Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1605
Peer-review started: June 14, 2023
First decision: July 7, 2023
Revised: July 15, 2023
Accepted: August 2, 2023
Article in press: August 2, 2023
Published online: September 15, 2023
The current prognostic significance of perigastric tumor deposits (TDs) in gastric cancer (GC) remains unclear.
To assess the prognostic value of perigastric TDs and put forward a new TNM staging framework involving TDs for primary GC.
This study retrospectively analyzed the pathological data of 6672 patients with GC who underwent gastrectomy or surgery for GC with other diseases from January 1, 2012 to December 31, 2017 at the Chinese PLA General Hospital. According to the presence of perigastric TDs or not, the patients were divided into TD-positive and TD-negative groups by using the method of propensity score matching. The differences between TD-positive and TD-negative patients were analyzed using binary logistic regression modeling. The Kaplan-Meier method was used to plot survival curves. Multivariate Cox regression modeling and the log-rank test were used to analyze the data.
Perigastric TDs were found to be positive in 339 (5.09%) of the 6672 patients with GC, among whom 237 were men (69.91%) and 102 were women (30.09%) (2.32:1). The median age was 59 years (range, 27 to 78 years). Univariate and multivariate survival analyses indicated that TD-positive GC patients had a poorer prognosis than TD-negative patients (P < 0.05). The 1-, 3-, and 5-year overall survival rates of GC patients with TDs were 68.3%, 19.6%, and 11.2%, respectively, and these were significantly poorer than those without TDs of the same stages. There was significant variation in survival according to TD locations among the GC patients (P < 0.05). A new TNM staging framework for GC was formulated according to TD location. When TDs appear in the gastric body, the original stages T1, T2, and T3 are classified as T4a with the new framework, and the original stages T4a and T4b both are classified as T4b. When TDs appear in the lesser curvature, the previous stages N0, N1, N2, and N3 now both are classified as N3. When TDs appear in the greater curvature or the distant tissue, the patient should be categorized as having M1. With the new GC staging scheme including TDs, the survival curves of patients in the lower grade TNM stage with TDs were closer to those of patients in the higher grade TNM stage without TDs.
TDs are a poor prognostic factor for patients with primary GC. The location of TDs is associated with the prognosis of patients with primary GC. Accordingly, we developed a new TNM staging framework involving TDs that is more appropriate for patients with primary GC.
Core Tip: The aim of this study was to assess the prognostic value of perigastric tumor deposits (TDs) and put forward a new TNM staging framework involving TDs for primary gastric cancer (GC). This study indicated that TDs serve as a bad prognostic factor in patients with primary GC and the new TNM staging system incorporating TDs is more suitable for patients with primary GC.
- Citation: Li Y, Li S, Liu L, Zhang LY, Wu D, Xie TY, Wang XX. Incorporation of perigastric tumor deposits into the TNM staging system for primary gastric cancer. World J Gastrointest Oncol 2023; 15(9): 1605-1615
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1605.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1605
Gastric cancer (GC) remains the fifth most common cancer worldwide. More than 70% of GC cases occur in developing countries, including Japan, Korea, and China. GC ranks as the fourth leading cause of cancer-related deaths both in men and women. It was estimated that in 2012, the GC mortality rate in East Asia was highest (24 male deaths and 9.8 female deaths per 100000 people) and lowest in North America (2.8 male deaths and 1.5 female deaths)[1].
The prognosis of GC varies according to pathological TNM (tumor, lymph node, and metastasis) stage, and the staging of GC is critical for its treatment and prognosis. GC pathological TNM stage is determined by the extent of primary tumor infiltration depth (T), the number of metastatic lymph nodes (N), and distant metastasis (M)[2]. In the past few years, some other predictors-for example, histological types, lymphatic vessel infiltration, and lymphatic wall carcinoma-have been identified as important or even independent predictors of survival[3].
Gabriel was the first clinician to discover tumor deposits (TDs) in 1935[4]. TDs were initially defined as peritumoral nodule clusters in the primary adipose tissue of GC, with no histological evidence of residual lymph nodes remaining in the nodules. It was speculated that TDs may reflect discontinuous spread, venous invasion, and extravascular spread, or complete replacement of lymph nodes[2]. The prognostic significance of TDs in colorectal cancer has been confirmed by several studies[5-9]. A series of studies has indicated that TDs are associated with other gastrointestinal tumors, including biliary tract cancer, GC, and pancreatic cancer[10,11].
The eighth edition of the American Joint Committee on Cancer (AJCC) Gastric Staging System considers all gastric metastatic nodules without residual lymph node tissue as regional lymph node metastases[5]. However, the AJCC TNM staging system for GC fails to distinguish between lymph node metastasis and TDs. The prognostic value of TDs in GC has not been extensively studied or confirmed. To date, no studies have investigated the prognostic significance of TDs in GC in detail[12-14]. In this study, we aimed to assess the prognostic value of TD location and to put forward a new TNM staging framework considering TDs for primary GC.
This study was approved by the Medical Ethics Committee of the Chinese PLA General Hospital (S2023-065-01). Patients provided written informed consent before being included. A retrospective cohort study was conducted to evaluate the clinicopathologic data of 6672 GC patients who underwent surgical procedures at the Chinese PLA General Hospital between January 2012 and December 2017. According to the presence of TDs or not, the patients were divided into TD-positive and TD-negative groups by using the method of propensity score matching (PSM). The eighth edition of the AJCC TNM staging system for GC was adopted in this study. The following clinical data were collected: Sex, age, time of gastrectomy, histologic grade, location, T stage, number of lymph node metastases, N stage, and type of operation. Patients with or without TDs were compared in terms of overall survival rates. The survival curves associated with different pathological TNM stages were compared, including comparisons between TD-positive and TD-negative patients. An amendment to the eighth edition of the AJCC TNM staging system for GC was proposed and validated. A multivariate analysis included the following variables: Clinicopathological characteristics, including sex, age, operative method, histological grade, TNM stage, and TD status, and survival data.
In the previous research of colorectal cancer, TDs were defined as isolated tumor foci found in the pericolonic or perirectal fat or the adjacent mesentery (mesocolonic fat) away from the invasive margin of the tumor without evidence of residual lymphatic tissue[13,14]. In our study, TDs were defined as isolated tumor nodules located in the subserosal, perigastric adipose, or omental tissues away from the margin of primary tumor without histologic evidence of residual lymph nodes[15]. Postoperative adjuvant therapy was performed as needed according to the Japanese Gastric Cancer Treatment Guidelines.
In this study, TDs locations were categorized as the gastric body, lesser curvature, greater curvature, and distant tissue. When tumor nodules appeared in the subserosal tissue of the stomach but away from the primary tumor, we defined the location as the gastric body. When tumor nodules appeared in the perigastric adipose or omental tissues, we defined it as the lesser or greater curvature according to the two curvature sides of the stomach. When tumor nodules appeared in the adipose or omental tissues far away from stomach, we defined it as distant tissue.
GC patients who underwent gastrectomy were obligatorily followed every 6 mo during the first year and every 6 or 12 mo thereafter. Follow-up included physical examination, laboratory tests, chest X-ray, abdominal and pelvic ultrasonography, and computed tomography, as previously reported. Overall survival was calculated from the date of diagnosis to the last contact or the date of death.
All statistical analyses (and generation of graphics) were performed using SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, United States). For the comparisons of clinicopathologic characteristics between the two propensity-score-matched groups, logistic regression analysis was used for categorical variables as appropriate. Overall survival rates were determined using the Kaplan-Meier method. The log-rank test was used to identify differences between the survival curves of different patient groups. In the univariate and multivariate analyses, Cox proportional hazard modeling was used to identify independent factors correlated with prognosis. Confidence intervals (CIs) were used in the analysis of the predictive accuracy estimates for models that either included or did not include TDs. All P values were two-sided, with P values < 0.05 considered statistically significant.
This study included 6672 patients with GC, among whom 339 (5.09%) had TDs detected. Among the patients with TDs, 237 were men, and 102 were women (P = 0.527). Among the patients without TDs, 256 were men, and 83 were women (P = 0.492). The clinical characteristics of the patients with and without TDs were comparable.
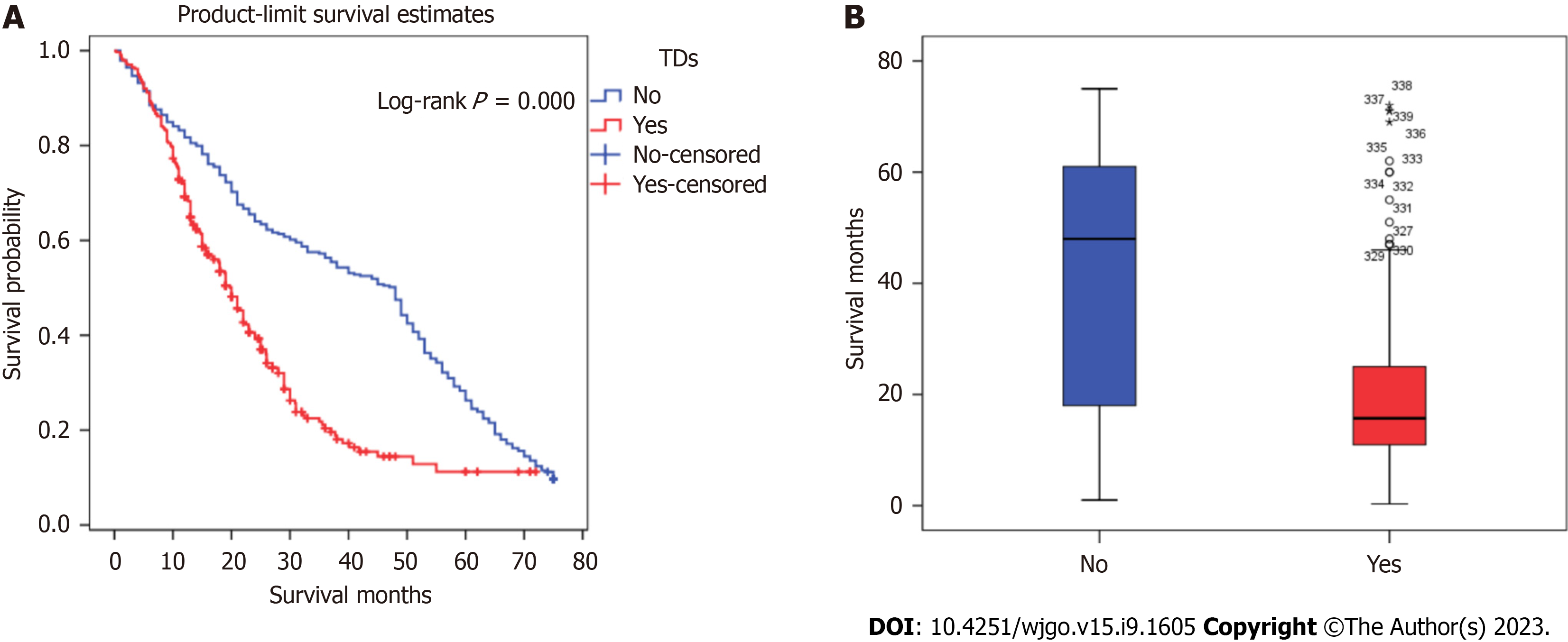
Kaplan-Meier analysis was performed to calculate the overall survival rates for patients with and without TDs. The 1-, 3-, and 5-year survival rates of patients with TDs were 68.3%, 19.6%, and 11.2%, respectively, and those for patients without TDs were 81.7%, 56.3%, and 26.3%, respectively (Figure 1).
Univariate analysis indicated that survival was significantly correlated with age (P = 0.011), operative method (P ≤ 0.001), histologic grade (P = 0.003), depth of invasion (P ≤ 0.001), lymph node metastasis (P ≤ 0.001), distant metastasis (P ≤ 0.001), and TD status (P ≤ 0.001). Cox multivariate analysis revealed that age, depth of invasion, lymph node metastasis, distant metastasis, and the presence of TDs were independent prognostic factors for GC patients (P < 0.05) (Table 1).
| Characteristic | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.772 | |||
| Male | 0.97 (0.80, 1.18) | |||
| Female | 1.00 (Reference) | |||
| Age | 0.011 | 0.009 | ||
| ≥ 65 yr | 1.26 (1.05, 1.50) | 1.27 (1.06, 1.53) | ||
| < 65 yr | 1.00 (Reference) | 1.00 (Reference) | ||
| Operation method | ≤ 0.001 | 0.076 | ||
| Proximal gastrectomy | 0.56 (0.45, 0.70) | ≤ 0.001 | 0.76 (0.60, 0.97) | 0.026 |
| Distal gastrectomy | 0.57 (0.46, 0.70) | ≤ 0.001 | 0.84 (0.67, 1.05) | 0.118 |
| Total gastrectomy | 1.00 (Reference) | 1.00 (Reference) | ||
| Histologic grade | 0.003 | 0.456 | ||
| Low | 1.41 (1.12, 1.76) | 1.09 (0.86, 1.39) | ||
| High | 1.00 (Reference) | 1.00 (Reference) | ||
| AJCC 8 TNM T category | ≤ 0.001 | 0.034 | ||
| T4b | 2.28 (1.58, 3.28) | ≤ 0.001 | 1.19 (0.79, 1.78) | 0.410 |
| T4a | 1.92 (1.34, 2.75) | ≤ 0.001 | 0.92 (0.62, 1.37) | 0.679 |
| T3 | 1.65 (1.15, 2.36) | 0.006 | 0.85 (0.58, 1.27) | 0.429 |
| T2 | 0.93 (0.59, 1.48) | 0.766 | 0.70 (0.44, 1.12) | 0.138 |
| T1 | 1.00 (Reference) | 1.00 (Reference) | ||
| AJCC 8 TNM N category | ≤ 0.001 | ≤ 0.001 | ||
| N3 | 3.64 (2.86, 4.64) | ≤ 0.001 | 2.72 (2.07, 3.59) | ≤ 0.001 |
| N2 | 2.01 (1.54, 2.63) | ≤ 0.001 | 1.66 (1.24, 2.22) | 0.001 |
| N1 | 1.43 (1.06, 1.94) | 0.019 | 1.37 (1.00, 1.89) | 0.052 |
| N0 | 1.00 (Reference) | 1.00 (Reference) | ||
| AJCC 8 TNM M category | ≤ 0.001 | ≤ 0.001 | ||
| M1 | 3.70 (2.89, 4.74) | 2.78 (2.15, 3.60) | ||
| M0 | 1.00 (Reference) | 1.00 (Reference) | ||
| Tumor Deposits | ≤ 0.001 | ≤ 0.001 | ||
| Yes | 2.09 (1.72, 2.53) | 1.64 (1.32, 2.03) | ||
| No | 1.00 (Reference) | |||
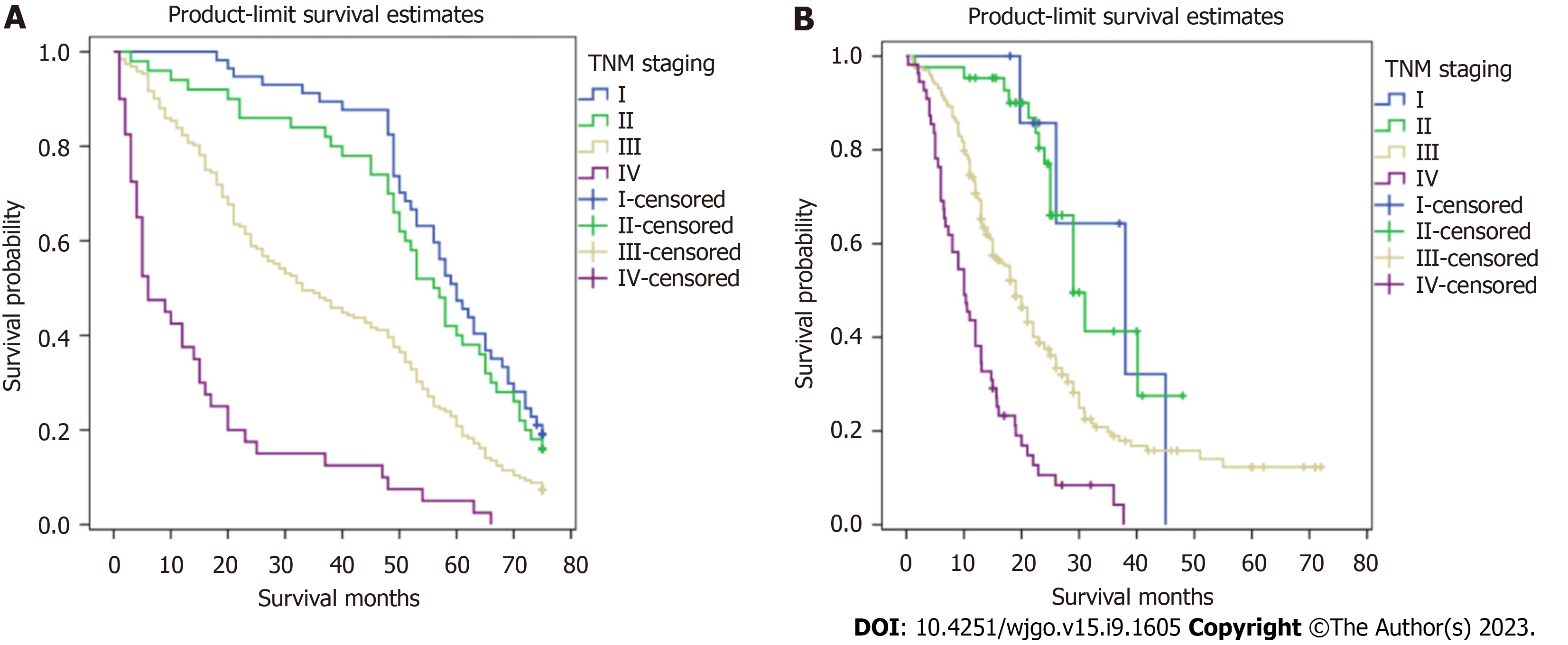
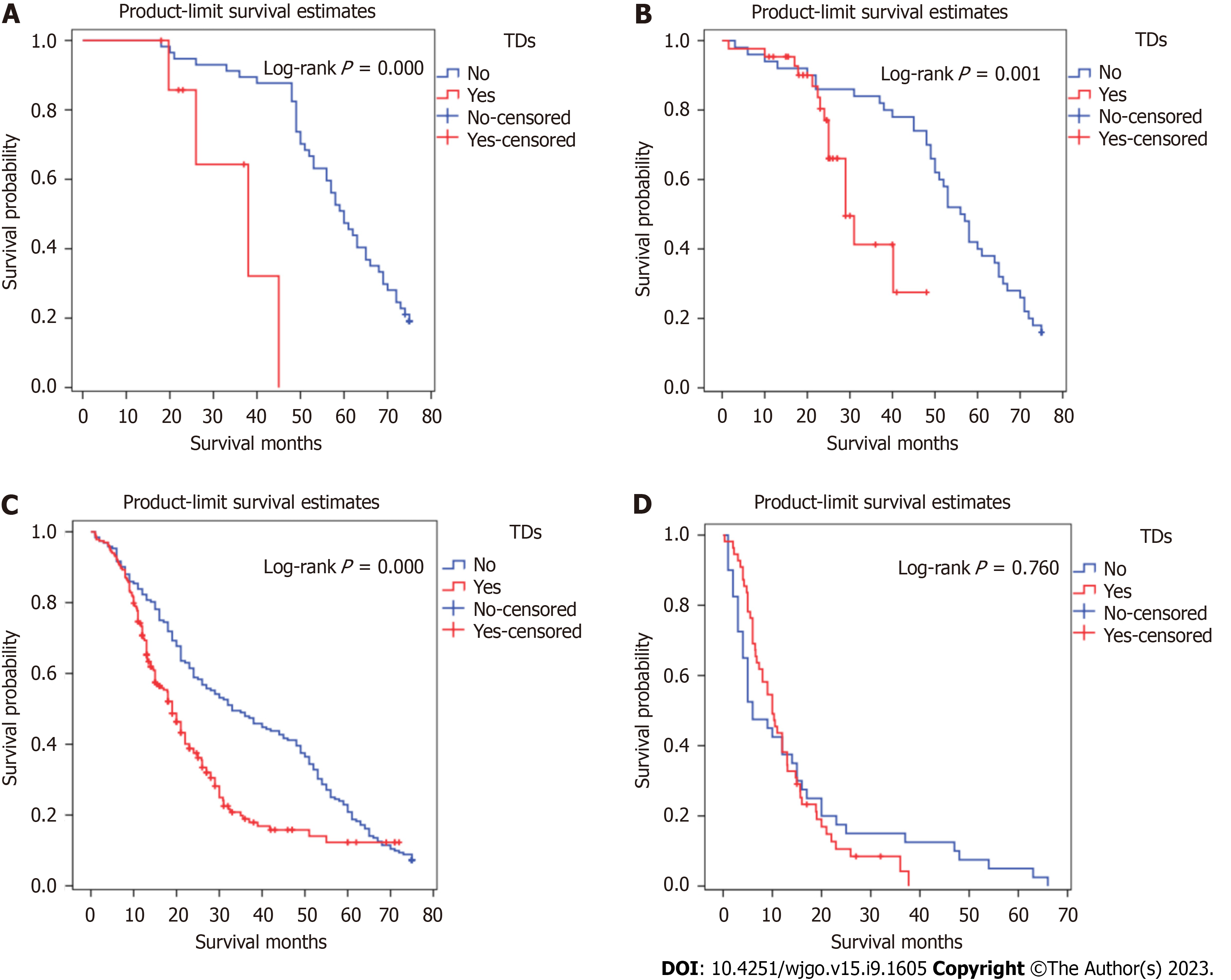
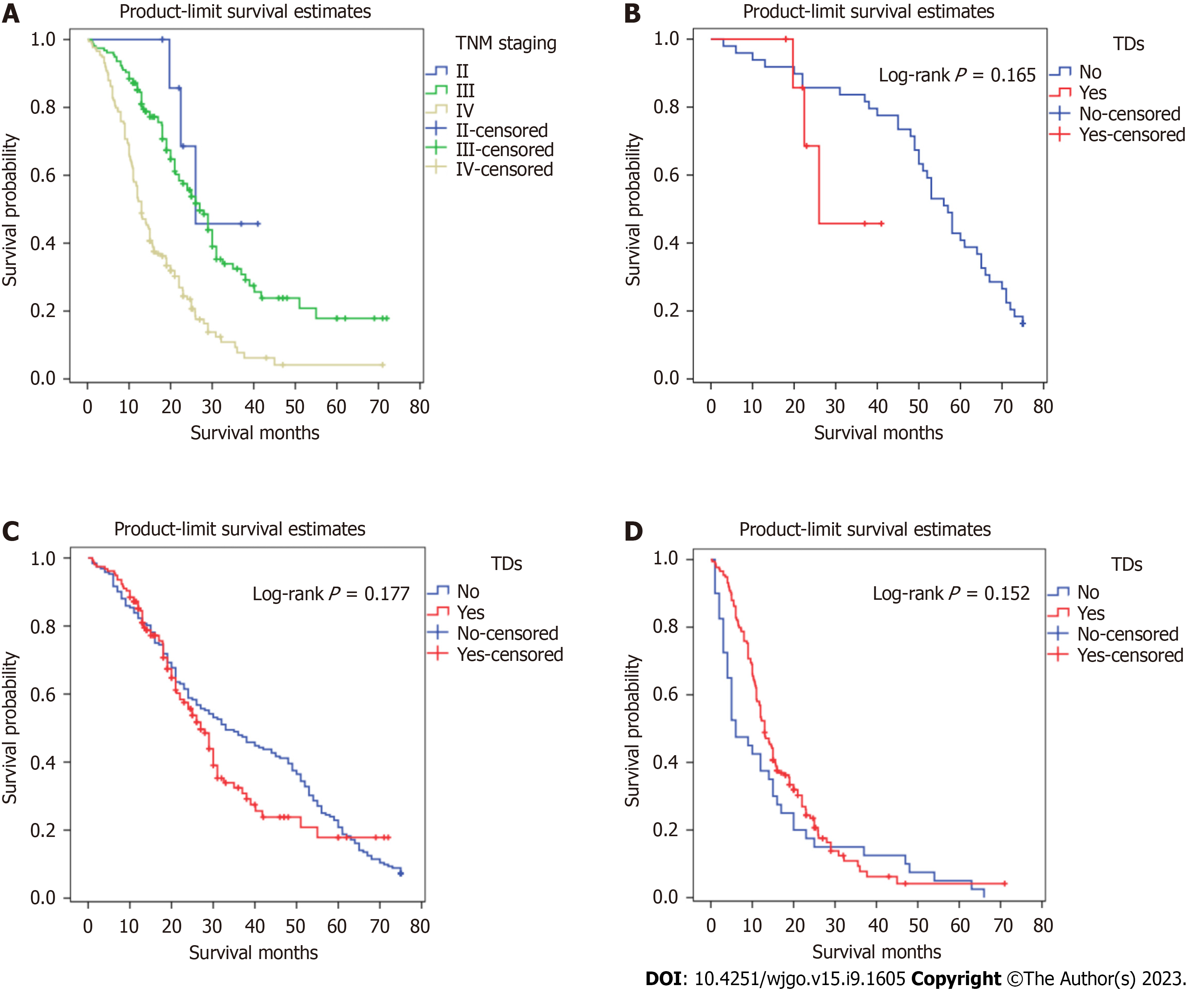
Figures 2 and 3 show survival curves of GC patients with and without TDs according to pathological TNM stage. The Kaplan-Meier survival curves indicate significant differences among the TNM stages (except stage IV) between patients with and without TDs. Patients with TDs had poorer survival than those without in each stage.
Kaplan-Meier survival curves were plotted to assess the prognostic value of TDs. In Figure 4, patients with TDs and those with stage TNM, T, or M without TDs did not have similar survival rates, but patients with TDs and those with stage N3 without TDs had similar survival rates.
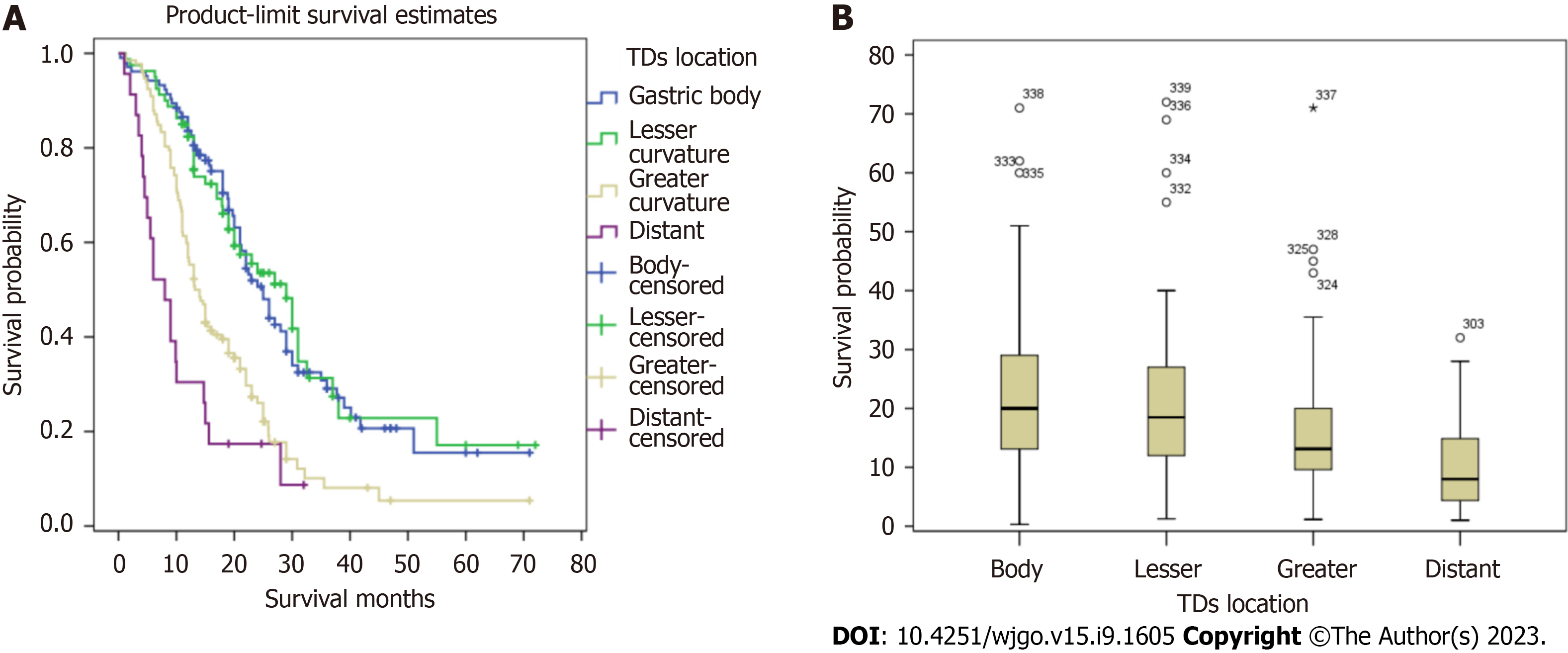
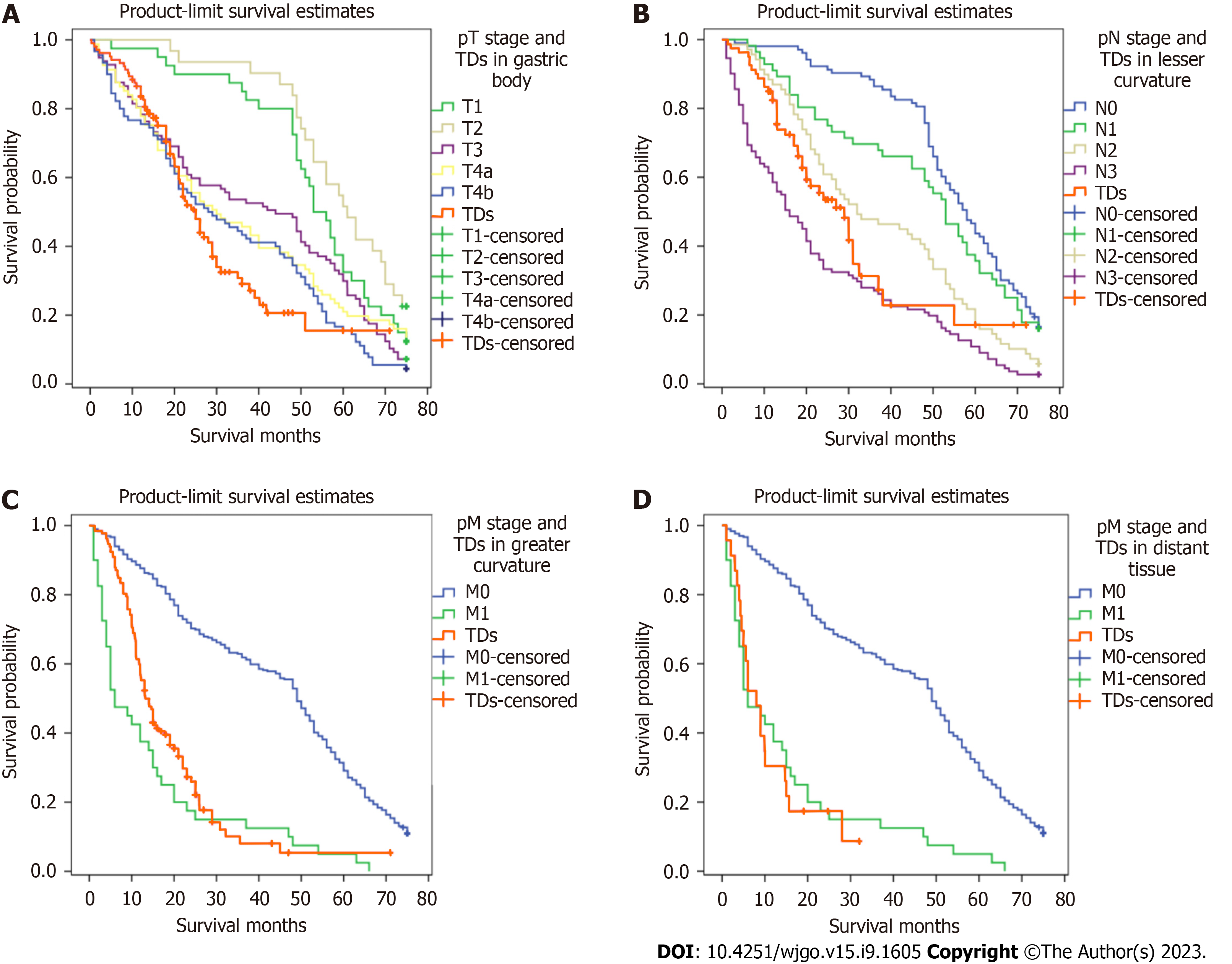
Figure 5 illustrates the comparative survival analysis among the locations. Figure 6 shows the survival curves of the patients with different TD locations, as well as the survival curves of patients without TDs with different TNM pathological stages.
We proposed an amendment to the eighth edition of the UICC/AJCC TNM staging system for GC. When TDs appear in the gastric body, the original T1, T2, and T3 stages correspond to T4a with the new system, and T4a changes to T4b. When TDs appear in the lesser curvature, the previous N0, N1, N2, N3 Labels correspond to N3 under the new system. When TDs appear both in the greater curvature and distant tissue, the patient should be categorized as having M1. Figure 7 shows Kaplan-Meier survival curves generated using the new pathological TNM staging framework for GC patients with TDs.
TDs first appeared in the fifth edition of UICC/AJCC Tumor Staging Guide for colorectal cancer staging in 1997, followed by the sixth and seventh editions of the colorectal cancer staging guide. However, the definitions of TDs vary between colorectal cancer stages. The criteria and histological features of TDs have been modified several times. In the seventh edition of the UICC/AJCC colorectal cancer staging guide, TDs are defined as non-contiguous with the primary tumor and as lacking evidence of lymphoid tissue structure; however, in the lymph node drainage area, TDs included as an indicator of stage N1c were considered an independent factor affecting the prognosis[16]. However, there is no evidence regarding how to best classify TDs with consideration of the actual survival of the patients. In the eighth edition of the UICC/AJCC guide, TDs are classified as regional lymph node metastasis without residual evidence of lymph node tissue[11].
The pathophysiological causes of TDs are still unclear, and most studies have shown that the presence of TDs is associated with lymph node metastasis, neurovascular invasion, and microvascular spread[5,8]. There is no credible evidence identifying the causes of TDs in GC. A colorectal cancer study observed four types of invasive non-continuous tumor infiltrations: Scattered, vascular, neurological, and nodular[17]. Subsequently, Goldstein and Turner[7] classified TDs into three types: The nerve disseminated type, the vascular disseminated type, and the intravascular tumor[7]. It has also been reported that when TDs appear in the mesorectum, they should be divided into intravascular, intratympanic, perineural, and isolated TDs[18]. Some studies have found that the formation of TDs may be related to the de-interstitialization of tumor cells[13]. Changes in the secretion of snail, twist, and epithelial cadherin promote the ability of the tumors to metastasize and spread through lymph nodes[19]. In summary, we found that the formation of TDs is associated with invasive tumor growth. In three previous reports, the probabilities of developing TDs in GC patients were 17.8%, 23.9%, and 24%, respectively (ordered according to publication date). It has been reported that the probability of TD development is associated with tumor size, Borrmann classification, the extent of tumor infiltration of lymphatic vessels, and lymphatic metastasis and expansion, and that the survival of GC patients is significantly correlated with TDs[20].
In this study, we analyzed the status of TDs and the clinicopathological characteristics of GC patients, and found that the presence of TDs was significantly associated with tumor infiltration (T), lymph node metastasis (N), tumor location, and neurovascular invasion. The associations of TD status with patient age (> 61 years), sex, body mass index, TNM pathological stage, and degree of differentiation were not statistically significant. This study demonstrated that TDs are associated with the invasive ability of tumor cells and that tumor cells in TDs are capable of migration, which may be via lymphatic pathways or the sudden infiltration of tumor cells of unknown causes.
Although the overall survival of patients with GC has improved significantly over the past few decades, there are still many questions to be answered about histopathology and predictive factors. Studies have shown that TDs have independent prognostic value in colorectal cancer; however, few studies have investigated gastric TDs. Several studies have shown that the appearance of TDs predicts a poor prognosis, which is similar to our findings. Kaplan-Meier survival curves were used to plot the survival of patients with or without TDs, and the two groups were significantly different from one another. Multivariate Cox regression analysis revealed that TD positivity was not independently associated with survival in GC but that the presence of TDs may be associated with late-stage disease. Cox regression survival analysis of all patients revealed lymph node metastasis (N) and age (> 61 years old) as the only significant factors. One study (that also used Cox regression analysis) found that the presence or absence of TDs was not an independent predictor of survival[13]. The studies identified that perigastric TDs as an important prognostic indicator, and previous investigators hoped to incorporate TDs into the staging of lymph node metastasis. Additionally, they classified TDs as metastatic lymph nodes, restaged patients with colorectal cancer and advanced GC using the seventh edition of the UICC/AJCC guidelines, and found TDs in patients with the same stage of lymph node metastasis (N). The presence of TDs can lead to a worse prognosis. However, the study was unconvincing because there were only six patients with TDs with stage T1 or T2 disease[14]. In a recent study, the histological tumor type and the extent of vascular invasion were identified to be important causes of TDs, and TDs were more common in intestinal tumors. With all current research considered, there is insufficient evidence supporting TDs in GC as an independent prognostic factor.
This study included 6672 patients who underwent surgery for GC, and a total of 339 patients were TD-positive. The rate of TD positivity was 5.09%, lower than previously reported. After dividing the patients into two groups by using PSM, there were 193 patients with positive deposits and 297 with negative deposits. Among 193 patients with positive deposits, 5 (2.59%) were in stage I, 28 (14.51%) in stage II, 139 (72.02%) in stage III, and 21 (10.88%) in stage IV. The proportion of TD-positive GC patients with late-stage disease was high, suggesting that the presence of TDs may indicate later-stage disease and a worse prognosis. We found that the median survival of patients with the same TNM stage in the TD-positive group was lower than that in the TD-negative group, but there was no intergroup difference in survival among patients with stage IV disease. Kaplan-Meier survival curves were used to evaluate the survival of the two groups. The prognosis of patients in stages I, II, and III with TDs was lower than that of the TD-negative group (P < 0.001). The prognosis of the patients with TDs in stage IV was better than that of the TD-negative group, but this difference was not statistically significant. At the same time, the median overall survival durations among patients with TDs in the gastric body, lesser curvature, greater curvature, and distant tissue were 36.0 mo, 37.0 mo, 15.2 mo, and 9.9 mo, respectively; the variation among these four groups was statistically significant.
The significance of TD location in the prognosis of GC patients has not been studied. TD locations are not routinely included in histopathology reports. It was found that when TDs appeared in the greater curvature of the stomach or the omental fat connective tissue, patient survival was significantly decreased. When TDs appeared in the lesser curvature of the stomach or the gastric body, there was no significant difference in the survival between the two groups. Therefore, we speculated that when the TDs appear in the lesser curvature side of the stomach or the gastric body, the range of invasion may be limited by the anatomical positional relationship of the small omental sac and the lymphatic drainage pathway (which limits the possibility of distant metastasis). When TDs appear in the greater curvature of the stomach, the tumor cells may be transferred distally through the gastric colon ligament. When TDs appear in the distal fat connective tissue or lymph nodes, this should not be considered in N staging; rather, this scenario should be directly classified as M1. Among patients with TDs in different TNM stages, only the Kaplan-Meier survival curves of patients with stage III disease were significantly different, and the Kaplan-Meier survival curves of patients classified in the other three stages were not significantly different. In order to verify the influence of TD location on the staging of GC patients, and to find a staging framework that better aligns with the actual survival of TD-positive patients, we performed the following steps. We compared the T and N stages of TD-positive patients with TDs in the lesser curvature with those of the TD-negative group and found that the survival curve of the TD-positive patients with TDs in the lesser curvature was similar to those of the TD-negative patients classified as stages N1 and N2, respectively. Comparing the T and N stages of the TD-positive group with those of the negative group, we found that the survival curve of the TD-positive group was similar to those of the TD-negative patients classified as having T3, T4b, and N2, respectively. We determined that, when TDs appeared in the gastric body, the T stage should not be lower than T3, but there was no similar definitive conclusion that could be made for N stage. Comparing N stages of TD-positive patients with TDs in the greater curvature with those of TD-negative patents, we found favorable survival among both the TD-positive patients with TDs in the greater curvature and the N3 patients in the TD-negative group. In this case, the N stage of patients with TDs was N3 regardless of the number of metastatic lymph nodes. The M stages of the TD-positive patients with TDs in the distant tissue were compared with those of the TD-negative patients, and the survival curve of the TD-positive patients with TDs in the distant tissue was similar to that of M1 patients in the TD-negative group. In summary, we believe that when the pathological report suggests that TDs appear in the gastric body, the T stage should be no less than T3; when TDs appear in the greater curvature of the stomach, the N stage should be no less than N3; when TDs are in the distant tissue, the M stage should be M1.
Although our study confirmed the adverse effects of TDs on the prognosis of GC and put forward a new TNM staging method containing TDs innovatively, the following limitations remained in this study. First, TDs were more common with diffuse histological type compared with intestinal type, but the results of Lauren typing were not used in the pathological report of our center, and it would be better if this part was added. Second, because of the single-center retrospective design, the number of cases was limited, and the follow-up time was insufficient. Third, the study was performed in Asian population, so the data might not be extrapolated to North American or European populations. Therefore, valid incorporation of TDs into the TNM staging system for primary GC requires multicenter and more large-scale clinical analyses including American and European data, as well as more in-depth basic research and exploration of the mechanism underlying TD development.
TDs are a poor prognostic factor in patients with primary GC, and a new TNM staging system combining TDs would be suitable for such patients.
The current prognostic significance of perigastric tumor deposits (TDs) in gastric cancer (GC) remains unclear.
The prognostic value of TDs in GC has not been extensively studied or confirmed. To date, no studies have investigated the prognostic significance of TDs in GC in detail. This study aimed to assess the prognostic value of perigastric TDs and put forward a new and appropriate TNM staging framework involving TDs for primary GC.
This study aimed to assess the prognostic value of perigastric TDs and put forward a new and appropriate TNM staging framework involving TDs for primary GC.
We retrospectively analyzed the pathological data of 6672 patients with GC who underwent gastrectomy or surgery for GC with other diseases from January 1, 2012 to December 31, 2017 at the Chinese PLA General Hospital. The patients were divided into TD-positive and TD-negative groups by using the method of propensity score matching. The differences between TD-positive and TD-negative patients were analyzed using binary logistic regression modeling. The Kaplan-Meier method was used to plot survival curves. Multivariate Cox regression modeling and the log-rank test were used to analyze the data.
With the new GC staging scheme including TDs, the survival curves of patients in the lower grade TNM stage with TDs were closer to those of patients in the higher grade TNM stage without TDs.
TDs are a poor prognostic factor in patients with primary GC, and a new TNM staging system combining TDs would be suitable for such patients.
From a clinical point of view, we found deficiencies in the current TNM staging system for GC and conducted this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dambrauskas Z, Lithuania; Shang L, China; Win TT, Malaysia S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Xu ZH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20863] [Article Influence: 2318.1] [Reference Citation Analysis (2)] |
| 2. | He X, Wu W, Lin Z, Ding Y, Si J, Sun LM. Validation of the American Joint Committee on Cancer (AJCC) 8th edition stage system for gastric cancer patients: a population-based analysis. Gastric Cancer. 2018;21:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Gurzu S, Orlowska J, Sugimura H, Bara T, Szentirmay Z, Januszewicz W, Bara T Jr, Szederjesi J, Jung I. Immunohistochemical features and staging of early gastric cancer. Arch Med Sci. 2017;13:1373-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Gabriel WB, Dukes CE, Bussey HJ. Biopsy of the rectum. Br J Surg. 1951;38:401-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ono C, Yoshinaga K, Enomoto M, Sugihara K. Discontinuous rectal cancer spread in the mesorectum and the optimal distal clearance margin in situ. Dis Colon Rectum. 2002;45:744-9; discussion 742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Tateishi S, Arima S, Futami K, Kawahara K, Tachikawa D, Naritomi K, Iwashita A. A clinicopathological investigation of "tumor nodules" in colorectal cancer. Surg Today. 2005;35:377-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Goldstein NS, Turner JR. Pericolonic tumor deposits in patients with T3N+MO colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000;88:2228-2238. [PubMed] [Cited in This Article: ] |
| 8. | Belt EJ, van Stijn MF, Bril H, de Lange-de Klerk ES, Meijer GA, Meijer S, Stockmann HB. Lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as stage III. Ann Surg Oncol. 2010;17:3203-3211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Tong LL, Gao P, Wang ZN, Song YX, Xu YY, Sun Z, Xing CZ, Xu HM. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann Surg. 2012;255:208-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, Solmi M, Capelli P, Scarpa A. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016;28:205-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Liang Y, Wu L, Liu L, Ding X, Wang X, Liu H, Meng J, Xu R, He D, Liang H. Impact of extranodal tumor deposits on prognosis and N stage in gastric cancer. Surgery. 2019;166:305-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Lee HS, Lee HE, Yang HK, Kim WH. Perigastric tumor deposits in primary gastric cancer: implications for patient prognosis and staging. Ann Surg Oncol. 2013;20:1604-1613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Sun Z, Wang ZN, Xu YY, Zhu GL, Huang BJ, Xu Y, Liu FN, Zhu Z, Xu HM. Prognostic significance of tumor deposits in gastric cancer patients who underwent radical surgery. Surgery. 2012;151:871-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Ersen A, Unlu MS, Akman T, Sagol O, Oztop I, Atila K, Bora S, Ellidokuz H, Sarioglu S. Tumor deposits in gastric carcinomas. Pathol Res Pract. 2014;210:565-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Nagtegaal ID, Tot T, Jayne DG, McShane P, Nihlberg A, Marshall HC, Påhlman L, Brown JM, Guillou PJ, Quirke P. Lymph nodes, tumor deposits, and TNM: are we getting better? J Clin Oncol. 2011;29:2487-2492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Koelzer VH, Lugli A, Dawson H, Hädrich M, Berger MD, Borner M, Mallaev M, Galván JA, Amsler J, Schnüriger B, Zlobec I, Inderbitzin D. CD8/CD45RO T-cell infiltration in endoscopic biopsies of colorectal cancer predicts nodal metastasis and survival. J Transl Med. 2014;12:81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Ueno H, Mochizuki H. Clinical significance of extrabowel skipped cancer infiltration in rectal cancer. Surg Today. 1997;27:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ratto C, Ricci R, Rossi C, Morelli U, Vecchio FM, Doglietto GB. Mesorectal microfoci adversely affect the prognosis of patients with rectal cancer. Dis Colon Rectum. 2002;45:733-42; discussion 742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Fan XJ, Wan XB, Yang ZL, Fu XH, Huang Y, Chen DK, Song SX, Liu Q, Xiao HY, Wang L, Wang JP. Snail promotes lymph node metastasis and Twist enhances tumor deposit formation through epithelial-mesenchymal transition in colorectal cancer. Hum Pathol. 2013;44:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Puppa G, Ueno H, Kayahara M, Capelli P, Canzonieri V, Colombari R, Maisonneuve P, Pelosi G. Tumor deposits are encountered in advanced colorectal cancer and other adenocarcinomas: an expanded classification with implications for colorectal cancer staging system including a unifying concept of in-transit metastases. Mod Pathol. 2009;22:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |