Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.968
Peer-review started: October 8, 2023
First decision: December 2, 2023
Revised: December 23, 2023
Accepted: January 19, 2024
Article in press: January 19, 2024
Published online: March 15, 2024
Traditional treatments for pancreatic cancer (PC) are inadequate. Photodynamic therapy (PDT) is non-invasive, and proven safe to kill cancer cells, including PC. However, the mitochondrial concentration of the photosensitizer, such as verteporfin, is key.
To investigate the distribution of fluorescence of verteporfin in PC cells treated with antitumor drugs, post-PDT.
Workable survival rates of PC cells (AsPC-1, BxPC-3) were determined with chemotherapy [doxorubicin (DOX) and gemcitabine (GEM)] and non-chemotherapy [sirolimus (SRL) and cetuximab (CTX)] drugs in vitro, with or without verteporfin, as measured via MTT, flow cytometry, and laser confocal microscopy. Reduced cell proliferation was associated with GEM that was more enduring compared with DOX. Confocal laser microscopy allowed observation of GEM- and verteporfin-treated PC cells co-stained with 4’,6-diamidino-2-phenylindole and MitoTracker Green to differentiate living and dead cells and subcellular localization of verteporfin, respectively.
Cell survival significantly dropped upon exposure to either chemotherapy drug, but not to SRL or CTX. Both cell lines responded similarly to GEM. The intensity of fluorescence was associated with the concentration of verteporfin. Additional experiments using GEM showed that survival rates of the PC cells treated with 10 μmol/L verteporfin (but not less) were significantly lower relative to nil verte
Verteporfin was observed in living cells. In GEM -treated human PC cells, verteporfin was particularly prevalent in the mitochondria. This study supports further study of PDT for the treatment of PC after neoadjuvant chemotherapy.
Core Tip: Photodynamic therapy (PDT) for pancreatic cancer (PC) is more effective when the photosensitizer is concentrated in the mitochondria. This study showed that 10 μmol/L verteporfin treatment at various times significantly reduced the survival of human PC cells, but lower concentrations had no discernible results. After gemcitabine treatment, verteporfin was located primarily in the mitochondria. Verteporfin was more prevalent in living than dead cells, and in the latter was found mainly in the mitochondria. This study provides a significant theoretical foundation for using PDT in the comprehensive treatment of PC after neoadjuvant chemotherapy.
- Citation: Zhang YQ, Liu QH, Liu L, Guo PY, Wang RZ, Ba ZC. Verteporfin fluorescence in antineoplastic-treated pancreatic cancer cells found concentrated in mitochondria. World J Gastrointest Oncol 2024; 16(3): 968-978
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/968.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.968
Pancreatic cancer (PC) is extremely malignant. It develops quickly, has a short disease course, and mortality is high. Many patients have already developed metastasis by the time PC is diagnosed. Furthermore, by the year 2030, PC may be the second leading cause of death from malignant tumors, as annual incidence rates are increasing from 0.5% to 1.0%[1]. Traditional treatment methods for PC are inadequate, and new multimodal comprehensive methods are required[2].
Photodynamic therapy (PDT) is a non-invasive medical technology that kills cancer cells. The essential components of PDT are an excitation light (laser), a drug activated by the light (i.e., the photosensitizer or photosensitizing agent), and an appropriate concentration of oxygen. When the photosensitizer is activated by blue fluorescent light from the laser, it reacts with the surrounding oxygen to produce reactive oxygen species (ROS) that can cause cell apoptosis and necrosis[3]. Relative to normal tissue, a photosensitizer is preferentially distributed and absorbed by tumor tissue[4], and when activated by the light source, the tumor tissue is effectively labeled with fluorescence.
In recent years, PDT has been employed to treat various tumors including esophageal, gastric, bladder, lung, and nasopharyngeal cancers, and basal cell carcinoma[5-9]. PDT has also been confirmed safe and effective for the treatment of PC[10,11]. However, the effectiveness of PDT for treating PC can be limited by shallow penetration, lack of targeted photosensitizer, and insufficient oxygen, and toxic side effects preclude the clinical application of various novel photosensitizers that have achieved satisfactory results in vitro and in vivo.
The subcellular site of the photosensitizer also influences the benefit of PDT. Specifically, PDT has a stronger antitumor effect when the photosensitizer is concentrated in the mitochondria[12,13]. It is well accepted that mitochondria control cell growth and the cell cycle[14], and serve as the energy source for highly prolific cancer cells[15]. The ROS that results from the activated photosensitizer has a short half-life and affects only the immediate area[16]. Thus, because the location of the photosensitizer determines both the site of ROS production and photodynamic damage, the photosensitizer is best targeted to the mitochondria.
Verteporfin is a second-generation porphyrin photosensitizer, a benzoporphyrin derivative monoacid. Used for PDT, verteporfin is a dark green-to-black solid that is activated by laser irradiation with 689 nm wavelength[17]. Verteporfin-mediated PDT can be used to treat PC[18]. Combined with traditional treatments for PC, PDT may be both safe and effective.
This preliminary in vitro study investigated the effectiveness of the photosensitizer verteporfin in human PC cells, and tested whether its use in combination with other common treatments is viable. First, an investigation was conducted into the respective effects of 4 antineoplastic drugs (non-chemotherapy and chemotherapy), with or without verteporfin, on the proliferation and apoptosis of the cells. From the results, further experiments located the verteporfin within the living and dead PC cells via fluorescence imaging assays.
Human metastatic PC AsPC-1 cells (derived from human PC ascites cells xenograft in nude mice) and human pancreatic PC BxPC-3 cells were purchased from the American Type Culture Collection. The cells were grown and maintained in RPMI1640 (Roswell Park Memorial Institute 1640) supplemented with 10% fetal bovine serum and antibiotics (penicillin-streptomycin; all from Gibco, United States) in a humidified incubator containing 5% CO2 at 37 °C.
A well plate (i.e., a 12 × 8 well format with a see-through lid, Corning Costar) was prepared containing a dilution series of the photosensitizer verteporfin. An in vivo imaging system (IVIS) spectrum was used to measure the fluorescence intensity of verteporfin. The following concentrations of verteporfin were applied in succession: Nil, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 μmol/L.
Cells of the above stated cell lines were seeded in 96-well plates (8000 AsPC-1 cells and 10000 BxPC-3 cells per well, respectively). The cells were incubated in complete cell culture medium (200 μL/well) overnight. The liquid at the top was discarded, the wells were rinsed with phosphate-buffered saline (PBS), and complete cell culture medium was added again.
The wells were treated respectively with verteporfin (United States), doxorubicin (DOX; Pfizer, United States), gemcitabine (GEM; Eli Lilly, France), sirolimus (SRL; Pfizer, Ireland), or cetuximab (CTX; Merck, Germany). Specifically, verteporfin was applied at the following concentrations for incubations times 1, 3, 6, 12, or 24 h: Nil, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 μmol/L. The incubation times for DOX were 24, and 48 h, at concentrations of nil, 0.2, 0.6, 0.8, 1.0, 1.2, or 1.4 μmol/L. The incubation times for GEM were 3, 6, 12, 24, 48, or 72 h, at concentrations of nil, 10, 20, 30, 40, 50, 60, or 70 μmol/L. Cells were treated with SRL for 24, 48, and 72 h, at concentrations of nil, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10 nmol/L. CTX was applied for 24, 48, or 72 h at concentrations of nil, 20, 40, 60, 80, or 100 μmol/L.
MTT (5 mg/mL, 12.5 μL/well; Sigma-Aldrich GmbH, Germany) was added to each well, and incubated for at least 4 h. The upper liquid was discarded and 100 μL of dimethyl sulfoxide (DMSO) was added to each well. A microplate reader was used to measure the optical density at 540 nm.
Different concentrations of DOX (0.6 μmol/L, 0.8 μmol/L) in AsPC-1 and BXPC-3 cells, and 70 μmol/L in GEM-treated AsPC-1 and BXPC-3 cells, were applied for up to 72 h. Eppendorf (EP) tubes were prepared. The PC cells were added (1 × 106), treated with drugs, and centrifuged (1000 rpm, 3 min). The waste liquid was discarded. The cells were double-fixed with 2.5% glutaraldehyde and 1% osmium tetroxide, dehydrated, and embedded after fixation. The samples were cut into ultrathin sections with a Reichert Jung UltraCut ultramicrotome, and viewed under a Hitachi H-7650 transmission electron microscope.
Based on the results of the MTT assays, the appropriate drug parameters were chosen according to the dose that reduced the survival rate of the PC cells. Cells were treated for 48 h with a series of DOX concentrations (nil, 0.2, 0.4, 0.6, 0.8, and 1 μmol/L). GEM was applied to AsPC-1 and BxPC-3 cells at concentrations of nil, 10, 30, 50, and 70 μmol/L for 72 h.
EP tubes were prepared. Each was filled with 106 drug-treated cells and centrifuged for 3 min at 1000 rpm. The top liquid was removed and Annexin V-FITC was added. This was mixed to resuspend the cells. The samples were incubated for 10 min at room temperature in the dark. Propidium iodide (PI; 5 μL/tube) was added and flow cytometry assays were conducted.
DOX (0.4, 0.8 μmol/L) and GEM (30, 50 μmol/L) were applied to the AsPC-1 and BxPC-3 cells, for 48 and 72 h, respectively. After the GEM or DOX treatment, the AsPC-1 and BxPC-3 cells (106/mL) were suspended in PBS. Annexin V-FITC was added to a final concentration of 2 nmol/L, and incubated for 10 min in the dark before adding 1 μg/mL of PI. The cells were washed to remove superfluous dyes that had not adhered to the cells. Under a Zeiss LSM 510 Meta laser confocal microscope, the images were examined and recorded.
AsPC-1 and BxPC-3 cells were treated with DOX (0.6 and 0.8 μmol/L, respectively) for 48 h, and with 70 μmol/L GEM for 72 h. Then each EP tube was filled with 106 of the drug-treated cells and centrifuged for 3 min at 1000 rpm, and the waste liquid was discarded. The cells in each EP tube underwent a double fixation with 1% osmium tetroxide and 2.5% glutaraldehyde. After fixation, these PC cells were dehydrated and embedded. A Reichert-Jung UltraCut ultra-thin microtome was used to section the samples, which were viewed through a Hitachi H-7650 transmission electron microscope.
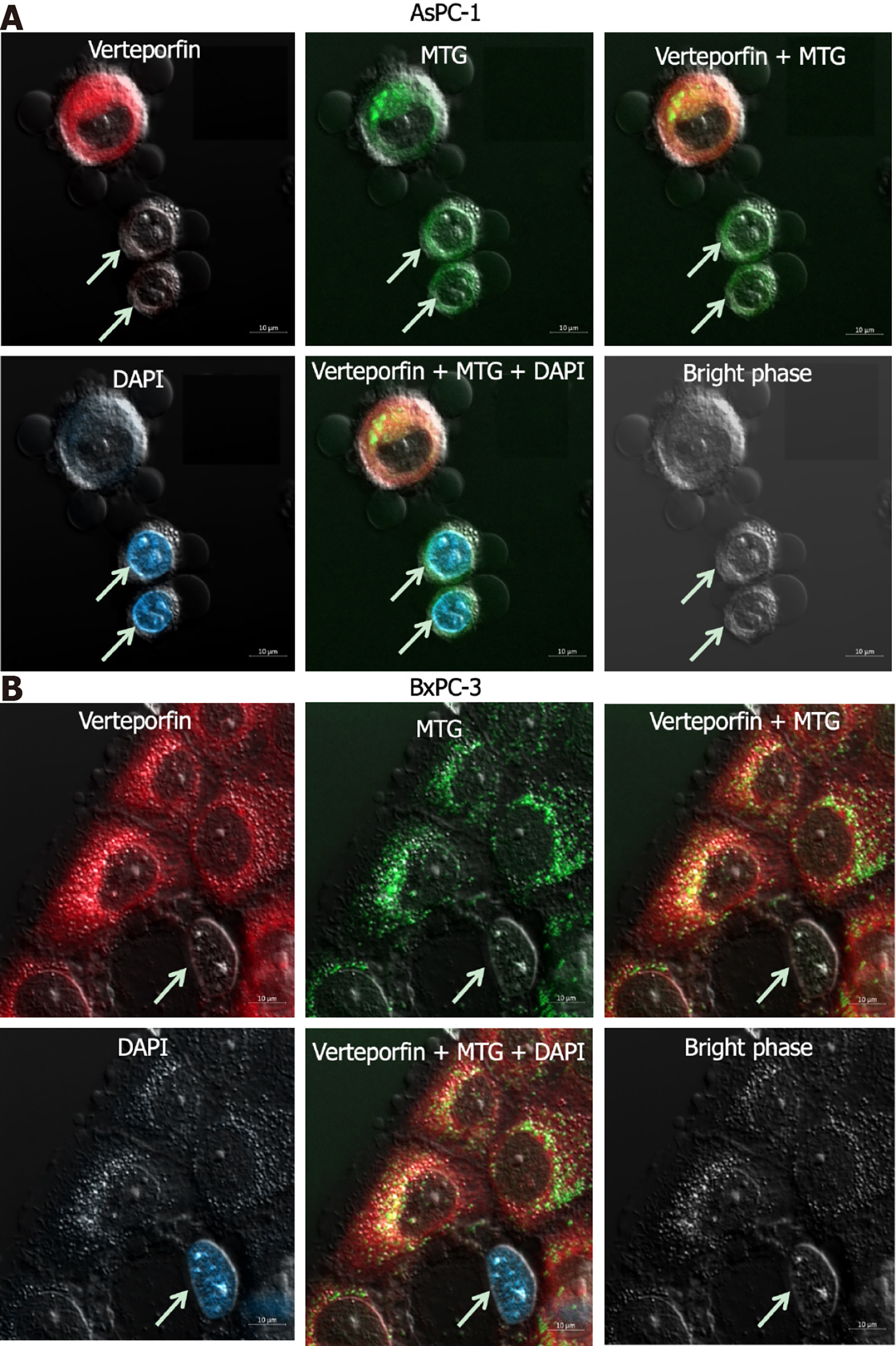
AsPC-1 and BxPC-3 cells were exposed to 30 μmol/L GEM for 72 h, and incubated for 2 h with 1 mol/L verteporfin. Anhydrous DMSO was used to make MitoTracker Green (MTG; a green fluorescent dye specific to mitochondria), at a final concentration of 1 mmol/L. One liter of MTG was added to either a 50-mL or 5-mL cell culture solution to make a working solution (20 to 200 nmol/L) of MTG.
Two milliliters of deionized water were used to dissolve 4’,6-diamidino-2-phenylindole (DAPI), yielding a 14.3 mmol/L DAPI storage solution. By mixing 2.1 μL of 14.3 mmol/L DAPI with 100 μL of PBS, a 300 μmol/L DAPI solution was created. DAPI was diluted in a 1:1000 ratio from 300 μmol/L to 300 nmol/L DAPI dye solution. The 300 nmol/L DAPI dye solution was added to cover the cells after 1 to 3 rounds of washing with PBS. The cells were incubated without light for 5 min, which were viewed finally using a confocal laser microscope.
SPSS 26.0 statistical software (IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM) was used for statistical analyses. All the data are reported as independent experiments that were repeated for reproducibility. The experimental results are reported as mean ± SD. The t-test was used for statistical analyses, and P < 0.05 was considered statistically significant.
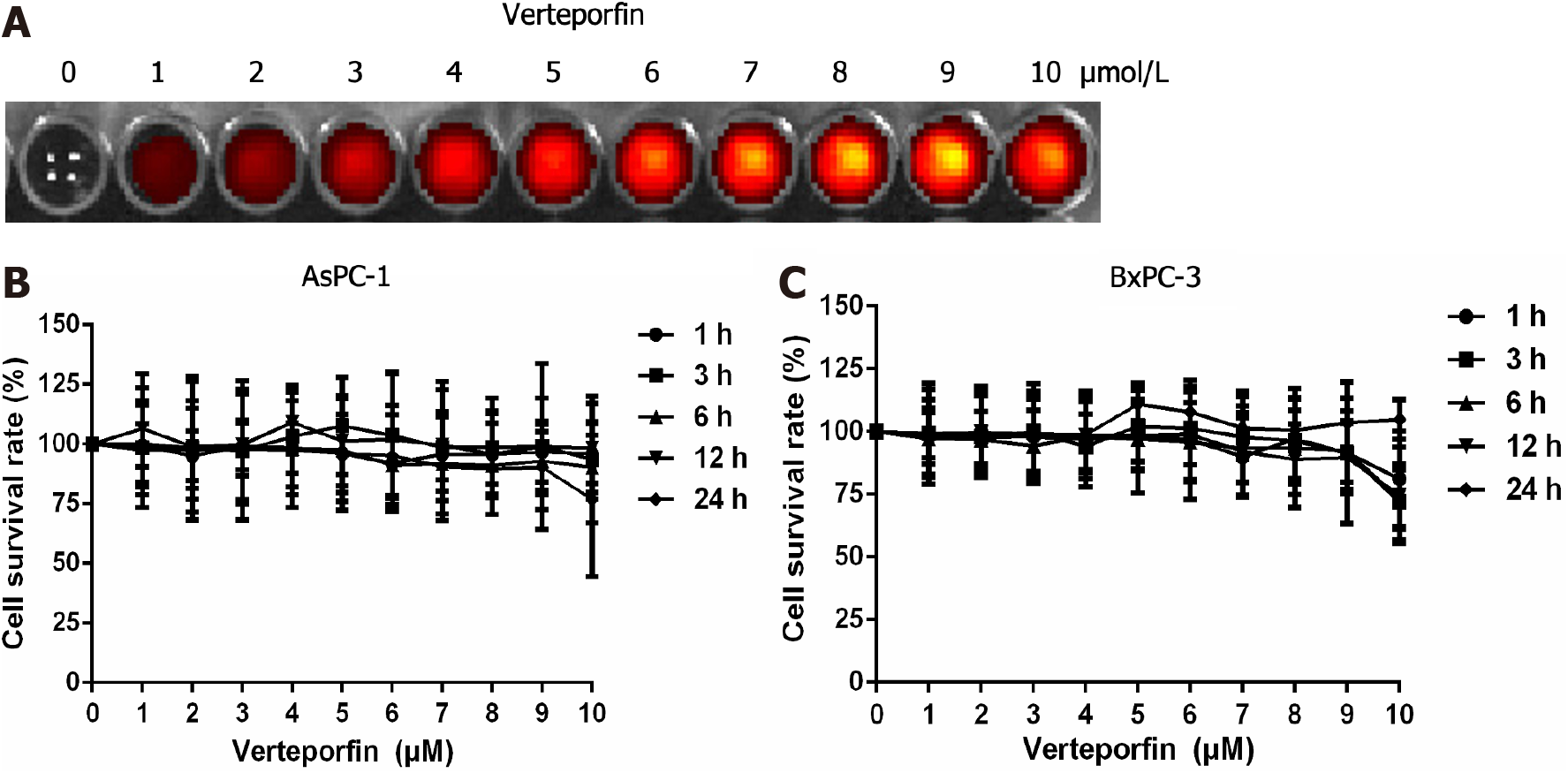
An IVIS spectrum small animal imaging system was used to measure the fluorescence intensity of verteporfin in different wells at the various concentrations. The intensity of the red fluorescence gradually increased as the verteporfin concentration increased (Figure 1A).
Cells of the 2 cell lines were respectively exposed to various concentrations of verteporfin for varying periods of time (see methods). Regarding the AsPC-1 cells, the cell survival rate was 76.94% after 24 h of treatment with 10 mol/L verteporfin, but at lower concentrations there was no appreciable change (Figure 1B). In the BxPC-3 cells, at 10 mol/L verteporfin and 24 h, the cell survival rate was significantly less than that of cells treated with lower concentrations. At 1-9 mol/L verteporfin, the cell survival rate was unchanged at any treatment time (Figure 1C).
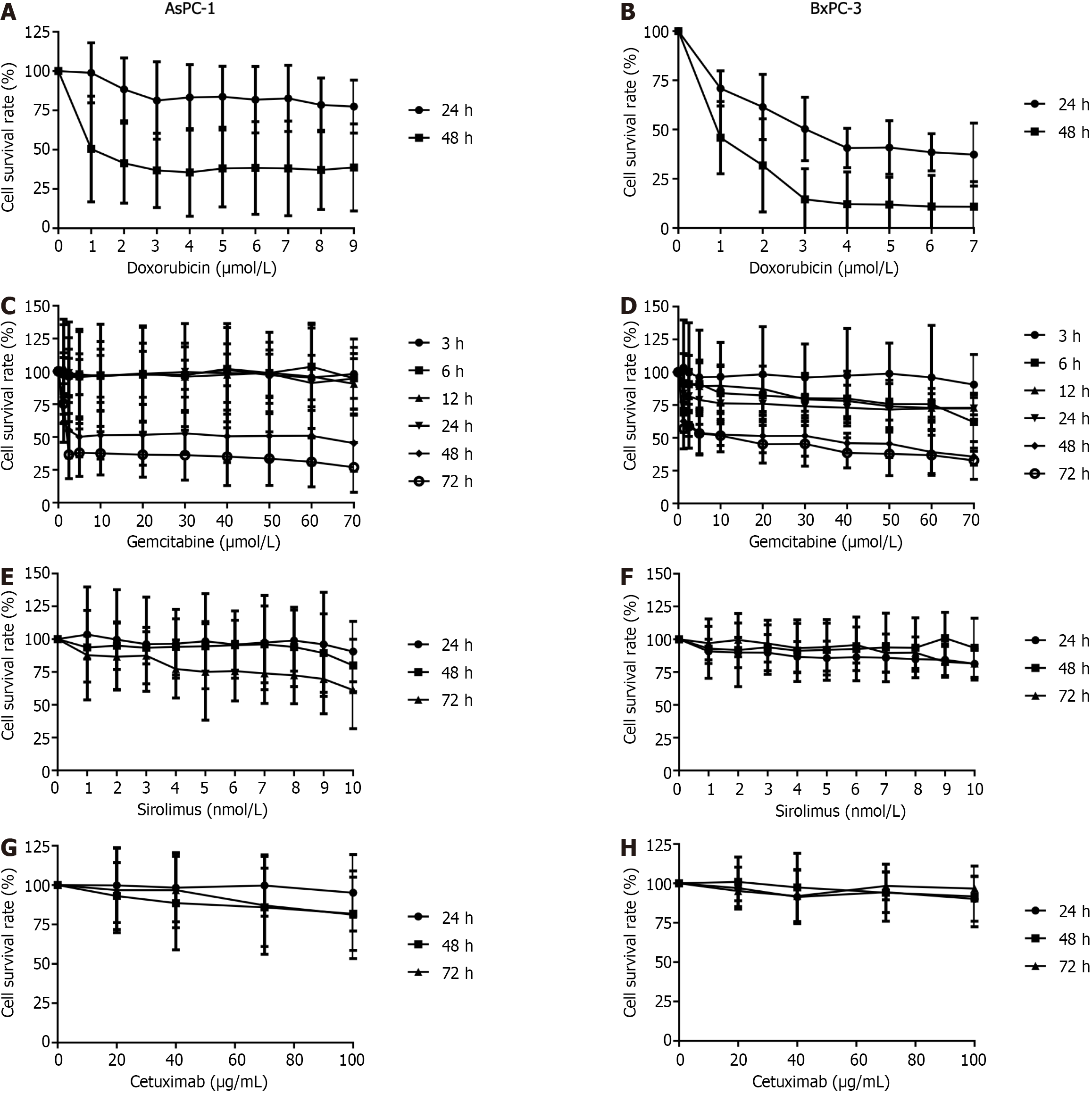
When AsPC-1 cells were treated with DOX for 24 h, the survival rate was lower than that of the non-treated cells, but similar over the range of DOX concentrations (Figure 2A). The cell survival rate was lowest (77.49% ± 16.94%) at the maximum concentration (1.4 mol/L). At 48 h incubation, the survival rate had continued to decrease compared with that of the non-treated cells; at the maximum concentration of DOX, survival was 38.66% ± 27.71%.
Under DOX treatment, at all timepoints, the survival rates of the BxPC-3 cells were significantly less than that of the AsPC-1 cells (Figure 2B). At 24 h incubation, the survival rates decreased gradually with increasing DOX concentration, reaching its lowest value (37.28% ± 16.04%) at 1.4 mol/L. At 48 h incubation, the survival rates also decreased as the DOX concentration increased, and was lowest (10.79% ± 12.79%) at 1.4 mol/L.
Concerning AsPC-1 cells treated with GEM, at all concentrations and at 3, 6, 12, and 24 h, there was no discernible effect on the survival of the cells (Figure 2C). However, at 48 and 72 h, cell survival was significantly lower. At 48 h, nearly half of the cells had died. At 72 h, the survival rates gradually reduced from 75.48% ± 15.20% at 1.25 μmol/L, to 26.93% ± 19.05% at 70 μmol/L.
In the BxPC-3 cells treated with GEM, at 3 h the survival rates under various concentrations were comparable (Figure 2D), but dropped at 6, 12, and 24 h when the cell survival was approximately 75%. At 48 and 72 h, the cell survival rate had significantly declined compared with the earlier incubation times.
In AsPC-1 cells treated with SRL, at all concentrations, at 24 and 48 h, cell proliferation had not changed (Figure 2E). However, at 72 h survival was negatively associated with SRL concentration. In the BxPC-3 cells treated with SRL, at all concentrations and timepoints, proliferation was comparable (Figure 2F). In AsPC-1 cells treated with CTX, at all concentrations, at 24, 48, and 72 h the survival rates decreased with time (Figure 2G). In BxPC-3 cells, the survival rates were similar at all concentrations and timepoints (Figure 2H).
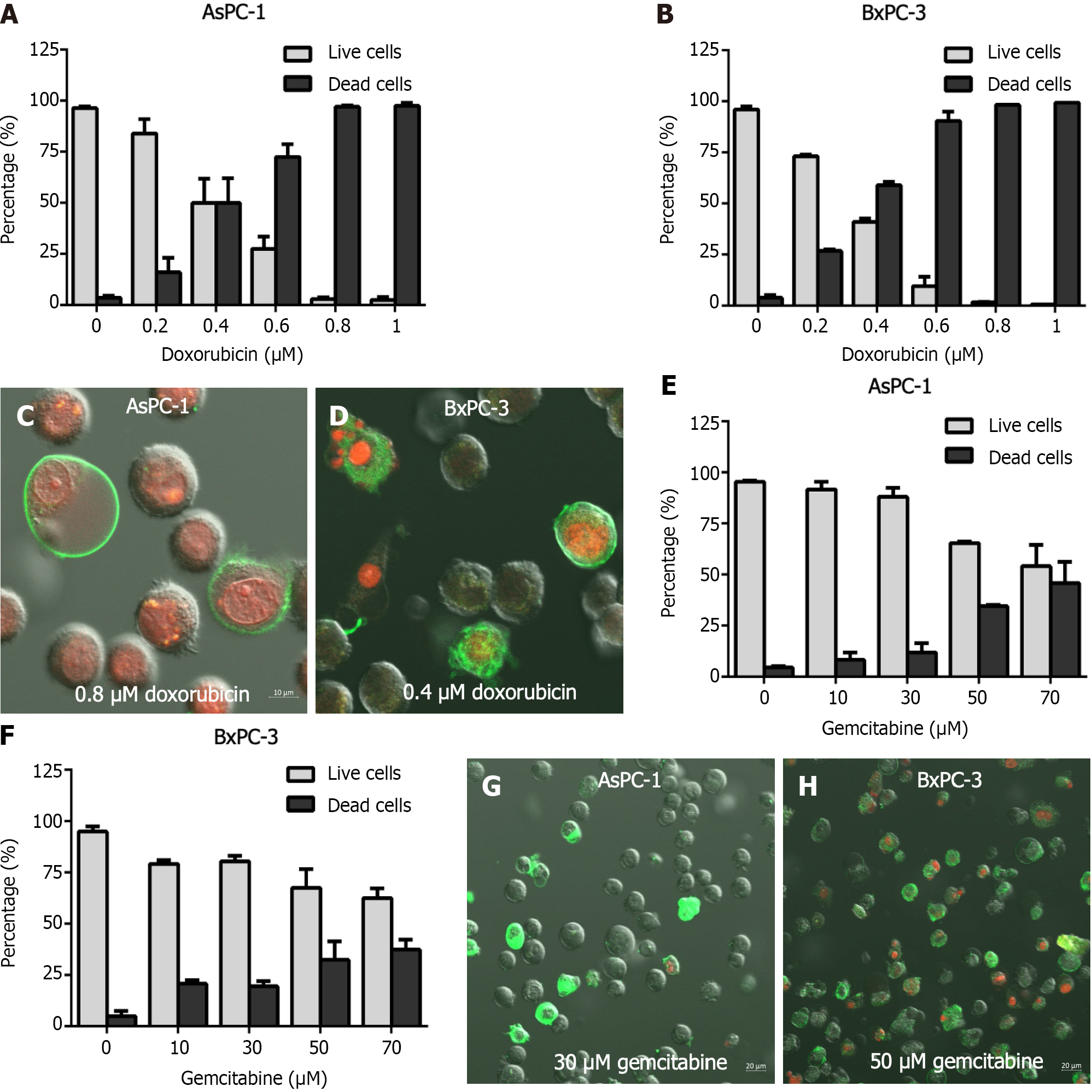
In the AsPC-1 (BxPC-3) cells treated with DOX, at 48 h the survival rates were negatively associated with DOX concentration, decreasing from 50% (95%) at 0.4 μmol/L to less than 1% (both) at 1 μmol/L (Figure 3A). After exposure to DOX at concentrations of 0.4 or 0.8 μmol/L for 48 h, AsPC-1 cells were stained with Annexin V-FITC and PI. Late apoptotic cells showed a green exterior and a red interior; cells that were entirely red were necrotic (Figure 3C and D). At 72 h, AsPC-1 cells subjected to GEM treatments at concentrations of nil, 10, 30, 50, and 70 μmol/L showed mean mortality rates of, respectively, 4.52, 8.24, 11.97, 34.55, and 45.87% (Figure 3E); the corresponding rates in the BxPC-3 cells were 5.05, 20.77, 19.49, 32.45, and 37.45% (Figure 3F).
After exposure to 30 or 50 μmol/L of GEM for 72 h, the PC cells were co-stained with PI and Annexin V-FITC. Most of them had only green peripherals and were early apoptotic cells. A small number of late apoptotic cells appeared red in the interior and green in the periphery. There were, however, a few necrotic cells that were entirely red (Figure 3G and H).
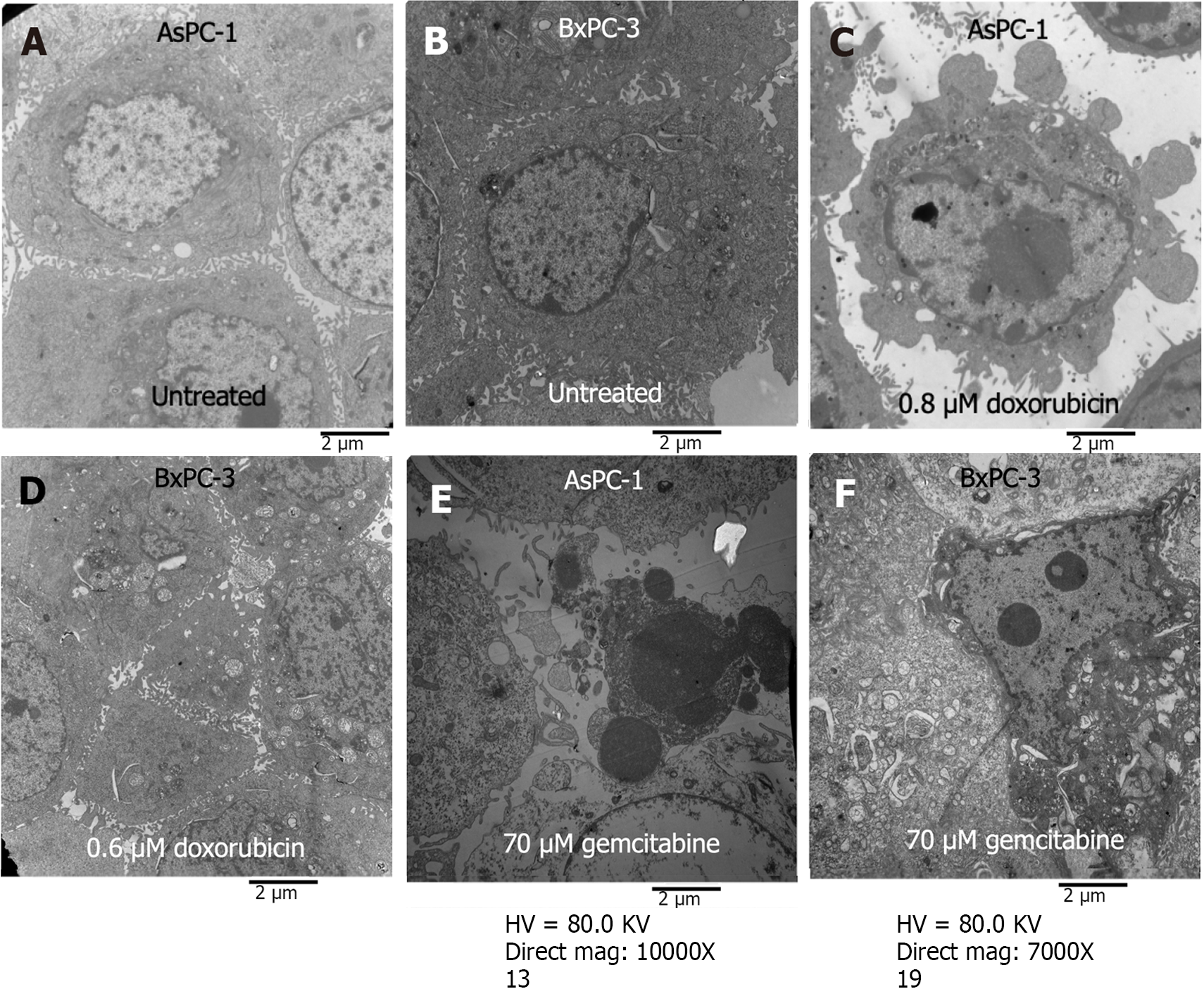
Under the transmission electron microscope, fine microvilli were visible on the surfaces of the untreated AsPC-1 and BxPC-3 cells, and numerous organelles were visible inside the cells (Figure 4A and B). The cells treated with DOX (48 h) or GEM (72 h) appeared apoptotic, cells were enlarged, and the nuclear and plasma membranes were intact (Figure 4C-F). The intercellular contacts and the pericellular microvilli were not apparent. Apoptotic bodies, chromatin aggregation, and cytoplasmic condensation were observed.
At the GEM concentration 30 mol/L, at 72 h, the number of living cells exceeded the number of dead cells. Thus, these parameters were chosen to track how the photosensitizer verteporfin is distributed within the cells. The GEM-treated cells were stained with the verteporfin, DAPI, and MTG. The living and dead cells could be distinguished by staining GEM-treated cells with low concentrations of DAPI. In both cell lines exposed to verteporfin, the photosensitizer was found primarily in the living cells, but comparatively few in the dead cells. Within the cells, verteporfin was observed primarily in the cytoplasm, specifically in the mitochondria, but not in the nucleus (Figure 5).
This study contributes to investigations into the viability of PDT for treating PC, in combination with the traditional anti-tumor drugs GEM and DOX. Because PDT is considered most effective when the photosensitizer is concentrated in the mitochondria, the drugs and parameters that were found most detrimental to PC cell survival in in vitro preliminary experiments were chosen for further imaging studies. It was found that the specific photosensitizer verteporfin, combined with GEM, was present in both living and dead PC cells, and located principally in the mitochondria of the dead cells.
GEM, a nucleoside analogue, is a common antimetabolite antitumor medication. GEM was approved by the United States Federal Drug Administration in 1996 and is offered under the trade name Gemzar[19]. It is still an important drug in the treatment of PC[20]. DOX, a powerful chemotherapeutic agent that is a member of the anthracycline class, is frequently prescribed alongside other medications to treat different types of cancer[21], including hematologic, gastric, pancreatic, ovarian, and breast cancers[22]. In the present study, human PC cells were exposed to GEM and DOX in varying concentrations, and the cell survival rate significantly dropped under either treatment. The two PC cell lines (AsPC-1, BxPC-3) responded differently to DOX, but were similar in declining proliferation under GEM, and the effect of GEM was more enduring compared with DOX. The AsPC-1 and BxPC-3 cells were also exposed to varying concentrations of non-chemotherapeutic drugs (SRL and CTX), with no appreciable changes in their proliferation. Thus, after establishing a workable concentration and incubation time, GEM was used for the imaging studies of verteporfin.
Forster et al[23] conducted a meta-analysis to assess the potential advantages of adding CTX to neoadjuvant therapy, adjuvant therapy, or palliative therapy for PC. They concluded that CTX was not clinically beneficial, but did increase associated toxic side effects and treatment costs. Yet, Fiore et al[24] tested the feasibility and tolerability of combined CTX and GEM radiotherapy for locally advanced PC, and the results were encouraging. Due to resistance to GEM, its clinical effectiveness in the treatment of PC is insufficient. CTX has poor sensitivity in the treatment of PC, but did have some effects when combined with GEM and radiotherapy. Therefore, comprehensive treatment such as GEM combined with other agents can be beneficial in PC. PC that is resistant to GEM may respond to PDT. Celli et al[25] discovered that the photosensitizer verteporfin was highly toxic to PC cells that were otherwise resistant to GEM.
In recent years, PDT in the field of tumor therapy has evolved, and the potential of photosensitizers is noteworthy. PDT is non-invasive and causes less damage than surgery, and is safer. If the effectiveness of PDT can be improved by more targeted photosensitizers, when combined with neoadjuvant therapy this technology has bright prospects for treating resectable PC. In earlier investigations into the distribution of photosensitizers in cells, Zhao et al[26] employed the dihydroporphyrin photosensitizer KAE in HeLa cells, and found that most of the photosensitizer was distributed in the mitochondria and there was strong anti-tumor activity. In addition, Saczko et al[27] observed verteporfin in both normal and malignant endothelial cells, (mainly in the latter) and preferentially accumulated in the mitochondria. The results of the present study are largely consistent with these studies of other cell lines. Thus, the development of photosensitizers that locate to the mitochondria can improve the efficiency of PDT against malignant tumors.
In the present study, we investigated the distribution of the photosensitizer verteporfin in human PC cells treated with GEM. Verteporfin was found primarily in the cytoplasm of GEM-treated human PC cells, but particularly in the mitochondria. What is more, verteporfin was more prevalent in living cells than in dead cells, and thus more able to maximize the photodynamic effect of PDT on tumor cells. Because PC is a difficult disease to treat and drug resistance is common, verteporfin-mediated PDT after neoadjuvant therapy for PC may be an effective and safe treatment. PDT can also be used in the treatment of esophageal, bladder, and gastric cancers. It is possible to apply neoadjuvant therapy combined with PDT and antitumor drugs and photosensitizers for different cancers. Our study provides a theoretical basis for conducting PDT after neoadjuvant chemotherapy in the treatment of PC.
The proliferation of human PC cells was significantly reduced by treatment with GEM or DOX, but unaffected by the non-chemotherapy drugs SRL or CTX. Verteporfin was found preferentially in the mitochondria of the human PC cells and the concentration of verteporfin was higher in living cells than in dead cells. This research establishes a theoretical foundation for administering PDT after neoadjuvant chemotherapy as comprehensive treatment for PC.
Traditional treatments for pancreatic cancer (PC) are inadequate. Photodynamic therapy (PDT) is a non-invasive technology proven safe to kill cancer cells, including PC, but subcellular concentration of the photosensitizer to the mitochondria is key.
This study investigated the distribution of fluorescence of verteporfin in PC cells treated with antitumor drugs.
This preliminary in vitro study was to investigate the effectiveness of the photosensitizer verteporfin in human PC cells, and tested whether its use in combination with other common treatments is viable.
Workable survival rates of PC cells were determined with chemotherapy and non-chemotherapy drugs in vitro, with or without verteporfin, as measured via MTT, flow cytometry, and laser confocal microscopy. Confocal laser microscopy allowed observation of gemcitabine (GEM)- and verteporfin-treated PC cells co-stained with 4’,6-diamidino-2-phenylindole and MitoTracker Green to differentiate living and dead cells and subcellular localization of verteporfin, respectively.
Cell survival significantly dropped upon exposure to either chemotherapy drug, but not to sirolimus or cetuximab. Both cell lines responded similarly to GEM. Additional experiments using GEM showed that survival rates of the PC cells treated with 10 μmol/L verteporfin (but not lesser concentrations) were significantly lower relative to nil verteporfin. After GEM treatment, verteporfin was observed primarily in the mitochondria.
Verteporfin was observed in living cells. In GEM-treated human PC cells, verteporfin was particularly prevalent in the mitochondria. This study supports further study of PDT for the treatment of PC after neoadjuvant chemotherapy.
In the future, more study can be investigated in using PDT in the comprehensive treatment of PC after neoadjuvant chemotherapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Nakano H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326:851-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 589] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 2. | Silvestris N, Brunetti O, Vasile E, Cellini F, Cataldo I, Pusceddu V, Cattaneo M, Partelli S, Scartozzi M, Aprile G, Casadei Gardini A, Morganti AG, Valentini V, Scarpa A, Falconi M, Calabrese A, Lorusso V, Reni M, Cascinu S. Multimodal treatment of resectable pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol. 2017;111:152-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Sobhani N, Samadani AA. Implications of photodynamic cancer therapy: an overview of PDT mechanisms basically and practically. J Egypt Natl Canc Inst. 2021;33:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Gomez S, Tsung A, Hu Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules. 2020;25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Ikeda N, Usuda J, Maehara S. Photodynamic therapy for central-type early-stage lung cancer. Gen Thorac Cardiovasc Surg. 2020;68:679-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Fargnoli MC, Peris K. Photodynamic therapy for basal cell carcinoma. Future Oncol. 2015;11:2991-2996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Yano T, Wang KK. Photodynamic Therapy for Gastrointestinal Cancer. Photochem Photobiol. 2020;96:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Civantos FJ, Karakullukcu B, Biel M, Silver CE, Rinaldo A, Saba NF, Takes RP, Vander Poorten V, Ferlito A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv Ther. 2018;35:324-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Kubrak T, Karakuła M, Czop M, Kawczyk-Krupka A, Aebisher D. Advances in Management of Bladder Cancer-The Role of Photodynamic Therapy. Molecules. 2022;27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Karimnia V, Slack FJ, Celli JP. Photodynamic Therapy for Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Wang H, Zhou L, Lu J, Jiang B, Liu C, Guo J. Photodynamic therapy of pancreatic cancer: Where have we come from and where are we going? Photodiagnosis Photodyn Ther. 2020;31:101876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Lv W, Zhang Z, Zhang KY, Yang H, Liu S, Xu A, Guo S, Zhao Q, Huang W. A Mitochondria-Targeted Photosensitizer Showing Improved Photodynamic Therapy Effects Under Hypoxia. Angew Chem Int Ed Engl. 2016;55:9947-9951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 13. | Taba F, Onoda A, Hasegawa U, Enoki T, Ooyama Y, Ohshita J, Hayashi T. Mitochondria-Targeting Polyamine-Protoporphyrin Conjugates for Photodynamic Therapy. ChemMedChem. 2018;13:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Tan YQ, Zhang X, Zhang S, Zhu T, Garg M, Lobie PE, Pandey V. Mitochondria: The metabolic switch of cellular oncogenic transformation. Biochim Biophys Acta Rev Cancer. 2021;1876:188534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Mani S, Swargiary G, Singh KK. Natural Agents Targeting Mitochondria in Cancer. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45:6597-6626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1359] [Cited by in F6Publishing: 1160] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 17. | Battaglia Parodi M, La Spina C, Berchicci L, Petruzzi G, Bandello F. Photosensitizers and Photodynamic Therapy: Verteporfin. Dev Ophthalmol. 2016;55:330-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW, Pereira SP. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Pandit B, Royzen M. Recent Development of Prodrugs of Gemcitabine. Genes (Basel). 2022;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Di Costanzo F, Di Costanzo F, Antonuzzo L, Mazza E, Giommoni E. Optimizing First-Line Chemotherapy in Metastatic Pancreatic Cancer: Efficacy of FOLFIRINOX versus Nab-Paclitaxel Plus Gemcitabine. Cancers (Basel). 2023;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 21. | Benjanuwattra J, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Doxorubicin and its proarrhythmic effects: A comprehensive review of the evidence from experimental and clinical studies. Pharmacol Res. 2020;151:104542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1458] [Cited by in F6Publishing: 1531] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 23. | Forster T, Huettner FJ, Springfeld C, Loehr M, Kalkum E, Hackbusch M, Hackert T, Diener MK, Probst P. Cetuximab in Pancreatic Cancer Therapy: A Systematic Review and Meta-Analysis. Oncology. 2020;98:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Fiore M, Trodella L, Valeri S, Borzomati D, Floreno B, Ippolito E, Trecca P, Trodella LE, D'Angelillo RM, Ramella S, Coppola R. Prospective study of cetuximab and gemcitabine in combination with radiation therapy: feasibility and efficacy in locally advanced pancreatic head cancer. Radiat Oncol. 2015;10:255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg Med. 2011;43:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Zhao H, Liu H, Kang L, Sun T, Liu Y, Chen D, Li K, Qiu H, Wang Y, Tan Y, Zeng J, Gu Y. In vitro and in vivo evaluation of a chlorin-based photosensitizer KAE® for cancer treatment. Photodiagnosis Photodyn Ther. 2022;38:102759. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 27. | Saczko J, Mazurkiewicz M, Chwiłkowska A, Kulbacka J, Kramer G, Ługowski M, Snietura M, Banaś T. Intracellular distribution of Photofrin in malignant and normal endothelial cell lines. Folia Biol (Praha). 2007;53:7-12. [PubMed] [Cited in This Article: ] |