Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.894
Peer-review started: September 22, 2023
First decision: December 4, 2023
Revised: December 19, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: March 15, 2024
Volatile organic compounds (VOCs) are a promising potential biomarker that may be able to identify the presence of cancers.
To identify exhaled breath VOCs that distinguish pancreatic ductal adenocarcinoma (PDAC) from intraductal papillary mucinous neoplasm (IPMN) and healthy volunteers.
We collected exhaled breath from histologically proven PDAC patients, radiological diagnosis IPMN, and healthy volunteers using the ReCIVA® device between 10/2021-11/2022. VOCs were identified by thermal desorption-gas chromatography/field-asymmetric ion mobility spectrometry and compared between groups.
A total of 156 participants (44% male, mean age 62.6 ± 10.6) were enrolled (54 PDAC, 42 IPMN, and 60 controls). Among the nine VOCs identified, two VOCs that showed differences between groups were dimethyl sulfide [0.73 vs 0.74 vs 0.94 arbitrary units (AU), respectively; P = 0.008] and acetone dimers (3.95 vs 4.49 vs 5.19 AU, respectively; P < 0.001). After adjusting for the imbalance parameters, PDAC showed higher dimethyl sulfide levels than the control and IPMN groups, with adjusted odds ratio (aOR) of 6.98 (95%CI: 1.15-42.17) and 4.56 (1.03-20.20), respectively (P < 0.05 both). Acetone dimer levels were also higher in PDAC compared to controls and IPMN (aOR: 5.12 (1.80-14.57) and aOR: 3.35 (1.47-7.63), respectively (P < 0.05 both). Acetone dimer, but not dimethyl sulfide, performed better than CA19-9 in PDAC diagnosis (AUROC 0.910 vs 0.796). The AUROC of acetone dimer increased to 0.936 when combined with CA19-9, which was better than CA19-9 alone (P < 0.05).
Dimethyl sulfide and acetone dimer are VOCs that potentially distinguish PDAC from IPMN and healthy participants. Additional prospective studies are required to validate these findings.
Core Tip: Participants with pancreatic ductal adenocarcinoma (PDAC) exhibit distinct exhaled breath volatile organic compounds (VOC) from those with intraductal papillary mucinous neoplasm (IPMN) or the general population. Dimethyl sulfide and acetone dimer are VOCs that could potentially identify PDAC from IPMN and healthy subjects.
- Citation: Tiankanon K, Pungpipattrakul N, Sukaram T, Chaiteerakij R, Rerknimitr R. Identification of breath volatile organic compounds to distinguish pancreatic adenocarcinoma, pancreatic cystic neoplasm, and patients without pancreatic lesions. World J Gastrointest Oncol 2024; 16(3): 894-906
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/894.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.894
Pancreatic ductal adenocarcinoma (PDAC), or pancreatic cancer, is the most common pancreatic malignant lesion, accounting for the seventh highest total of cancer-related deaths worldwide[1]. The incidence of PDAC is on the rise and is considered one of the most deadly cancers[1]. Due to the non-specific symptoms of PDAC, most patients are diagnosed at an advanced stage with a 5-year survival rate of less than 8%[1-4]. Current diagnostic techniques rely on imaging, endoscopic ultrasonography, and biopsy to determine tumor histology. Unfortunately, the performance of these techniques is suboptimal for detecting tumors smaller than 2 cm in diameter. Additionally, biomarkers commonly used, particularly CA19-9, have shown poor performance[5,6].
In addition to pancreatic cancer, another pancreatic lesion that is being detected more frequently due to advances in diagnostic imaging technology is intraductal papillary mucinous neoplasm (IPMN)[7]. The reported prevalence of incidental pancreatic cysts, including IPMN, identified on multi-detector computed tomography (CT) and magnetic resonance imaging (MRI), is as high as 2.6% and 13.5%, respectively[8,9]. These lesions carry the risk of malignant transformation to pancreatic cancer, which occurs 76% of the time in main duct type IPMN (MD-IPMN), 16% in branch duct type IPMN (BD-IPMN), and 46% in mixed type IPMN[10]. Similar to PDAC, there are no biomarkers currently available to predict the malignant transformation of IPMN.
Volatile organic compounds (VOC) are organic compounds generated by the metabolism of cells and released into the blood and other body fluids, with high vapor pressure under room temperature conditions[11]. VOCs can be found in exhaled breath, urine, bile, feces, or saliva, and represent biochemical reactions caused by biological activities, such as apoptosis, oxidative stress, or inflammation[11]. According to accumulating evidence, the analysis of VOC profiles is a promising novel cancer biomarker to diagnose many types of cancer, such as lung, breast, gastrointestinal, and urological cancers[12]. One of the simplest biological specimens to access is breath samples. Samples can be collected in large volumes completely noninvasively and with high patient acceptability. Previous studies have shown satisfactory results in the ability of exhaled breath to distinguish VOC profiles between cancer and noncancer participants[13-15]. We hypothesized that biomarkers for PDAC exist in exhaled breath and that these VOC profiles in PDAC patients differ from those with IPMN and those without pancreatic lesions. We also believe that VOC profiles may have the potential as an early detection biomarker and screening tool for patients at risk of this malignancy.
Although a few studies have suggested that VOCs could be employed as new biomarkers, the VOCs reported in each investigation varied. This was owing to the fact that the type and quantity of VOCs discovered depended on the methods employed[16]. Because of their high resolution and sensitivity, gas chromatography-mass spectrometry (GC-MS) and selective ion flow tube mass spectrometry (SIFT-MS) were the most often used techniques[12]. However, these techniques are expensive, time-consuming, and require expertise to operate, making them unsuitable for point-of-care screening programs[17,18]. Field asymmetric ion mobility spectrometry (FAIMS), which has an equivalent sensitivity to GC-MS and SIFT-MS but is less expensive and less technical, has recently been recommended as being more suitable for clinical study[18,19]. To date, no study has been conducted to differentiate VOCs for PDAC using FAIMS. Our objective in this study was to identify VOCs of exhaled breath that could be used to distinguish PDAC from IPMN and controls without pancreatic lesions using the FAIMS technique and to investigate the diagnostic performance of VOCs in comparison to CA19-9.
We conducted a single-center, cross-sectional study between October 2021 and January 2023 at the Center of Excellence for Innovation and Endoscopy in Gastrointestinal Oncology, Division of Gastroenterology, Department of Medicine, Chulalongkorn University. The study protocol was reviewed and approved by the Institutional Research Committee, Faculty of Medicine, Chulalongkorn University (IRB No.0482/65) and registered in the Thai Clinical Trials Registry (TCTR20211109002).
Participants with cytological or histological confirmation of PDAC and radiological confirmation of IPMN were enrolled. The stages of PDAC were classified as early, locally advanced, and advanced stages according to the American Joint Committee on Cancer/Union for International Cancer Control TNM classification of malignant tumors[20]. IPMN patients were classified as MD-IPMN, BD-IPMN, and mixed-type IPMN according to the Sendai and Fukuoka consensus guidelines[7]. Any PDAC or IPMN participant who had undergone previous treatment, including surgical intervention, pancreatic radiation, systemic chemotherapy, and endoscopic local ablations, were excluded from the study to avoid possible effects of the treatment. Healthy volunteers without pancreatic lesions confirmed by abdominal imaging, including transabdominal ultrasound, endoscopic ultrasonography, abdominal CT, or MRI, with a minimum age of 18 years were also recruited as the control group. Participants with a history of other malignancies, breathing through tracheostomy tubes, pregnant at the time of recruitment, concurrent active infection, history of infection within 3 wk prior to breath sample collection, diagnosed with cirrhosis, end-stage renal disease, or participants with poorly controlled diabetes, defined as HbA1C of ≥ 8%, were also excluded from the study.
All eligible participants were provided with details of the study. Verbal and written informed consent were obtained from every participant before enrollment. Baseline characteristics including age, sex, smoking status, alcohol consumption, co-morbidities, such as chronic viral hepatitis B and C, nonalcoholic fatty liver disease, ischemic heart disease, diabetes, current medication, and previous history of endoscopic treatment were obtained from participants and electronic medical records. Laboratory data associated with pancreatic diseases including liver function test, amylase, lipase, and the tumor biomarker CA19-9 were also obtained prior to breath sampling. Information on tumor location, size, staging, and metastasis sites was gathered from electronic medical records.
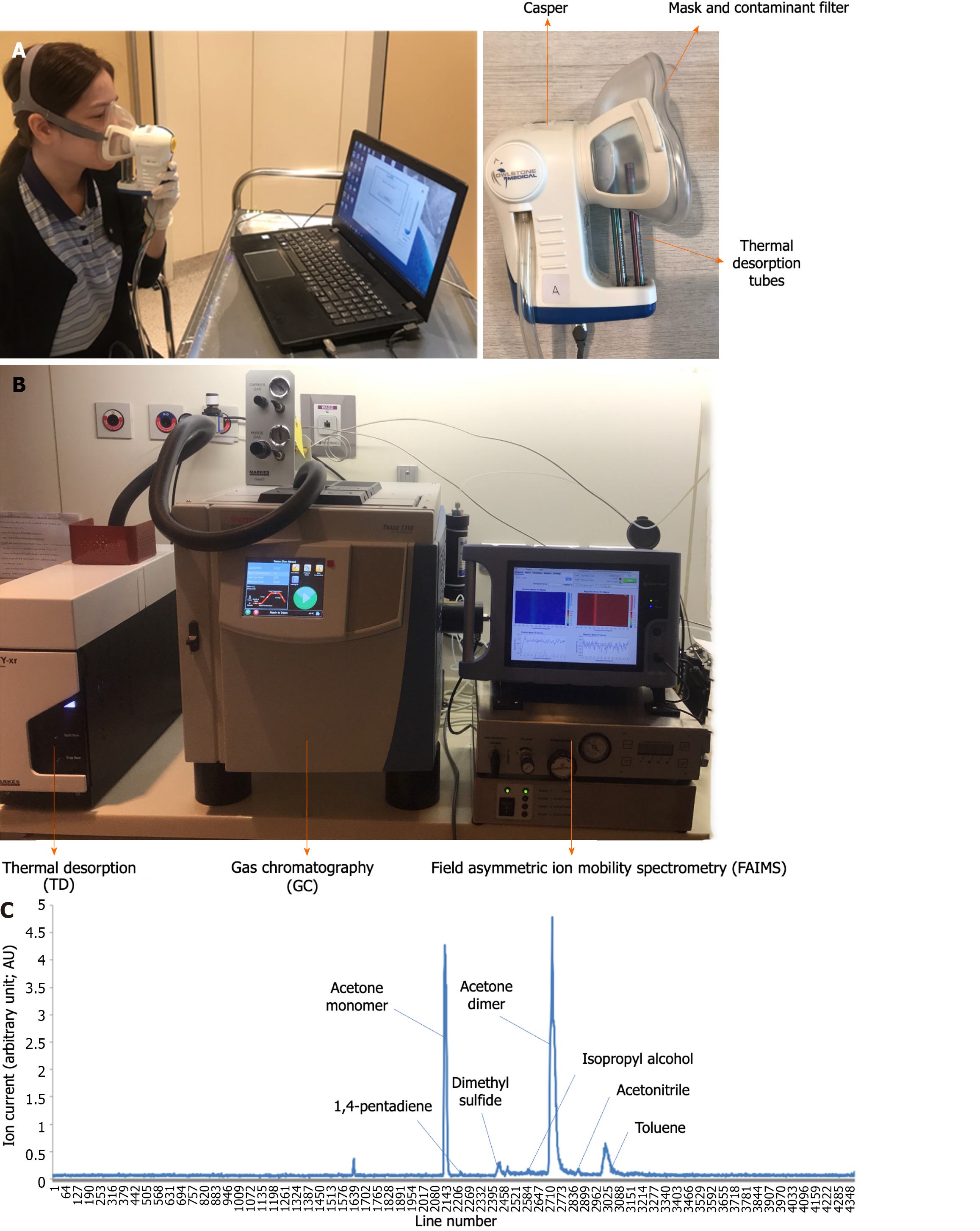
All participants had been advised to fast, stop smoking, exercise, and withhold current medication for 6 hours prior to breath sample collection to minimize confounders in exhaled breaths. Participants were instructed to breathe normally through a disposable face mask that covered both the nose and mouth for 2-3 min. The face mask was connected to the ReCIVA™ breath sample system (Owlstone Medical, Cambridge, United Kingdom), which was coupled with software to monitor real-time progression of the breath sample collection (Figure 1A). During this process, a pure oxygen gas supply was provided through the face masks to avoid contamination of confounding surrounding gas into the system. Approximately 100 mL of exhaled air was collected and distributed equally into 4 thermal desorption (TD) tube (UnityTM-XR; Markes International Ltd, Llantrisant, United Kingdom) prior to the VOC extraction process.
The VOC extraction protocol was carried out using a previously validated method[21]. Extraction of VOCs from TD tubes was performed using a thermal desorption unit (Unity™-XR, Markes International Ltd, Llantrisant, United Kingdom). The collected TD tubes were heated from 10°C to 250°C in split flow mode to release the VOCs collected inside. The VOC profiling was then analyzed by gas chromatography (GC) using the Thermo Scientific TRACE1310 GC system (Waltham, MA, United States), which is equipped with an HP-PLOT U gas chromatography column of 30 m length × 0.32 mm of inner diameter × 10 µm of film thickness (Agilent Technologies, Santa Clara, CA, United States). The GC column was initially programmed to heat at 40°C for 2 min and ramp to 130°C at the rate of 10°C/min for 10 min. Helium was used as the carrier gas at a flow rate of 1 mL/min during the VOC separation. The extracted VOCs then flowed through a transfer line at 130°C to final extraction using a FAIMS system (Owlstone Medical Lonestar VOC Analyzer FAIMS system, Cambridge, United Kingdom) (Figure 1B). After final extraction, VOCs extracted from each participant’s group were shown in the form of chromatograms (Figure 1C).
VOC identification was achieved by calibrating the retention time of standard solutions with the chromatogram and comparing them with the in-house VOC data library[21]. The in-house VOC data library was created with XGBoost to identify important features of VOC profiles, and the algorithm chose the optimal combination of VOCs to yield optimal statistical parameters. The XGBoost algorithm is an AI algorithm established in the previous VOC study[21]. XGBoost enabled the minimization of false predictions by continuously learning from the model that was previously constructed as a series and thus maximizing the prediction model's efficiency. System calibration was performed daily to ensure the accuracy of all instruments. The difference in types of VOCs extracted and relative amounts of the VOCs, expressed as arbitrary unit (AU), were analyzed and compared between groups.
The sample size was calculated to achieve 80% power with a two-sided type I error of 0.05 based on data from the previous VOC study to distinguish between PDAC and the control population[15]. With a 1:1:1 ratio among PDAC, IPMN, and control groups, a minimum sample size of 41 in each group was estimated.
The descriptive statistics of the categorical variables were expressed as frequency (percentage) and the descriptive statistics of the continuous variables were expressed as mean ± standard deviation or median and range, as appropriate. Pearson’s Chi-square test was used to compare categorical variables. The Mann-Whitney U test or ANOVA was used to compare differences between continuous variables. Multivariate logistic regression analysis was performed to assess the association between VOC levels and the participant’s diagnosis. A receiver-operating characteristic (ROC) curve was constructed to identify the optimal cutoff point for VOCs to distinguish PDAC from other diagnoses. Statistical significance was established at P < 0.05. All statistical tests were performed with SPSS version 22.0.0 (IBM Corp., Armonk, NY, United States).
A total of 156 participants (44% male) with a mean age of 62.6 ± 10.6 years were enrolled. Among these, 54 participants were PDAC patients, 42 were IPMN patients, and 60 were selected for the control group. PDAC patients consisted of 3 (5%) early stage, 27 (50%) locally advanced stage, and 24 (44%) advanced stage. The advanced-stage PDAC metastatic sites included 17 (32%) liver, 12 (22.6%) lymph nodes, 8 (15%) peritoneum, and 4 (7%) lungs. In the IPMN group, 5 (11.9%) participants were classified as MD-IPMN, 35 (83.3%) as BD-IPMN, and 6 (14.2%) were malignant IPMN.
Baseline characteristics in terms of age, sex, smoking and alcohol consumption, and participant co-morbidities were not different among the three groups. PDAC patients, however, had statistically higher levels of bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), and international normalized ratio (INR). A lower level of albumin compared to IPMN and control groups was also reported (Table 1).
| Variables | PDAC, n = 54 | IPMN, n = 42 | Normal pancreas, n = 60 | P value |
| Age, mean ± SD | 64.8 ± 10.4 | 63.2 ± 10.3 | 60.0 ± 10.6 | 0.057 |
| Male, n (%) | 25 (46.3) | 20 (47.6) | 24 (40.0) | 0.695 |
| Smoking, n (%) | 7 (13.0) | 5 (11.9) | 8 (13.6) | 0.970 |
| Alcohol consumption, n (%) | 16 (29.6) | 14 (33.3) | 16 (26.7) | 0.768 |
| Participants’ comorbidity, n (%) | ||||
| HBV | 1 (1.9) | 4 (9.5) | 4 (6.8) | 0.258 |
| HCV | 0 (0.0) | 0 (0.0) | 2 (3.3) | 0.198 |
| NAFLD | 1 (1.9) | 3 (7.1) | 8 (13.3) | 0.071 |
| CAD | 2 (3.7) | 2 (4.8) | 5 (9.3) | 0.441 |
| ESRD | 0 (0.0) | 1 (2.4) | 0 (0.0) | 0.255 |
| DM | 3 (5.6) | 4 (9.5) | 0 (0.0) | 0.066 |
| HT | 2 (3.7) | 3 (7.1) | 0 (0.0) | 0.127 |
| Old CVA | 2 (3.8) | 3 (7.1) | 0 (0.0) | 0.128 |
| Laboratory results, mean ± SD | ||||
| Albumin in g/dL | 3.7 ± 0.7 | 3.8 ± 1.1 | 4.2 ± 0.3 | 0.002 |
| Total bilirubin in mg/dL | 5.7 ± 9.7 | 1.8 ± 4.6 | 0.7 ± 0.3 | 0.009 |
| Aspartate aminotransferase in U/L | 73.4 ± 115.1 | 26.0 ± 18.0 | 23.0 ± 7.4 | 0.001 |
| Alanine aminotransferase in U/L | 86.9 ± 156.1 | 23.4 ± 11.9 | 25.9 ± 14.8 | < 0.001 |
| Alkaline phosphatase in U/L | 279.8± 412.6 | 95.2 ± 91.3 | 73.5 ± 22.5 | < 0.001 |
| INR | 1.2 ± 0.2 | 1.2 ± 0.6 | 1.0 ± 0.1 | 0.009 |
| Amylase | 120.8 ± 160.0 | 82.0 ± 52.2 | 74.1 ± 16.3 | 0.879 |
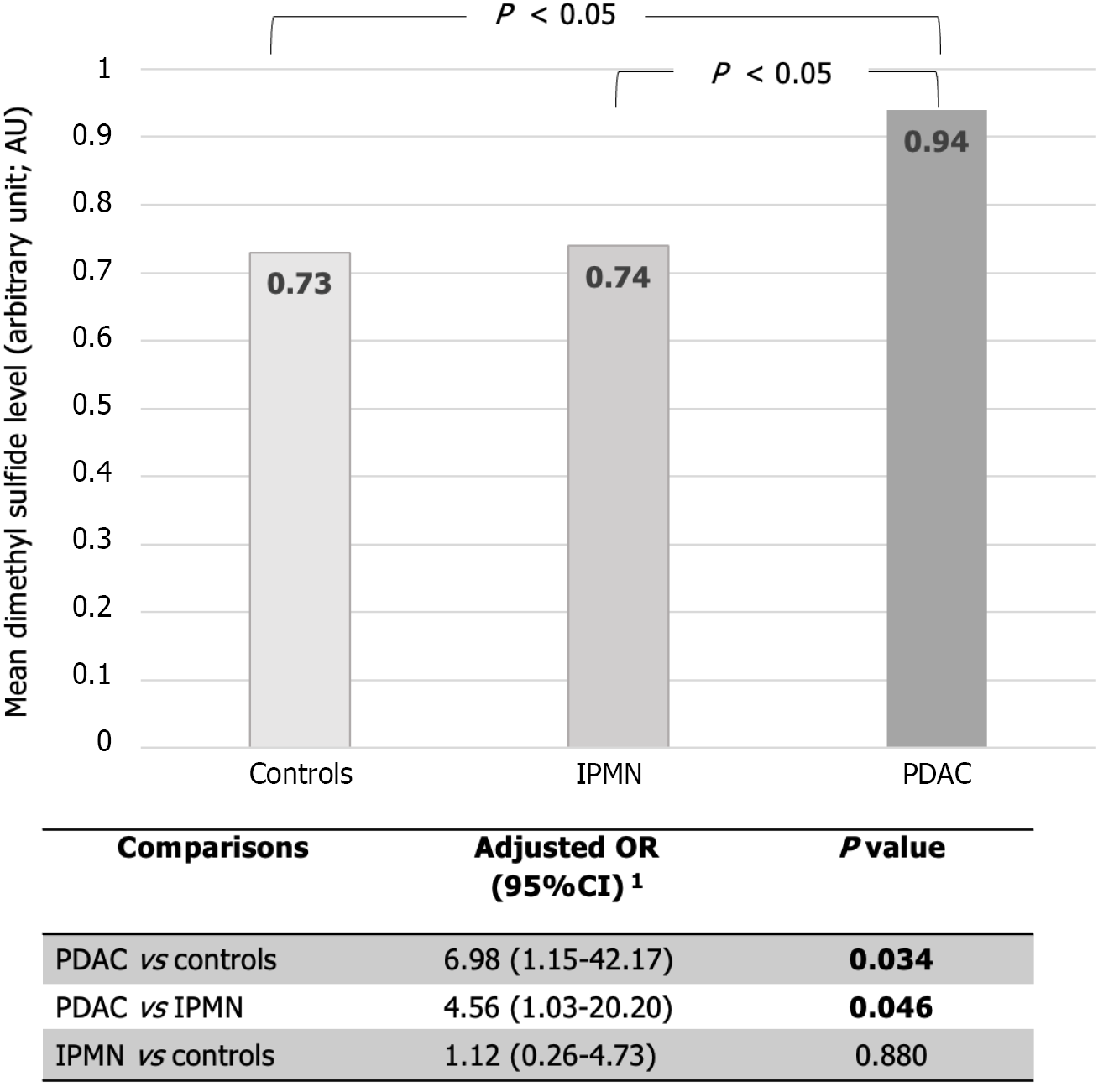
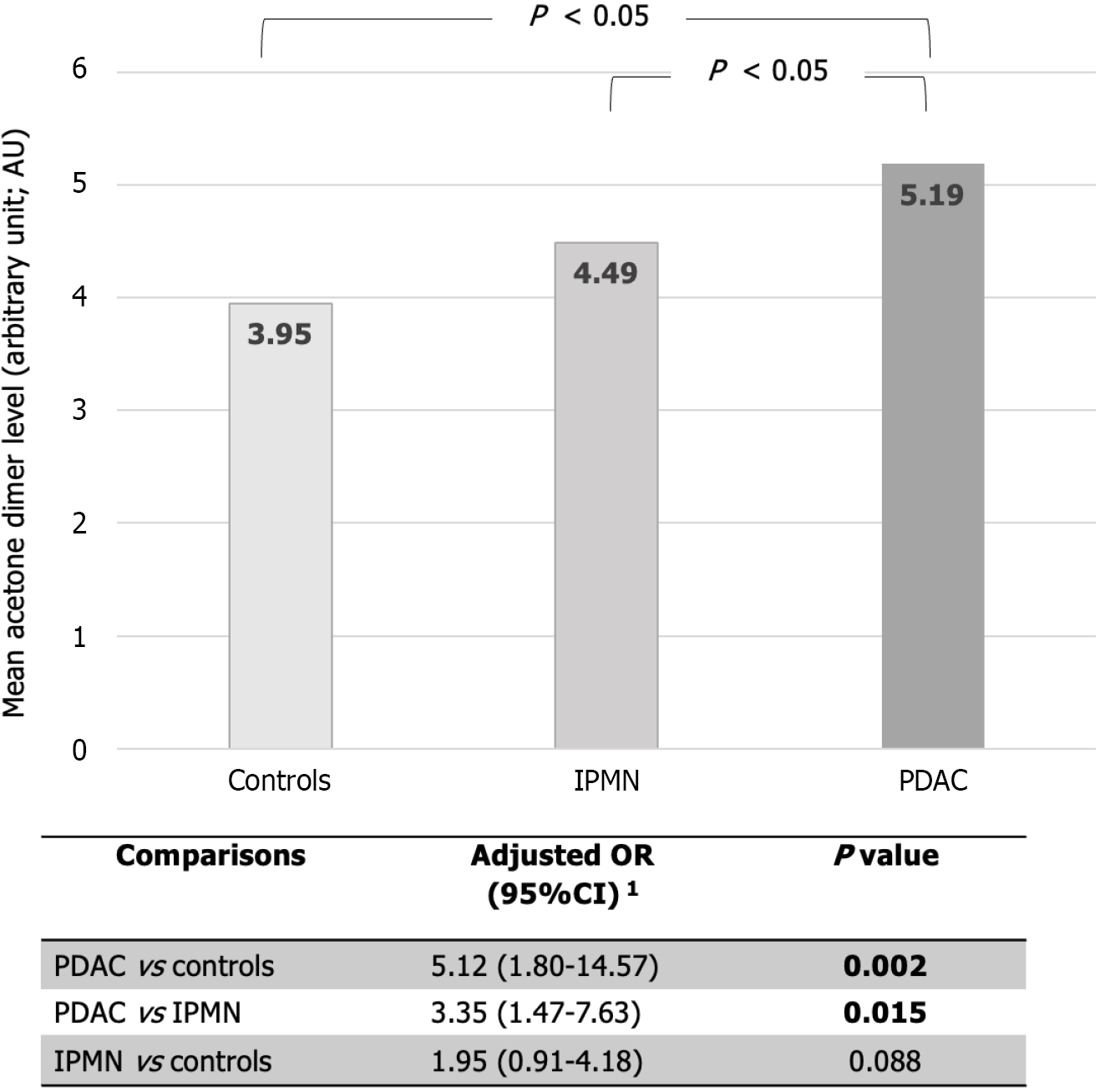
A total of 9 VOCs from exhaled breath were identified (Table 2). Two VOCs, dimethyl sulfide and acetone dimer, demonstrated a statistically significant difference between the PDAC, IPMN, and control groups (0.94 vs 0.74 vs 0.73 AU; P = 0.008 for dimethyl sulfide; 5.19 vs 4.49 vs 3.97 AU; P < 0.001 for acetone dimer, respectively).
| Variable | PDAC, n = 54 | IPMN, n = 42 | Normal pancreas, n = 60 | P value2 |
| 1,4-pentadiene | 0.34 ± 0.34 | 0.32 ± 0.28 | 0.32 ± 0.21 | 0.626 |
| Acetone dimer | 5.19 ± 0.74 | 4.49 ± 0.81 | 3.95 ± 0.86 | < 0.001 |
| Acetone monomer | 3.99 ± 0.79 | 4.21 ± 0.59 | 4.07 ± 0.69 | 0.411 |
| Acetonitrile | 0.13 ± 0.05 | 0.14 ± 0.10 | 0.13 ± 0.06 | 0.544 |
| Benzene | 0.20 ± 0.15 | 0.19 ± 0.11 | 0.25 ± 0.19 | 0.336 |
| Dimethyl sulfide | 0.94 ± 0.40 | 0.74 ± 0.38 | 0.73 ± 0.40 | 0.008 |
| Ethanol | 0.25 ± 0.06 | 0.26 ± 0.05 | 0.26 ± 0.07 | 0.726 |
| Isopropyl alcohol | 0.31 ± 0.45 | 0.19 ± 0.23 | 0.20 ± 0.27 | 0.731 |
| Toluene | 0.56 ± 0.42 | 0.57 ± 0.52 | 0.50 ± 0.37 | 0.655 |
After adjusting for imbalanced factors between groups, including levels of bilirubin, AST, ALT, ALP, INR, and albumin levels using multivariate logistic regression, the PDAC group showed a significantly higher level of dimethyl sulfide compared to IPMN with an adjusted odd ratio (aOR) of 4.56 (95%CI: 1.03-20.20, P = 0.046), and the control group with aOR of 6.98 (95%CI: 1.15-42.17, P = 0.034), respectively (Figure 2).
The acetone dimer also showed a significantly higher level in the PDAC group compared to the IPMN group (aOR 3.35; 95%CI 1.47-7.63, P = 0.015), and the control group (aOR 5.12; 95%CI 1.80-14.57, P = 0.002) (Figure 3). However, when comparing the levels of dimethyl sulfide and acetone dimer of the IPMN group with those of controls, no statistical difference in VOC levels was identified.
In subgroup analyses, dimethyl sulfide levels were higher in the metastatic PDAC than in the localized PDAC group (0.96 vs 0.92 AU; P = 0.016) as shown in Table 3. However, the acetone dimer level did not differ between PDAC stages.
| Variable | Metastasis, n = 30 | Non-metastasis, n = 24 | P value2 |
| 1,4-pentadiene | 0.36 ± 0.24 | 0.31 ± 0.47 | 0.741 |
| Acetone dimer | 5.10 ± 0.92 | 5.26 ± 0.57 | 0.403 |
| Acetone monomer | 3.90 ± 0.82 | 4.08 ± 0.75 | 0.497 |
| Acetonitrile | 0.14 ± 0.05 | 0.12 ± 0.05 | 0.315 |
| Benzene | 0.19 ± 0.09 | 0.22 ± 0.20 | 0.932 |
| Dimethyl sulfide | 0.96 ± 0.41 | 0.92 ± 0.38 | 0.016 |
| Ethanol | 0.24 ± 0.05 | 0.26 ± 0.08 | 0.321 |
| Isopropyl alcohol | 0.39 ± 0.56 | 0.19 ± 0.18 | 0.308 |
| Toluene | 0.56 ± 0.45 | 0.57 ± 0.39 | 0.676 |
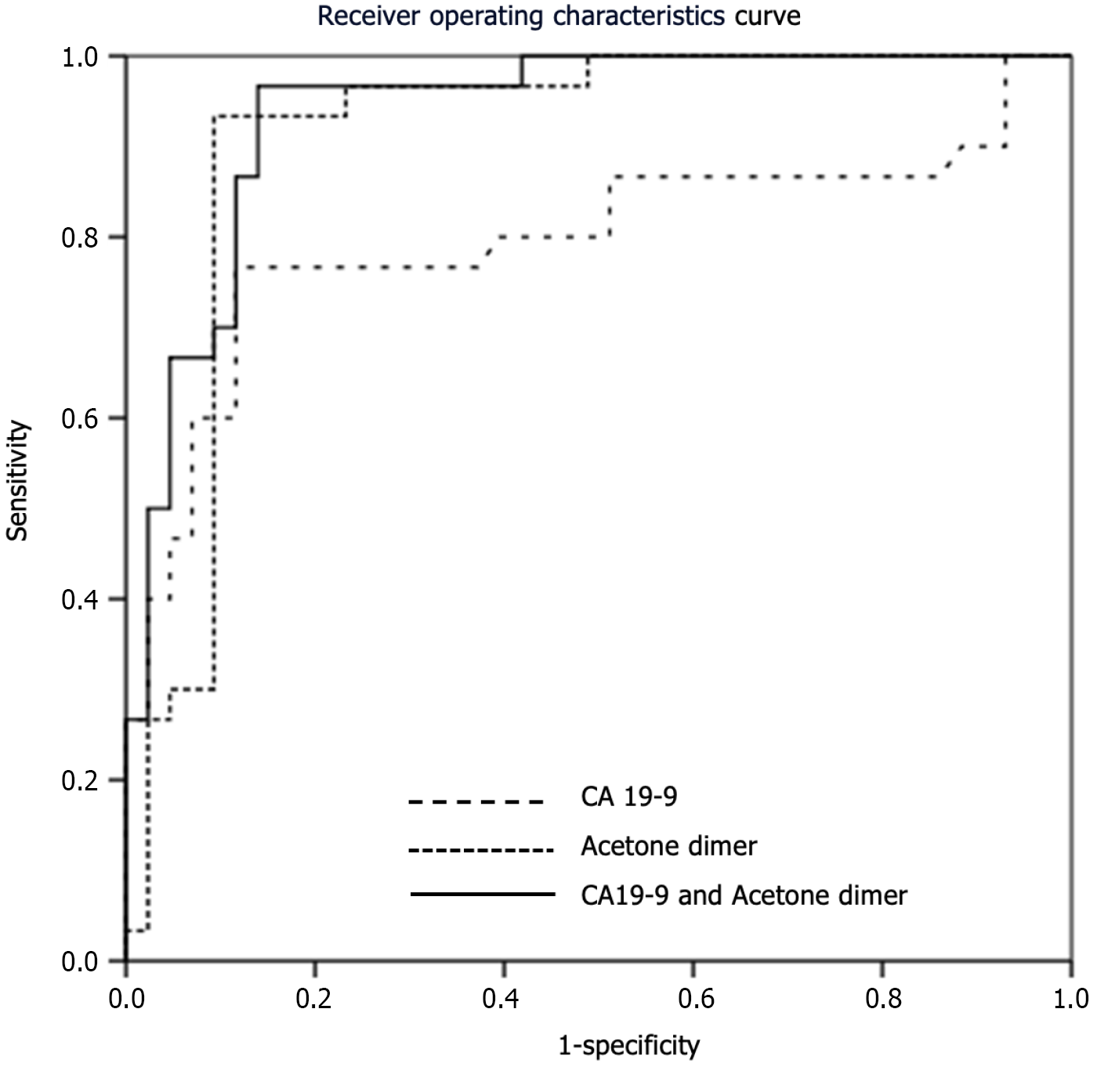
The mean value of the PDAC biomarker CA19-9 was significantly different between PDAC, IPMN and healthy participants (mean ± SD of 307 ± 467, 43 ± 89, and 11 ± 5.4, respectively; P < 0.001). When looking at the ROC curves, CA19-9 with a cutoff value of 28.75 was able to distinguish PDAC patients from non-PDAC participants with an AUROC of 0.796.
Dimethyl sulfide using a cutoff value of 0.78 AU distinguished PDAC from non-PDAC participants with an AUROC of 0.671 and 68.5% sensitivity, 59.8% specificity, 47.4% positive predictive value (PPV), 78.2% negative predictive value (NPV) and 62.8% accuracy. These results were not statistically significantly different than the performance of CA19-9 (P = 0.115).
Acetone dimer with a cutoff value of 4.97 AU outperformed CA19-9 with an AUROC of 0.910 and 87.0% sensitivity, 92.2% specificity, 85.5% PPV, 93.1% NPV, and 90.4% accuracy. When combining CA19-9 with acetone dimer level at a cutoff point of 0.29 AU, the AUROC increased to 0.936, which was significantly better than the performance of CA19-9 alone (P = 0.020) and reported a sensitivity, specificity, PPV, NPV, and accuracy of 96.7%, 86.0%, 82.9%, 97.4%, and 90.4%, respectively (Table 4 and Figure 4).
| Biomarker | AUC | Cut off | Sensitivity, % | Specificity, % | Accuracy, % | NPV, % | PPV, % | P value1 |
| CA19-9 | 0.796 | 28.75 | 76.7 | 88.4 | 85.3 | 83.3 | 88.4 | - |
| Dimethyl sulfide | 0.671 | 0.78 | 68.5 | 59.8 | 62.8 | 78.2 | 47.4 | 0.115 |
| Dimethyl sulfide + CA19-9 | 0.823 | 0.31 | 80 | 76.7 | 57.0 | 84.6 | 70.6 | 0.555 |
| Acetone dimer | 0.910 | 4.97 | 87.0 | 92.2 | 90.4 | 93.1 | 85.5 | 0.116 |
| Acetone dimer + CA19-9 | 0.936 | 0.29 | 96.7 | 86.0 | 90.4 | 97.4 | 82.9 | 0.020 |
Our study reported that the exhaled breath VOCs dimethyl sulfide and acetone dimer showed statistically higher concentrations in the PDAC group compared to the non-PDAC and control groups. The significant discriminatory performance of VOCs, particularly acetone dimer, supported previous evidence that exhaled VOCs have the potential to be new biomarkers for PDAC detection[13-15].
Our findings of higher acetone dimer levels in PDAC patients were consistent with previous findings[14]. Acetone dimer levels in human secretions have been reported to be higher in a variety of digestive cancers, including colorectal cancer[22,23], gastric cancer[22], hepatocellular carcinoma (HCC)[21,24], and cholangiocarcinoma[25]. The dysregulation of ketone metabolism caused by cancer cells is thought to be one possible explanation for increased acetone levels. Acetone is a ketone compound derived from the spontaneous degradation of acetoacetate, a ketone body generated from acetyl-CoA, mainly obtained from the beta-oxidation of long-chain fatty acids[22,26]. Because of their ability to divide uninhibitedly, cancer cells cause an abnormal increase in glucose metabolism and deplete the availability of glucose for the surrounding cells, shifting the energy source toward the lipid metabolism pathway, resulting in increased ketone body production[27]. These extra ketone bodies can be metabolized by the mitochondria of both cancer and surrounding cells via the tricarboxylic acid cycle and provide energy to the cell in addition to the normal glucose pathways[22].
Despite the findings that acetone dimer levels were higher in PDAC groups, acetone monomer levels failed to show significant differences. Acetone is typically reported without labeling the ionized form (whether it is acetone monomer or acetone dimer) when using GC-MS or SIFT-MS methods[14,28]. However, by utilizing FAIMS, we were able to discriminate between the two ionized forms, as the FAIMS analyzer contains a beta radiation source (Ni-63) that emits electrons that ionize the air and moisture that flows through the analyzer. The process generates positive and negative hydronium ions, which react with molecules with a strong proton affinity, such as acetone, to produce distinct ion peaks of an acetone monomer or dimer[29]. The amount of monomer or dimer produced varies by factors such as temperature and, more importantly, substance concentration. A previous study revealed that proton-bound dimers form when the gas concentration is sufficiently high[30]. As a result, the acetone dimer may have a stronger association with the amount of acetone generated than the monomer.
Another source of acetone is the alcohol dehydrogenase enzyme, which uses an oxidation-reduction reaction to convert isopropyl alcohol to acetone[31]. Not surprisingly, previous research showed that acetone is directly related to isopropyl alcohol, and these two VOCs usually co-exist[32,33]. While acetone levels were higher in PDAC, our study failed to show statistical differences in isopropyl alcohol levels between PDAC and non-PDAC participants. This suggests that in contrast to other cancers, the alcohol dehydrogenase enzyme may not be the primary energy pathway in IPMN and PDAC.
Our findings also revealed that PDAC patients produced more dimethyl sulfide compared to non-PDAC patients and controls, which is one of the volatile sulfur compounds produced by the metabolism of sulfur-containing amino acids like methionine and cysteine[34]. Dimethyl sulfide is normally detected in very low concentrations in normal population serum. Higher concentrations were found in patients with pulmonary hypertension, cirrhosis, and HCC[24]. The proposed mechanism of dimethyl sulfide production is the methylation of methanethiol by thiol S-methyltransferase, which occurs endogenously or is synthesized by the microbiota in the intestinal tract (e.g., Streptococcus, Fusobacterium, Salmonella, Enterobacter, and Helicobacter)[35]. A previous study reported significantly lower levels of dimethyl sulfide in cholangiocarcinoma exhaled breath, which could reflect a reduced formation of the antioxidant glutathione linked to cancer pathways[25]. In another study, dimethyl sulfide was found to be one of the discriminatory VOCs in HCC when compared to cirrhosis[24].
To the best of our knowledge, no previous study has demonstrated statistical differences in dimethyl sulfide levels between PDAC and non-PDAC populations. In this study, we discovered a correlation between dimethyl sulfide levels and PDAC metastasis status. This finding suggested that dimethyl sulfide could be used as a biomarker for PDAC metastasis. However, more data on symptom-free survival and response to treatment after follow-up is required to confirm these findings. Based on the findings of the HCC study, one of our hypotheses is that higher dimethyl sulfide levels in the metastasis group is due to liver lesions, as liver metastasis was the most common type of metastasis. Another possibility is that cancer cells' protein methylation has changed or that the gut microbiota has changed as a result of duodenal invasion. While hypotheses can be offered, the reasons why PDAC patients demonstrated a higher level of dimethyl sulfide remain unanswered and require further investigation.
Acetone dimer at a cut-off value of 4.97 is the only VOC that demonstrated a better PDAC discriminating performance when compared to CA19-9, a well-known tumor marker for PDAC. Acetone dimer with AUROC of 0.910 in our study was consistent with previous reports of exhaled breath acetone shown to differentiate PDAC from controls with AUROCs of 0.757-0.901[14,36]. We also showed that acetone dimer, when combined with CA19-9 at a cutoff value of 0.29, can improve the AUROC to 0.936. This intriguing finding highlighted not only the possibility of using acetone dimer as a sole biomarker, but also the importance of combining acetone dimer with the previous CA19-9 to improve diagnostic accuracy. External validation of our findings is required before applying them to real-world clinical practice.
The strength of this study compared to previous research is the method used for alveolar air collection and the use of FAIMS in combination with the XGBoost algorithm VOC library for VOC profile analysis. Previous VOC studies employed a variety of methods for collecting breath samples, such as aluminum bags or the Bio-VOC sample device[14,15], which potentially introduced ambient air into the samples more than alveolar air. We selected the ReCIVATM breath sample system with software to monitor the real-time progression of the breath sample collection with a pure oxygen gas supply to avoid contamination with confounding gas. We also collected the gas in thermal desorption tubes, which are more stable than aluminum bags.
A second difference compared to previous studies was the use of FAIMS instead of complex analytic techniques for VOC extraction such as GC-MS, ion-molecule reaction mass spectrometry, or electrical nose[13-15]. FAIMS can distinguish VOCs by using specific mobilities in the electrical field of the substances[37]. The advantages of this method include better VOC discrimination performance at low concentrations, which we believe is more appropriate in exhale breath samples, and a shorter runtime (approximately 10 min) compared to GC-MS (up to 45 min)[19,37]. As a result, it is regarded as a promising method in real clinical practice. FAIMS does have limitations, specifically the difficulty in consistency between different instruments, resulting in minor variations in waveform between units[37]. To ensure the accuracy of the VOCs extracted, we created our own VOC library by standardizing gas processing and comparing VOC chromatograms on the same machine. We also used continuous pure oxygen gas flow during breath sample collection to prevent environmental VOC contamination.
Aside from the VOC collection and analysis method, another study strength was the homogeneity of non-PDAC participants. Most of the previous data used a combination of patients with benign pancreatic diseases including chronic pancreatitis, pancreatic cysts, pancreatic pseudocysts, IPMN, and healthy volunteers as the controls[14,36,38]. The heterogeneity in benign pancreatic pathophysiology within the control groups may have affected the ability to detect differences in VOCs between groups in these studies. To prevent these confounding factors in our study, we inclusively enrolled only IPMN participants who had potential malignant transformation to serve as a second comparison group and enrolled standard healthy volunteers for the control group.
Our study did have several limitations. First, our recruited PDAC population was primarily comprised of patients with locally advanced and advanced stage PDAC. This resulted from the limitation of current PDAC diagnosis, since most PDAC patients are diagnosed in the advanced stage. The VOCs collected in our study may not be representative of VOCs detected in the early-stages of PDAC. The study could not demonstrate whether early stage PDAC VOC levels were significantly different than at later disease stages and might not be effective as a screening method. A larger longitudinal study with more early-stage PDAC patients is needed. Secondly, because of the small number of malignant IPMN patients in our study, a conclusion that the VOCs can distinguish malignant from benign IPMN is problematic. Further research with larger numbers of malignant IPMN is still needed. Third, we cannot conclude which PDAC was developed from IPMN in our study. However, no PDAC patients had any cystic component or other pancreatic cystic lesion that represented an area of current or previous IPMN. Fourth, this is a "proof-of-concept” study. We intended to identify PDAC VOCs from potentially malignant pancreatic lesions like IPMN. Other benign pancreatic tumors with no malignant potential such as serous cystic neoplasm, as well as other peri-ampullary cancers such as distal cholangiocarcinoma, ampullary cancer, or duodenal cancer, were not included in the comparison group. The specific VOCs that distinguish PDAC from these populations require further investigation.
Multiple exogenous confounding factors such as environmental factors, occupational exposure, and underlying diseases could possibly alter the VOC profiles in real-world practice. In addition, the VOC analysis in this study was derived from a single cohort, and the model performance was evaluated in a cohort of participants from the same center. The generalizability of our study results should be interpreted with caution. Lastly, the external validation of these findings using an independent cohort is required before applying this approach to clinical practice.
The use of alveolar VOC composition offers potential as a biomarker to help distinguish PDAC from IPMN and normal pancreas. The two VOCs that showed promising results were dimethyl sulfide and acetone dimer. The combination of acetone dimer with CA19-9 showed the highest AUROC to distinguish PDAC from the non-PDAC population. More prospective diagnostic research is required to validate our findings.
In various gastrointestinal malignancies, including pancreatic adenocarcinoma, volatile organic compounds (VOC) have demonstrated a good result in distinguishing patients with cancer and those without cancer.
No previous research has been conducted to capture exhaled breath VOCs using thermal desorption-gas chromatography/field-asymmetric ion mobility spectrometry (TD-GC/FAIMS) methods to distinguish pancreatic ductal adenocarcinoma (PDAC). The control population in the previous study was heterogeneous and reduced the validity of the results.
To identify exhale breath VOCs that can distinguish PDAC patients from those with intraductal papillary mucinous neoplasm (IPMN) and those with no pancreatic lesions using TD-GC/FAIMS.
Exhaled breath was collected using the ReCIVA® device and VOCs were identified using TD-GC/FAIMS.
Dimethyl sulfide and acetone dimer were higher in PDAC patients when compared to IPMN and healthy participants. Dimethyl sulfide levels have been linked to PDAC metastasis status. Combining acetone dimer with the CA19-9 biomarker improved PDAC diagnostic accuracy.
Dimethyl sulfide and acetone dimer were two VOCs that can potentially distinguish PDAC from IPMN and healthy participants.
Further validation with a larger cohort using a longitudinal approach is needed to confirm these findings.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koganti SB, United States; Ling Q, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 893] [Cited by in F6Publishing: 1228] [Article Influence: 245.6] [Reference Citation Analysis (0)] |
| 2. | Kanno A, Masamune A, Hanada K, Maguchi H, Shimizu Y, Ueki T, Hasebe O, Ohtsuka T, Nakamura M, Takenaka M, Kitano M, Kikuyama M, Gabata T, Yoshida K, Sasaki T, Serikawa M, Furukawa T, Yanagisawa A, Shimosegawa T; Japan Study Group on the Early Detection of Pancreatic Cancer (JEDPAC). Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 3. | Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 4. | Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg. 2013;257:17-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Chhoda A, Lu L, Clerkin BM, Risch H, Farrell JJ. Current Approaches to Pancreatic Cancer Screening. Am J Pathol. 2019;189:22-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry SJ, Doubeni CA, Epling JW Jr, Kubik M, Landefeld CS, Mangione CM, Pbert L, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2019;322:438-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 971] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 8. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 409] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 9. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 10. | Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Janfaza S, Khorsand B, Nikkhah M, Zahiri J. Digging deeper into volatile organic compounds associated with cancer. Biol Methods Protoc. 2019;4:bpz014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Agarwal SM, Sharma M, Fatima S. VOCC: a database of volatile organic compounds in cancer. RSC Advances. 2016;6:114783-114789. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Uslu HI, Dölle AR, Dullemen HM, Aktas H, Kolkman JJ, Venneman NG. Pancreatic ductal adenocarcinoma and chronic pancreatitis may be diagnosed by exhaled-breath profiles: a multicenter pilot study. Clin Exp Gastroenterol. 2019;12:385-390. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 14. | Markar SR, Brodie B, Chin ST, Romano A, Spalding D, Hanna GB. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br J Surg. 2018;105:1493-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Princivalle A, Monasta L, Butturini G, Bassi C, Perbellini L. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer. 2018;18:529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Schmidt K, Podmore I. Current Challenges in Volatile Organic Compounds Analysis as Potential Biomarkers of Cancer. J Biomark. 2015;2015:981458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Rattray NJ, Hamrang Z, Trivedi DK, Goodacre R, Fowler SJ. Taking your breath away: metabolomics breathes life in to personalized medicine. Trends Biotechnol. 2014;32:538-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2016;408:2759-2780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Crosby BT, Ridzuan-Allen A, O’Neill JP. Volatile organic compound analysis for the diagnosis of pancreatic cancer. Anna Pancreatic Cancer. 2021;4. [DOI] [Cited in This Article: ] |
| 20. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 430] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 21. | Sukaram T, Apiparakoon T, Tiyarattanachai T, Ariyaskul D, Kulkraisri K, Marukatat S, Rerknimitr R, Chaiteerakij R. VOCs from Exhaled Breath for the Diagnosis of Hepatocellular Carcinoma. Diagnostics (Basel). 2023;13. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 22. | Chung J, Akter S, Han S, Shin Y, Choi TG, Kang I, Kim SS. Diagnosis by Volatile Organic Compounds in Exhaled Breath in Exhaled Breath from Patients with Gastric and Colorectal Cancers. Int J Mol Sci. 2022;24:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 23. | Liu D, Ji L, Li M, Li D, Guo L, Nie M, Wang D, Lv Y, Bai Y, Liu M, Wang G, Li Y, Yu P, Li E, Wang C. Analysis of volatile organic compounds released from SW480 colorectal cancer cells and the blood of tumor-bearing mice. Transl Cancer Res. 2019;8:2736-2751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Miller-Atkins G, Acevedo-Moreno LA, Grove D, Dweik RA, Tonelli AR, Brown JM, Allende DS, Aucejo F, Rotroff DM. Breath Metabolomics Provides an Accurate and Noninvasive Approach for Screening Cirrhosis, Primary, and Secondary Liver Tumors. Hepatol Commun. 2020;4:1041-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Siriwong N, Sukaram T, Tansawat R, Apiparakoon T, Tiyarattanachai T, Marukatat S, Rerknimitr R, Chaiteerakij R. Exhaled volatile organic compounds for cholangiocarcinoma diagnosis. Liver Res. 2022;6:191-197. [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 26. | Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin Chim Acta. 2004;347:25-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 740] [Cited by in F6Publishing: 591] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Sukaram T, Tansawat R, Apiparakoon T, Tiyarattanachai T, Marukatat S, Rerknimitr R, Chaiteerakij R. Exhaled volatile organic compounds for diagnosis of hepatocellular carcinoma. Sci Rep. 2022;12:5326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Navaneethan U, Spencer C, Zhu X, Vargo JJ, Grove D, Dweik RA. Volatile organic compounds in bile can distinguish pancreatic cancer from chronic pancreatitis: a prospective observational study. Endoscopy. 2021;53:732-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Ireland CP, Ducati C. Investigating the photo-oxidation of model indoor air pollutants using field asymmetric ion mobility spectrometry. J Photochem Photobiol A Chem. 2015;312:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Tozihi M, Bahrami H, Farajmand B, Tabrizchi M. Ion mobility spectrometry and theoretical study for investigation of thermal decomposition, chemical ionization, and dimer formation of proline. Int J Mass Spectrom. 2020;448:116272. [DOI] [Cited in This Article: ] |
| 31. | Verma A, Yadav D, Singh A, Gupta M, Thapa KB, Yadav BC. Detection of acetone via exhaling human breath for regular monitoring of diabetes by low-cost sensing device based on perovskite BaSnO3 nanorods. Sens Actuators B Chem. 2022;361:131708. [DOI] [Cited in This Article: ] |
| 32. | Li W, Liu Y, Cheng S, Duan Y. Exhaled isopropanol: new potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017;7:17480-17488. [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Wang W, Zhou W, Wang S, Huang J, Le Y, Nie S, Wang W, Guo Q. Accuracy of breath test for diabetes mellitus diagnosis: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3366-3377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Mochalski P, Leja M, Gasenko E, Skapars R, Santare D, Sivins A, Aronsson DE, Ager C, Jaeschke C, Shani G, Mitrovics J, Mayhew CA, Haick H. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J Breath Res. 2018;12:046005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Navaneethan U, Parsi MA, Gutierrez NG, Bhatt A, Venkatesh PG, Lourdusamy D, Grove D, Hammel JP, Jang S, Sanaka MR, Stevens T, Vargo JJ, Dweik RA. Volatile organic compounds in bile can diagnose malignant biliary strictures in the setting of pancreatic cancer: a preliminary observation. Gastrointest Endosc. 2014;80:1038-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Zrodnikov Y, Davis CE. The Highs and Lows of FAIMS: Predictions and Future Trends for High Field Asymmetric Waveform Ion Mobility Spectrometry. J Nanomed Nanotechnol. 2012;3:109e. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Navaneethan U, Parsi MA, Lourdusamy D, Grove D, Sanaka MR, Hammel JP, Vargo JJ, Dweik RA. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig Dis Sci. 2015;60:2150-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |