Published online Jul 15, 2017. doi: 10.4251/wjgo.v9.i7.308
Peer-review started: January 23, 2017
First decision: February 17, 2017
Revised: March 15, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: July 15, 2017
Processing time: 173 Days and 11.4 Hours
Neuroendocrine neoplasms are the most common epithelial tumors among appendix tumors. Appendix tumors that are completely or partially composed of neuroendocrine cells are divided into two categories: Classic carcinoid tumors and goblet cell carcinoid tumors (GCCT). They are known to progress more aggressively than classic (neuro) endocrine tumors. In this study, three cases with acute appendicitis symptoms are presented, including their clinical and histopathological findings. Microscopic examination detected GCCT in two cases and mixed adenoneuroendocrine carcinoma in one case, in addition to acute appendicitis.
Core tip: Goblet cell carcinoid is a much more aggressive tumor than classic carcinoid, particularly if the tumor shows transmural involvement or if it has extended to the cecum at the time of the operation. This paper presents three cases clinical and histopathological features. Two were diagnosed as goblet cell carcinoid tumor and one was diagnosed as mixed adenoneuroendocrine carcinoma.
- Citation: Karaman H, Şenel F, Güreli M, Ekinci T, Topuz Ö. Goblet cell carcinoid of the appendix and mixed adenoneuroendocrine carcinoma: Report of three cases. World J Gastrointest Oncol 2017; 9(7): 308-313
- URL: https://www.wjgnet.com/1948-5204/full/v9/i7/308.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i7.308
Of appendix tumors, the most common epithelial tumors are neuroendocrine neoplasias[1]. Appendix tumors composed completely or partially of neuroendocrine cells are divided into two categories: Classic carcinoid tumors and goblet cell carcinoid tumors (GCCT) and their variants. GCCT of the appendix are rare and constitute approximately 6% of appendix carcinoids[1,2]. This kind of appendix tumor was first identified by Gagne in 1969[1,3]. Goblet cell carcinoids originate from pluripotent intestinal stem cells that show both neuroendocrine and mucinous differentiation[3-5]. Goblet cell carcinoid is known by various names such as adenocarcinoid, mucinous carcinoid, crypt cell carcinoid, and mucin-producingneuroendocrinetumor[3,6,7]. According to the neuroendocrine neoplasm classification published by the World Health Organization (WHO) in 2010, GCCTs are in the neuroendocrine tumor group[8-10]. They are known to progress more aggressively than classic (neuro)endocrine tumors[9,10].
In 2010, the WHO and the European Neuroendocrine Tumor Society (ENETS) divided the grade of neuroendocrine tumors (NET) into three groups based on the Ki-67 proliferation index and the number of mitoses. If there are less than two mitoses and a Ki-67 index of less than 3% in 10 HPF, it is called “NET, low grade (Grade 1)”. If there are 2-20 mitoses and a Ki-67 index of 3%-20%, it is called “NET, moderate-grade (Grade 2)”. If there are more than 20 mitoses with a Ki-67 index greater than 20%, it is classified as “neuroendocrine carcinoma, high grade (Grade 3)”[4,9]. The most important prognostic factor is the stage of disease. Appendectomy and right hemicolectomy are the main modalities of treatment, followed by adjuvant chemotherapy in select cases.
This paper presents three cases clinical and histopathological features. Two were diagnosed as goblet cell carcinoid and one was diagnosed as mixed adenoneuroendocrine carcinoma.
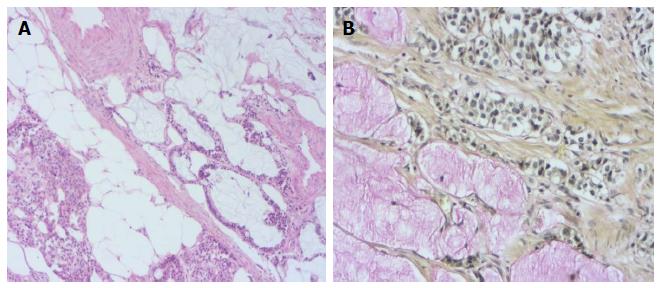
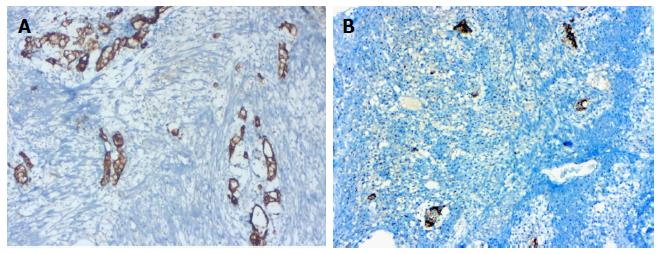
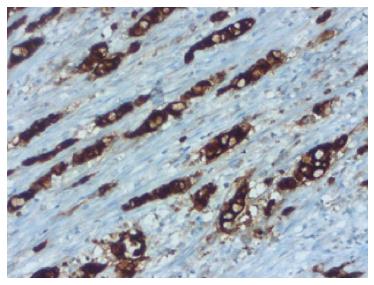
A 76-year-old male patient was admitted to the emergency room with abdominal pain and a fever. An appendectomy was performed with a pre-diagnosis of acute abdomen. The appendectomy material was submitted, which was 9 cm × 3 cm × 2 cm. There was a perforated area 2 cm in diameter. In section from the perforated area, tumour is composed of small, rounded nests of signet ring-like cells resembling normal intestinal goblet cells, except for the nuclear compression. The cells dispay mild-to-moderate atypia, low mitotic activity with a Ki 67 proliferation index < 20%, and infiltrated individually and in groups up to the subserosa and serosa by crossing the muscle layer from the mucosa on the appendix wall (Figure 1A). During the histochemical staining, mucicarmine and intra- and extra-cellular mucin deposition were identified in the tumor tissue (Figure 1B). Epithelial membrane antigen (Figure 2A), chromogranin-A (Figure 2B) and NSE (neuron-specific enolase) from the neuroendocrine markers, focal and CEA [monoclonal carcinoembryonic antigen (mCEA)], and wide spread immunoreaction were detected in the tumor cells via immunohistochemistry. Ten percent positive staining was obtained with a Ki 67 proliferation marker. There was no vascular and perineural invasion. Based on these findings, the diagnosis was goblet cell carcinoid and (pT3). Because of the tumor was potentially malignant right hemicolectomy were performed.
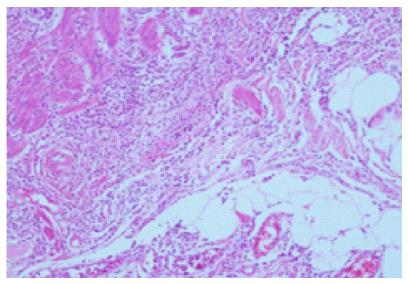
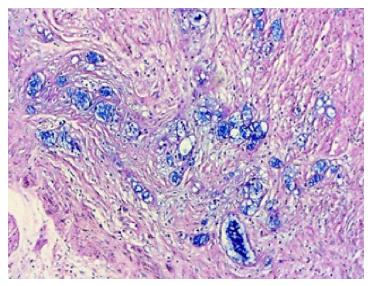
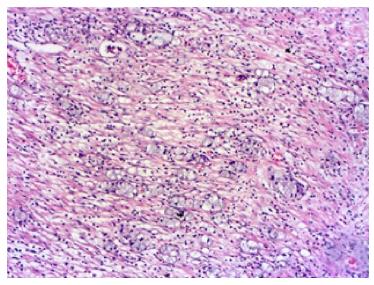
A 71-year-old man patient was admitted to the hospital emergency room with abdominal pain associated nausea and vomiting. The patient was operated for acute appendicitis. During surgery, the appendix was observed to be hyperemic and edematous. No significant tumor structure was observed. The appendix was completely removed for examination. In the examination of microscopic sections from an area of 5 cm, mucosa, submucosa, muscle layer, and tumor tissue invading the subserosa were identified, which were completely covering the tissue, starting from the basal part of the appendix lamina propria crypts (Figure 3). No tumor was identified on the proximal end of the incision. The tumor was wide, eosinophilic, had granular cytoplasm, and was characterized by small uniform nests formed by eccentric nuclei goblet cells and microglandular development. Perineural invasion was monitored. PAS-Alcian Blue pH 2.5 (Figure 4) and intra and extra-cellular mucin deposition were detected in the tumor tissue. In the immunohistochemicalstudiestumor was positive with the epithelial markers CK-20 and mCEA (Figure 5), and neuroendocrine cell component marker synaptophysin, chromogranin-A, CD56 and S100. With Ki-67, the proliferation index was 3%. There were acute appendicitis findings in the case as well.Overall the histological and immunohistochemical features were those of a goblet cell carcinoid tumor of the appendix tip with co-existing acute appendicitis. Because of the diffuse propagation of the tumor in an area of 5 cm on the appendix wall and the mesoappendix and its potential to be malignant, a right hemicolectomy was performed.
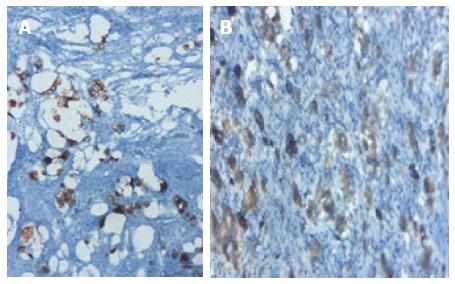
A 56-year-old man patient referred to the hospital with right lower quadrant pain.Physical examination revealed right lower quadrant abdominal tenderness and localized rebound tenderness. No palpable abdominal mass was present. In the emergency abdominal ultrasonography, the appendix diameter was 8.5 mm and markedly edematous. The neighboring mesenteric tissue was also edematous. Due to signs and symptoms typical of acute appendicitis, the patient underwent a simple appendectomy. Gross examination of the specimen showed the material measured 6 cm × 2 cm × 2 cm. The outer surface of the appendix was fibrinated in many areas. The area showing beige wall thickening was notablein macroscopic cross-section and measured 1.1 cm in diameter. In the surface of the cross-section, mucosa, submucosa, muscle layer, and tumor tissue infiltrated the subserosa were monitored, starting from the basal part of the appendix lamina propria crypts. No tumor was identified on the proximal end of the section. The tumor was significant cytologic atypia, wide, eosinophilic, had granular cytoplasm forming small nests and cords, and was characterized by small uniform nests with eccentric nuclei, signet-ring with micro glandular development, and cells in goblet cell appearance (Figure 6). No central lumen was observed in the tumor cell groups. In the serial sections, five mitoses were detected in 10 high-power fields (HPF) in the tumor. Perineural invasion was observed. PAS-Alcian Blue pH 2.5 and intra and extra-cellular mucin deposition were detected in the tumor tissue. In the immunohistochemical studies, mCEA, keratin 7, keratin 20, synaptophysin, chromogranin-A, CD56 and S100 from the common cytoplasmic and neuroendocrine markers, and strong focal positivity were detected in the tumor (Figure 7). With Ki-67, the proliferation index was 5%-10%. There were strong acute appendicitis findings in the case. Based on these findings, the case was diagnosed as “mixed adenoneuroendocrine carcinoma and acute appendicitis”. A right hemicolectomy was performed.
We considered these cases worth presenting, given their rarity, and for the opportunity to discuss malignancy criteria.
There are four types of appendix tumors: Epithelial tumors, mesenchymal tumors, lymphomas, and secondary tumors[1]. Epithelial tumors are grouped categorized as premalignant lesions, carcinoma, neuroendocrine tumors[2]. The most common epithelial tumors are neuroendocrine neoplasias. Appendix tumors that are completely or partially composed of neuroendocrine cells are divided into two different categories: Classic carcinoid tumors and GCCT, and their variants. Tumors showing both glandular and endocrine differentiation are called “amphicrine tumors”. The amphicrine tumor of the appendix is goblet cell carcinoid. In the WHO classification, this type of tumors that advance aggressively are called “mixed carcinoid adenocarcinoma” in the “mixed adenoneuroendocrine carcinoma” group, and “adenocarcinoma”, which develops on the goblet cell carcinoid[9]. The goblet cell carcinoid is called adenocarcinoid of goblet cell type[11], mucinous carcinoid tumor[12], and microglandular carcinoma[13], as well as crypt cell carcinoma[14]. Microscopically, it is characterized predominantly by submucosal growth. Extending into the muscle and serosa is common. However, the mucosa is typically preserved except tumor islets and obvious connections between crypt bases. The tumor itself is composed of small uniform goblet cell islets, which are generally arranged in the microglandular style and sometimes accompanied by extracellular mucus. The typical lesion consist of small uniform nests of goblet cells, often arranged in a microglandular fashion and sometimes accompanied by extracellular mucus[3].
GCCs usually occur in older patients, generally median age was 58 years (range 31-73) and no definite sex predominance[3]. Clinical presentation followed two distinct patterns: Acute appendicitis or chronic symptoms associated with a pelvic mass. The pathogenesis is unclear however the tumor likely arises from pluripotent intestinal epithelial crypt base stem cells. Loss of Notch signaling may be the driver mutation with other successive downstream mutations likely favors them into progressing and behavior similar to poorly differentiated adenocarcinoma with minimal neuroendocrine differentiation[9,15,16].
Goblet cell carcinoid is a more aggressive tumor than classic carcinoid, if the tumor shows transmural involvement or if it has extended to the cecum at the time of the operation[3,11,13]. In 2008, Tang et al[15], GCC patients are divided into three groups (A, B, C). Typical GCC (Group A); adenocarcinoma ex GCC, signet ring cell type (Group B); adenocarcinoma ex GCC, poorly differentiated carcinoma type (Group C). Typical GCC (Group A) was defined as well-defined goblet cells arranged in cluster or in a cohesive linear pattern, with minimal cytologic atypia and architectural distortion of the appendical wall, and minimal to no desmoplasia. Adenocarcinoma ex GCC, signet ring cell type (Group B) was defined as goblet cells or signet ring cells arranged in irregular large clusters, with the lack of confluent sheets of cells in a discohesive single file or single cell infiltrating pattern with significant cytologic atypia, and desmoplasia and associated destruction of the appendiceal wall[15,16]. Adenocarcinoma ex GCC, poorly differentiated carcinoma type (Group C) was defined with the least focal evidence of goblet cell morphology and a component (> 1 low power field or 1 mm2) that is not otherwise distinguishable from a poorly differentiated adenocarcinoma[15,16].
The tumor itself, as in our case study, is composed of small uniform goblet cell islets, which are generally arranged in the microglandular style and sometimes accompanied by extracellular mucus. The histochemical stainings, mucicarmine, and CEA stainings are always positive. There is generally CK20 in all cases and the focal style CK7 positivity in approximately 70% of the cases. In our case, in agreement with the literature, tumor cells were positive for CEA, CK7, CK20, and neuroendocrine markers.
Fifty percent of patients with these tumors, who are rarely seen, often go to hospitals in the 50s or 60s with abdominal pain and palpable masses[2]. Others cases, like ours, were detected incidentally in the appendectomy specimens removed from the patients, who arrived at the hospital with acute abdominal pain and symptoms of acute appendicitis and were taken to surgery diagnosed with acute appendicitis[3,4,12,14-18]. In addition to the characteristic histological appearance, as seen in our cases, they showed widespread invasion of the mesoappendix and perineural[19].
Adenocarcinoma developing on the goblet cell carcinoid ground constitutesa significant portion of mixed adenoneuroendocrine carcinoma. The adenocarcinoma component is in the form of signet ring cell carcinoma or poorly differentiated carcinoma. In these tumors, the goblet cell carcinoid tumor area should cover at least low-power fields (or 1 mm2) of the tumor. The signet ring cell type is composed of cells aligned in a single row or that form irregular groups with the appearance of a signet ring as well as goblet cell carcinoid areas[4]. Our case was diagnosed as “mixed adenoneuroendocrine carcinoma diagnosis” because of the presence of the signet ring cell component developing from the goblet cell carcinoid.
In our case, the Ki-67 labeling index was 3%-10%. In the ENETS-2007 pathologic TNM staging, < 1 cm tumors are T1, ≤ 2 cm tumors with subserosa or mesoappendix invasion of less than 3 mm are T2, > 2 cm tumors with subserosa or mesoappendix invasion of > 3 mm are T3, and tumors with the peritoneum or other organ invasion are T4. GCCT are more aggressive than classical carcinoid tumors. Metastases have been documented in 8%-20% of the cases[11,13,15,16]. There are liver, ovarian, and peritoneal metastases during the diagnosis in approximately 10% of patients. While five-year survival is between 50%-80% for the disease when limited to the appendix, it is less than 20% in the presence of distant metastases[16]. In the literature, it is reported that an appendectomy should be sufficient for the treatment of tumors smaller than 1 cm that do not have no serious mesoappendix involvement and a low Ki-67 index[4,20-22].
The recommended treatment for goblet cell carcinoid, whether pure or combined, is a right hemicolectomy, especially if the tumor has spread beyond the appendix and/or shows a high mitoticcount. Although many authors suggest routine right hemicolectomy for goblet cell carcinoids, this recommendation has been questioned, and some authors believe that appendectomy alone may be sufficient if the appendical margin is clear, there is no evidence of spread into the periappendiceal soft tissue, the mitotic count is no more than two mitoses per 10 HPF, and there are no features of mixed goblet cell carcinoid-adenocarcinoma[3,15,16]. Both European and North American Neuroendocrine tumor societies guidelines recommend right hemicolectomy after appendectomy due to the high rate of metastases and its impact on prognosis. Our cases underwent a right hemicolectomy after the diagnosis of GCC and mixed adenoneuroendocrine carcinoma and have been made. As a result, because it is known that GCCT are more aggressive than classical carcinoid tumors but they do not show malignant behavior like adenocarcinomas, their histological identification is important.
Finally, GCCs are unique to the appendix. Appendiceal NETs asymptomatic. Carcinoid synrome is very uncommon. The symptoms resemble acute appendicitis. Since the diagnosis is usually established post appendectomy, appendectomy materials should be examined carefully. NET and adenocarcinomas developing on the goblet cell carcinoid should definitely be kept in mind in terms of malignancy. Grading and staging play an important role in treatment and prognosis.
Abdominal pain.
Acute abdomen.
Appendiceal neoplasm.
All labs were within normal limits.
Acute appendicitis.
Goblet cell carcinoid.
Hemicolectomy mixed adenoneuroendocrine carcinoma.
Grading and staging play an important role in treatment and prognosis.
GCCT: Goblet cell carcinoid tumors.
Neuroendocrine tumors and adenocarcinomas developing on the goblet cell carcinoid should definitely be kept in mind in terms of malignancy.
The authors presented a rare pathology of appendix. The manuscript was well structured and written.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alkan A, Tanabe S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Rosai J. Appendix. In: Rosai and Ackerman’s Surgical Pathology. 10th ed. Edinburgh, Elsevier, Mosby, China 2011; 714. |
| 2. | Gramlich J, Petras R. Vermiform appendix. In: Mills S, eds. Histology for Pathologists. Lippincot Williams 2007; 648-657. |
| 3. | Doyle LA, Odze RW. Inflammatory disorders of the appendix. In: Odze RD, Goldblum JR, eds. Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas, 2nd ed. Philadelphia 2015; 512-529. |
| 4. | Paşaoğlu E. Apendiksin Tümörleri. In: Doğusoy G, eds. Gastroenteroloji Patoloji. İzmir. O’tıp kitabevi ve Yayıncılık 2015; 240-259. |
| 5. | Mederos R, Danielpour P, Christopher M, Middendorf BA, Willis I, Minervini D, Vicentelli C. Goblet cell carcinoid tumors of the appendix: Report of two cases and a short review of the literature. Surg Journal. 2007;2:33-36. |
| 6. | Haqqani MT, Williams G. Mucin producing carcinoid tumours of the vermiform appendix. J Clin Pathol. 1977;30:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Roy P, Chetty R. Goblet cell carcinoid tumors of the appendix: An overview. World J Gastrointest Oncol. 2010;2:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 749] [Article Influence: 49.9] [Reference Citation Analysis (2)] |
| 9. | Bosman F, Carneiro F, Hruban R, Theise N, editors . WHO classification of tumours of the digestive system. Lyon: IARC Press 2010; . |
| 10. | Schott M, Klöppel G, Raffel A, Saleh A, Knoefel WT, Scherbaum WA. Neuroendocrine neoplasms of the gastrointestinal tract. Dtsch Arztebl Int. 2011;108:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Warkel RL, Cooper PH, Helwig EB. Adenocarcinoid, a mucin-producing carcinoid tumor of the appendix: a study of 39 cases. Cancer. 1978;42:2781-2793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Klein HZ. Mucinous carcinoid tumor of the vermiform appendix. Cancer. 1974;33:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Wolff M, Ahmed N. Epithelial neoplasms of the vermiform appendix (exclusive of carcinoid). I. Adenocarcinoma of the appendix. Cancer. 1976;37:2493-2510. [PubMed] |
| 14. | Isaacson P. Crypt cell carcinoma of the appendix (so-called adenocarcinoid tumor). Am J Surg Pathol. 1981;5:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Tang LH, Shia J, Soslow RA, Dhall D, Wong WD, O’Reilly E, Qin J, Paty P, Weiser MR, Guillem J. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32:1429-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Shenoy S. Goblet cell carcinoids of the appendix: Tumor biology, mutations and management strategies. World J Gastrointest Surg. 2016;8:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Chetty R, Serra S. Lipid-rich and clear cell neuroendocrine tumors (“carcinoids”) of the appendix: potential confusion with goblet cell carcinoid. Am J Surg Pathol. 2010;34:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Plöckinger U, Couvelard A, Falconi M, Sundin A, Salazar R, Christ E, de Herder WW, Gross D, Knapp WH, Knigge UP. Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated tumour/carcinoma of the appendix and goblet cell carcinoma. Neuroendocrinology. 2008;87:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Vilchez RT, Martin DP, Blanco CQ, Aragones AM, Liebana RF, Donoso FJM, Conejo EA. Goblet cell carcinoid of the appendix. A 52 years old male with acute appendicitis. Oncologia. 2007;30:72-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Kahraman DS, Akin N. Goblet cell carcinoid of the appendix: a case report. Turk Patoloji Derg. 2015;31:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95:135-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 22. | Griniatsos J, Michail O. Appendiceal neuroendocrine tumors: Recent insights and clinical implications. World J Gastrointest Oncol. 2010;2:192-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |