeptide and somatostatin. Cells positive for glucagon, pancreatic polypeptide and somatostatin, still remained after 25 d in culture compared to controls although these cells were infrequent and did not express PDX1+ (not shown). PDX1+ cells were shown to be negative for vimentin staining (Figure 4) and CK19 (not shown). PDX1+ cells did however, co-stain with EpCAM (Figure 4) demonstrating that in our experimental conditions PDX1+ cells are localized to the epithelial cell population. PDX1 and insulin expression was verified by, insulin and PDX1 primary antibody staining (Figure 5). Nuclear and cytoplasmic PDX1 staining (Figure 5) was observed while insulin staining was confined to the cytoplasm (Figure 5).
Although several recent human studies that involve lineage tracing[13,14,20,26] have been conducted, the debate continues as to the origin of human β-cell progenitors. Several studies have proposed that β-cell progenitors can be derived from a mesenchymal cell intermediate via EMT. In our previous studies we were also able to expand MSCs from exocrine and endocrine cell explants[21,23]. We determined that when the culture medium contained 10% serum, pancreatic MSCs could be rapidly expanded from pancreatic cells and that these MSCs could be easily differentiated into mesoderm (bone, fat and cartilage)[21,28]. However, in those studies pancreatic MSCs could only be partially differentiated into endocrine cells and we were unable to restore the epithelial cell phenotype or detect insulin protein by immunostaining[21,23]. We have since hypothesized that in our differentiation conditions it is the low percentage of epithelial cells, which remain after mesenchymal cell expansion, that respond favorably to our differentiation protocol[21,23] and not MSCs or a MSC intermediate. Thus, the dedifferentiation of islet or IDPT cells during long-term culture results in the loss of epithelial cells thus making this population difficult to follow in vitro.
In this study we utilized a reduced serum medium supplemented with growth factors known to support epithelial cells (EGF, 3,5,3’-triiodo-l-thyronine, bovine brain extract) and were able to minimize EMT and preserve the epithelial phenotype (Table 2). We determined that by preventing cell dedifferentiation and MSC overgrowth we could maintain the epithelial phenotype for greater than 25 d. If in fact epithelial mesenchymal transition[15,20] is a necessary process where progenitors or β-cells must dedifferentiate to replicate and then be redifferentiated into insulin producing cells then an alternate cell culture model must be employed. However, it is unclear if dedifferentiation, expansion then redifferentiation is a preferential model for increasing β-cells since β-cells generated in this model do not secrete physiologic levels of insulin compared to normal β-cells[15,20].
Lentiviral tracing[26] in combination with our pancreatic epithelial expansion medium, allowed us for the first time to observe morphological changes during in vitro differentiation without MSC overgrowth. Compared to lentiviral infected controls (undifferentiated) we have concluded that it is within the adherent differentiated cellular aggregates where PDX1+ and insulin+ cells reside. In addition, it is within these cellular aggregates where epithelial cells (EpCAM) that are PDX1+ are located. More importantly we did not observe vimentin+ or ductal epithelial cells (CK19+) that were PDX1 or insulin positive within the cell aggregates. Although several citations have demonstrated that pancreatic ductal epithelial cells can generate new β-cells[9-12], we did not observe this in our differentiation model. In addition, the infrequent cells we observed that were pancreatic polypeptide, somatostatin, and glucagon positive were also negative for PDX1 and insulin. By relative quantification we observed increased gene expression of developmental transcription factors indicative of a β-cell progenitor[24-26]. Differentiated cells compared to starting islets had at least two fold higher expression of PDX1, insulin, PAX4 and RFX (Figure 2C).
In summary we describe a unique cell culture condition for long-term study of pancreatic epithelial progenitor cells that minimizes overgrowth of MSCs (vimentin+) and dedifferentiation of epithelial cells through EMT. We confirmed that during differentiation via lentiviral reporting that PDX1+ cells were confined to epithelial cell aggregates that form during differentiation and not vimentin+ cells suggesting that EMT is not a mechanism for generating pancreatic progenitor cells. Future studies will be to determine overall function of differentiated cells.
We thank the staff of the Edmonton Clinical Islet Transplant Program (Alberta Health Services) for providing islets and IDPT tissue, and Dr. James D. Johnson and Marta Szabat for providing lentiviral constructs. We thank Sheena Lesyk, Mohammed Ahmed, Alana Espheter, Lynnette Elder and Bethany Ostrowerka for technical assistance.
Islet transplantation is an attractive alternative to daily insulin injections. Identifying and understanding the origin of a potential human β-cell progenitor could alleviate the current shortage of donor islets and contribute to the overall knowledge of β-cell regeneration. However, the study of β-cell progenitors is fraught with controversy, as several conflicting models and mechanisms describing the origin and existence of these progenitor cells have been proposed.
A popular model describing the origin of human β-cell progenitors that has been proposed is β-cell dedifferentiation and the expansion of a mesenchymal stem cell (MSC) intermediate via epithelial-mesenchymal transition (EMT). However, there has been limited success when redifferentiating these mesenchymal cells back into functional β-cells.
The authors previously demonstrated that MSCs, could be expanded 12-fold from human islet depleted pancreatic tissue that remained following islet isolation and demonstrated that these pancreatic MSCs could be partially differentiated into islet-like cells in vitro. However, in a follow up study the authors could not restore an epithelial phenotype or generate functional endocrine cells. The authors determined that the few remaining epithelial cells after MSC expansion were the cells that responded to the authors differentiation protocol. In these culture conditions MSC overgrowth prevented pancreatic epithelial progenitor expansion and differentiation. In this study the authors report that by using a novel pancreatic epithelial medium, MSC expansion and epithelial cell dedifferentiation can be minimized and pancreatic epithelial cell progenitors can be successfully expanded and differentiated.
This study demonstrates that β-cell progenitors reside in the pancreatic epithelium and that EMT should be inhibited in vitro to successfully expand and differentiate these progenitors. The authors conclude that EMT is not a mechanism for generating pancreatic progenitor cells.
EMT occurs in vitro when epithelial cells de-differentiate and lose their phenotype. The resulting cells are MSCs otherwise known as multi-potent stromal cells.
The authors describe a new method that could in the future allow for in vitro generation of insulin-producing cells. The work is well done and appropriately presented.
P- Reviewers: Zoller M, Vaidya MM S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1275] [Cited by in F6Publishing: 1173] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 2. | Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107-3114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Korsgren O, Lundgren T, Felldin M, Foss A, Isaksson B, Permert J, Persson NH, Rafael E, Rydén M, Salmela K. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia. 2008;51:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1709] [Cited by in F6Publishing: 1651] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 5. | Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 731] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 6. | Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 365] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Lipsett MA, Castellarin ML, Rosenberg L. Acinar plasticity: development of a novel in vitro model to study human acinar-to-duct-to-islet differentiation. Pancreas. 2007;34:452-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999-8004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 751] [Cited by in F6Publishing: 808] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 10. | Heremans Y, Van De Casteele M, in’t Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915-19919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | White MG, Al-Turaifi HR, Holliman GN, Aldibbiat A, Mahmoud A, Shaw JA. Pluripotency-associated stem cell marker expression in proliferative cell cultures derived from adult human pancreas. J Endocrinol. 2011;211:169-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261-2264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 354] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 16. | Eberhardt M, Salmon P, von Mach MA, Hengstler JG, Brulport M, Linscheid P, Seboek D, Oberholzer J, Barbero A, Martin I. Multipotential nestin and Isl-1 positive mesenchymal stem cells isolated from human pancreatic islets. Biochem Biophys Res Commun. 2006;345:1167-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Davani B, Ariely S, Ikonomou L, Oron Y, Gershengorn MC. Human islet-derived precursor cells can cycle between epithelial clusters and mesenchymal phenotypes. J Cell Mol Med. 2009;13:2570-2581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Russ HA, Ravassard P, Kerr-Conte J, Pattou F, Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Russ HA, Sintov E, Anker-Kitai L, Friedman O, Lenz A, Toren G, Farhy C, Pasmanik-Chor M, Oron-Karni V, Ravassard P. Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS One. 2011;6:e25566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1345] [Cited by in F6Publishing: 1308] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 23. | Seeberger KL, Eshpeter A, Rajotte RV, Korbutt GS. Epithelial cells within the human pancreas do not coexpress mesenchymal antigens: epithelial-mesenchymal transition is an artifact of cell culture. Lab Invest. 2009;89:110-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development. 2010;137:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang J, Lynn FC. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Szabat M, Luciani DS, Piret JM, Johnson JD. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology. 2009;150:1627-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Fox JE, Seeberger K, Dai XQ, Lyon J, Spigelman AF, Kolic J, Hajmrle C, Joseph JW, Kin T, Shapiro AM. Functional plasticity of the human infant β-cell exocytotic phenotype. Endocrinology. 2013;154:1392-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Seeberger KL, Eshpeter A, Korbutt GS. Isolation and culture of human multipotent stromal cells from the pancreas. Methods Mol Biol. 2011;698:123-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with Image. J Biophotonics. 2004;11:36-42. [Cited in This Article: ] |
| 30. | Misiti S, Anastasi E, Sciacchitano S, Verga Falzacappa C, Panacchia L, Bucci B, Khouri D, D’Acquarica I, Brunetti E, Di Mario U. 3,5,3’-Triiodo-L-thyronine enhances the differentiation of a human pancreatic duct cell line (hPANC-1) towards a beta-cell-Like phenotype. J Cell Physiol. 2005;204:286-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun. 2007;358:698-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods Enzymol. 2006;407:703-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |