Published online Feb 18, 2017. doi: 10.4254/wjh.v9.i5.252
Peer-review started: August 24, 2016
First decision: September 27, 2016
Revised: December 29, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 18, 2017
To evaluate and compare the efficacy and safety of telaprevir (TVR)-and simeprevir (SMV)-based triple therapies in elderly patients, specifically patients aged 66 years or older.
The present study enrolled 112 and 76 Japanese patients with chronic hepatitis C virus genotype 1b infection who were treated with a 12-wk TVR-based or SMV-based triple therapy, respectively, followed by a dual therapy that included pegylated interferon α and ribavirin (RBV) for 12 wk. The patients were categorized into two groups according to age as follows: A younger group of patients aged ≤ 65 years old and an older group of patients aged > 65 years old. Among the patients treated with TVR-based triple therapy, 34 patients were included in the older group. The median ages were 56 years (range: 28-65 years) in the younger group and 69 years (range: 66-81 years) in the older group. Among the patients treated with SMV-based triple therapy, 39 patients were included in the older group. The median ages were 59 years (range: 36-65 years) in the younger group and 71 years (range: 66-86 years) in the older group. The clinical, biochemical and virological data were analyzed before and during treatment.
Among the patients treated with the TVR-based triple therapy, no significant difference in the sustained virological response (SVR) was found between the younger (80.8%) and older (88.2%) groups. The SVR rates for patients with the interleukin 28B (IL28B) (rs8099917) TG/GG-genotypes (73.9% and 60.0% in the younger and older groups, respectively) were significantly lower than for patients with the IL28B TT-genotype (86.3% and 92.9%, respectively). The cumulative exposure to RBV for the entire 24-wk treatment period (as a percentage of the target dose) was significantly higher in the younger group than in the older group (91.7% vs 66.7%, respectively, P < 0.01), but the cumulative exposure to TVR was not significantly different between the younger and older groups (91.6% vs 81.9%, respectively). A multivariate analysis identified the TT-genotype of IL28B (OR = 8.160; 95%CI: 1.593-41.804, P = 0.012) and the adherence of RBV (> 60%) (OR = 11.052; 95%CI: 1.160-105.273, P = 0.037) as independent factors associated with the SVR. Adverse events resulted in discontinuation of the treatment in 11.3% and 14.7% of the younger and older groups, respectively. Among the patients treated with the SMV-based triple therapy, no significant difference in the SVR rare was found between the younger (81.1%) and older (82.1%) groups. The SVR rates for patients with the IL28B TG/GG-genotypes (77.8% and 64.7% in the younger and older groups, respectively) were significantly lower than for patients with the IL28B TT-genotype (88.2% and 100%, respectively). A multivariate analysis identified the TT-genotype of IL28B as an independent factor associated with the SVR (OR = 9.677; 95%CI: 1.114-84.087, P = 0.040). Adverse events resulted in discontinuation of the treatment in 7.0% and 14.3% of patients in the younger and older groups, respectively.
Both TVR- and SMV-based triple therapies can be successfully used to treat patients aged 66 years or older with genotype 1b chronic hepatitis C. Genotyping of the IL28B indicates a potential to achieve SVR in these difficult-to-treat elderly patients.
Core tip: We evaluated the efficacy and safety of telaprevir (TVR)-and simeprevir (SMV)-based triple therapies for elderly patients with chronic hepatitis C, especially patients aged 66 years or older, in a real-world clinical setting. In both the TVR and SMV groups, no significant differences in the SVR and adverse events resulting in treatment discontinuation were found between the younger (aged ≤ 65) and older (aged > 65) patients. Both the TVR- and SMV-based triple therapies can be successfully used to treat patients aged 66 years or older with chronic hepatitis C virus genotype 1b infection. Genotyping of the interleukin-28B indicates a potential to achieve SVR in these difficult-to-treat elderly patients.
- Citation: Yamagiwa S, Ishikawa T, Waguri N, Sugitani S, Wakabayashi H, Ohkoshi S, Tsukishiro T, Takahashi T, Watanabe T, Terai S. Efficacy and safety of telaprevir- and simeprevir-based triple therapies for older patients with chronic hepatitis C. World J Hepatol 2017; 9(5): 252-262
- URL: https://www.wjgnet.com/1948-5182/full/v9/i5/252.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i5.252
Chronic hepatitis C virus (HCV) infections affect approximately 130-170 million people worldwide and are associated with an increased risk of developing liver cirrhosis and hepatocellular carcinoma (HCC)[1,2]. In Japan, an estimated 1.5-2 million people are infected with HCV[3]. Most of infected patients in Japan are infected with genotype 1 HCV and are older than the infected patients in Europe and the United States[4]. Although older patients with chronic HCV infection have a higher risk of developing HCC than younger patients even at the same liver fibrosis stage[5], older patients have been reported to show poor virological responses to antiviral treatments, especially postmenopausal women[6-8]. Because older patients often have reduced cardiovascular, pulmonary, and renal function and a decreased blood count, they are usually more susceptible to the toxic effects of antiviral treatments, which may lead to a higher rate and severity of adverse events and a poor adherence to the treatment[4]. Therefore, an evaluation of the safety and efficacy of antiviral treatments, especially in elderly patients with chronic HCV infections, is still necessary.
Before the introduction of direct-acting antiviral agents (DAA), pegylated interferon (PegIFN) α and ribavirin (RBV) were the standard of care for HCV genotype 1 infections. However, with the approval of telaprevir (TVR) that is an HCV non-structural (NS) 3/4A protease inhibitor, the optimum treatment regimen for chronic HCV genotype 1 infections was changed to a triple therapy with a protease inhibitor plus PegIFN α and RBV for 24 wk[9]. The TVR-based triple therapy has achieved an improved sustained virological response (SVR) rate compared to PegIFN monotherapy or PegIFNα plus RBV dual therapy[10,11]. However, the TVR-based triple therapy is associated with an increased rate and severity of adverse events, including pruritus, skin rash, anemia, and anorectal diseases, as well as increased rates of treatment discontinuation compared to patients receiving PegIFNα plus RBV dual therapy[10,11]. Because of the increased risk and severity of adverse events associated with the TVR-based triple therapy, it is difficult to use this therapy in older patients, and, therefore, reports describing the safety and efficacy of TVR-based triple therapy in elderly patients are limited[4].
Simeprevir (SMV) is a second-generation oral HCV NS3/4A protease inhibitor with antiviral activity against HCV genotype 1, 2, 4, 5 and 6 infections[12]. The QUEST 1 and QUEST 2 phase 3 clinical trials demonstrated the SVR rates of 80% and 81%, respectively, in patients treated with SMV-based triple therapy combined with PegIFNα and RBV[13]. In Japan, 4 phase 3 clinical trials (CONCERTO) were conducted, and the SVR rates were 88.6% and 91.7% for treatment-naïve patients; 35.8%, 50.9% and 38.5% for non-responders; and 89.8% and 96.6% for patients that relapsed[14-16]. Although the SMV-based triple therapy shows a favorable efficacy without inducing severe dermatologic and hematologic toxicities, the safety and efficacy of the SMV-based triple therapy for elderly patients has not yet been fully evaluated. Therefore, in the present study, we aimed to assess the efficacy and safety of TVR- and SMV-based triple therapies in elderly patients, specifically patients aged 66 years or older, in a real-world clinical setting.
This prospective and multicenter study enrolled 112 and 76 HCV genotype 1b Japanese patients who received 12 wk of TVR-based and SMV-based triple therapies, respectively, followed by a dual therapy that included PegIFNα and RBV for 12 wk. Nine hospitals in Niigata, Japan, including Niigata University Hospital, participated in this study. The patients were categorized into two groups according to age as follows: A younger group of patients aged ≤ 65 years old and an older group of patients aged > 65 years old. Among the patients treated with the TVR-based triple therapy, 34 patients were included in the older group. The median ages were 56 years (range: 28-65 years) in the younger group and 69 years (range: 66-81 years) in the older group. Among the patients treated with the SMV-based triple therapy, the older group consists of 39 patients. The median ages were 59 years (range: 36-65 years) in the younger group and 71 years (range: 66-86 years) in the older group. Liver biopsy samples were obtained from 34 (30.6%) and 42 patients (55.2%) in the TVR and SMV groups, respectively. For each sample, the fibrosis stage (F0-4) and activity grade (A0-3) were evaluated according to the Metavir score[17].
According to responses to prior treatments, relapse was defied as undetectable HCV during and at the end of treatment with positive HCV RNA detecting later on. Non-responder was defined as detectable HCV RNA for more than 24 wk. Patients with decompensated liver cirrhosis, hepatocellular carcinoma, co-infection with hepatitis B virus or human immunodeficiency virus, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, or Wilson’s disease were excluded. Patients with uncontrollable diabetes mellitus, chronic renal failure, depression, and those with a history of alcohol abuse, were also excluded. Information regarding patient profiles was shown in Tables 1 and 2.
| Factors (median, range) | Patients aged < 66 | Patients aged ≥ 66 | P value |
| n | 78 | 34 | |
| Gender, n (male/female) | 41/37 | 20/14 | 0.68 |
| Age (yr) | 56 (28-65) | 69 (66-81) | < 0.001 |
| Body weight (kg) | 61.1 (35.0-97.4) | 57.8 (41.0-74.8) | 0.105 |
| Body mass index (kg/m2) | 22.7 (15.8-32.2) | 22.9 (17.9-28.9) | 0.892 |
| Baseline HCV-RNA (log IU/mL) | 6.7 (3.9-7.7) | 6.7 (3.1-7.8) | 0.766 |
| White blood cell (/mm3) | 5000 (1900-8720) | 4500 (2700-7700) | 0.245 |
| Hemoglobin (g/dL) | 14.0 (9.1-18.6) | 13.5 (9.5-16.3) | 0.121 |
| Platelets (× 104/mm3) | 15.8 (6.5-28.7) | 13.4 (8.3-29.0) | 0.068 |
| Albumin (mg/dL) | 4.1 (2.7-5.9) | 3.9 (2.4-4.4) | 0.007 |
| AST (IU/L) | 40 (17-249) | 45 (20-163) | 0.909 |
| ALT (IU/L) | 48 (15-278) | 38 (15-189) | 0.486 |
| γ-GTP (IU/L) | 39 (11-717) | 25 (11-144) | 0.034 |
| Serum creatinine (mg/dL) | 0.7 (0.4-1.2) | 0.8 (0.4-1.0) | 0.036 |
| Estimated GFR (mL/min) | 79.0 (44.0-134.0) | 71.5 (39.0-101.9) | 0.006 |
| Prior treatment response, n (naïve/relapse/non-responder) | 45/26/7 | 15/15/4 | 0.403 |
| Liver histology (F0-2/3-4/ND) | 21/6/51 | 4/3/27 | 0.348 |
| IL28B SNP (rs8099917), n (TT/non-TT/ND) | 51/22/5 | 28/5/1 | 0.235 |
| HCV ISDR, n (0/1-3 /4-/NT) | 32/26/6/14 | 15/10/2/7 | 0.955 |
| HCV Core 70, n (Wild/Mutant/ND) | 46/18/14 | 18/10/6 | 0.751 |
| HCV Core 91, n (Wild/Mutant/ND) | 42/22/14 | 19/9/6 | 1 |
| Serum CXCL10 (pg/mL) | 510 (95-1794) | 543 (118-1218) | 0.445 |
| Factors (median, range) | Patients aged < 66 | Patients aged ≥ 66 | P value |
| n | 37 | 39 | - |
| Gender, n (%) (male/female) | 19/18 (48.6) | 14/25 (64.1) | 0.123 |
| Age (yr) | 59 (36-65) | 71 (66-86) | < 0.001 |
| Body weight (kg) | 62.0 (39.8-94.0) | 56.0 (37.5-76.6) | 0.011 |
| Body mass index (kg/m2) | 22.8 (17.2-30.3) | 22.7 (17.8-32.1) | 0.287 |
| Baseline HCV-RNA (log IU/mL) | 6.7 (5.4-7.8) | 6.6 (4.7-7.6) | 0.631 |
| White blood cells (/mm3) | 4620 (2600-7800) | 4300 (2400-8100) | 0.010 |
| Hemoglobin (g/dL) | 13.8 (11.0-16.7) | 13.1 (9.8-16.8) | < 0.001 |
| Platelets (× 104/mm3) | 16.4 (8.7-28.8) | 16.3 (7.3-31.7) | 0.291 |
| Albumin (mg/dL) | 4.2 (2.8-4.8) | 4.0 (3.1-4.6) | 0.002 |
| AST (IU/L) | 45 (21-159) | 34 (19-128) | 0.056 |
| ALT (IU/L) | 42 (16-316) | 29 (12-112) | 0.006 |
| γ-GTP (IU/L) | 29 (13-260) | 27 (9-171) | 0.388 |
| Serum creatinine (mg/dL) | 0.70 (0.44-1.01) | 0.70 (0.42-1.36) | 0.689 |
| Estimated GFR (mL/min) | 78.7 (50.0-112.6) | 77.4 (41.3-109.0) | 0.221 |
| Prior treatment response, n (naïve/relapse/non-responder) | 20/10/7 | 13/16/10 | 0.197 |
| Liver histology (F0-2/3-4/ND) | 12/6/19 | 19/5/15 | 0.483 |
| IL28B SNP (rs8099917), n (TT/non-TT/ND) | 17/19/1 | 18/17/4 | 1 |
| HCV ISDR, n (0/1-3/4-/ND) | 9/13/5/10 | 11/12/2/14 | 0.044 |
| HCV Core 70, n (Wild/Mutant/ND) | 17/13/7 | 15/8/16 | 1 |
| HCV Core 91, n (Wild/Mutant/ND) | 18/12/7 | 18/5/16 | 0.385 |
All patients received a 12-wk triple therapy that included either TVR [1500 or 2250 mg/d; the initial dose of TVR was determined by each attending physician based on each patient’s baseline characteristics such as bodyweight (BW)] (the dose of TVR was also reduced by each attending physician based on each patient’s adverse events such as anemia, malaise, and anorexia) (Telavic; Mitsubishi Tanabe Pharma, Osaka, Japan) or SMV (100 mg/d) (Sovriad; Janssen Pharmaceutical K.K., Tokyo, Japan) combined with PegIFNα2a (180 μg/wk) (Pegasys; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) or PegIFNα2b (1.5 μg/BW kg per week) (Peg-Intron; MSD, Tokyo, Japan) and RBV (600-1000 mg/d according to BW as follows: < 60 kg: 600 mg/d; 60-80 kg: 800 mg/d; > 80 kg: 1000 mg/d; if the patient’s hemoglobin was < 13 g/dL at the start of therapy, RBV was reduced by 200 mg) (Rebetol; MSD or Copegus; Chugai Pharmaceutical Co., Ltd.), followed by dual therapy of PegIFNα2a or PegIFNα2b with RBV for 12 wk.
This study was conducted in accordance with the Declaration of Helsinki. The study was reviewed and approved by the Niigata University Medical and Dental Hospital Institutional Review Board. Written informed consent was appropriately obtained from all of the individuals who enrolled in the study according to the institutional review board’s approved protocols (approval No. 1474) at the Niigata University Medical and Dental Hospital.
Laboratory and safety assessments were performed at initiation of treatment; at treatment weeks 2, 4, 8, 12, 16, 20 and 24; at the end of treatment; and at 12 and 24 wk after the end of treatment. Data on adverse events were collected, and physical examinations were performed at each visit, if clinically indicated.
The detection of HCV viremia was performed using a real-time polymerase chain reaction assay (COBAS TaqMan HCV test, Roche Diagnostic, Tokyo, Japan) with a lower limit of quantitation of 15 IU/mL and a linear dynamic range of 1.2-7.8 log IU/mL. The number of amino acid substitutions in the interferon sensitivity-determining region (in the range of 2209-2248 in the HCV NS5A) was determined using a direct sequencing method as reported previously[18]. The core amino acid substitutions at positions 70 and 91 of the HCV genome were determined by direct sequencing as reported previously[19].
SVR that is defined as undetectable serum HCV RNA at 24 wk after the end of treatment was successful treatment. Early virological responses during the first 12 wk of treatment were defined as rapid virological response (RVR), which was undetectable HCV RNA at week 4, and complete early virological response (cEVR), which was undetectable at week 12. End of treatment response (ETR) was defined as undetectable HCV RNA at the end of treatment. Relapse was defined as an ETR response but non-SVR.
Human genomic DNA was extracted from the peripheral blood. Single-nucleotide polymorphism (SNP) genotyping of the interleukin 28B (IL28B) (rs8099917) gene was performed using the TaqMan allelic discrimination demonstration kit (Applied Biosystems, Foster City, CA). The rs8099917 genotype was classified into the following 2 categories: TT (major genotype) and non-TT (minor genotype, TG or GG).
Continuous data from patients are expressed as the median with the interquartile range. The significance of the differences was analyzed statistically by the χ2, Fisher’s exact test, or Mann-Whitney U test, as appropriate, using SPSS software (Ver.18, SPSS Inc., Chicago, IL). To evaluate independent factors for predicting an SVR, variables that reached the P < 0.1 level in the univariate tests were used as candidate factors in a multivariate logistic regression analysis. In all of the cases, the level of significance was set as P value < 0.05.
The patient characteristics in the TVR group (n = 112) and SMV group (n = 76) are summarized by age in Tables 1 and 2. The analysis of the pretreatment factors revealed that serum albumin, γ-glutamyl-transpeptidase, and the estimated glomerular filtration rate in the older patients were significantly lower than those of the younger patients in the TVR group (Table 1). Pretreatment serum chemokine C-X-C motif ligand 10 (CXCL10) levels were not significantly different between the younger (543 pg/mL, range: 118-1218 pg/mL) and older (510 pg/mL, range: 95-1794 pg/mL) groups. In the SMV group, BW, white blood cell count, hemoglobin, serum albumin, and serum alanine aminotransferase (ALT) in the older patients were significantly lower than those of the younger patients (Table 2). No significant differences in the prior treatment response, HCV core 70/91 mutations, or IL28B SNPs were found between the younger and older group in both TVR and SMV groups.
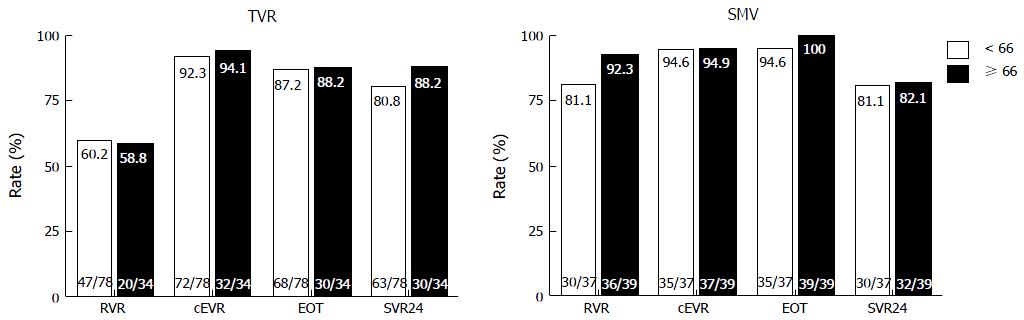
Figure 1 shows the virological responses by age. RVR, cEVR, ETR and SVR did not significantly differ between the younger and older patients in the TVR group (60.2% vs 58.8%, 92.3% vs 94.1%, 87.2% vs 88.2%, and 80.8% vs 88.2%, respectively). Similar to the TVR group, RVR, cEVR, ETR and SVR did not significantly differ between the younger and older patients in the SMV group (81.1% vs 92.3%, 94.6% vs 94.9%, 94.6% vs 100% and 81.1% vs 82.1%, respectively). In the older patients, SVR did not significantly differ between the TVR and SMV groups, although RVR was significantly higher in the SMV group than in the TVR group (92.3% vs 58.5%, P < 0.01).
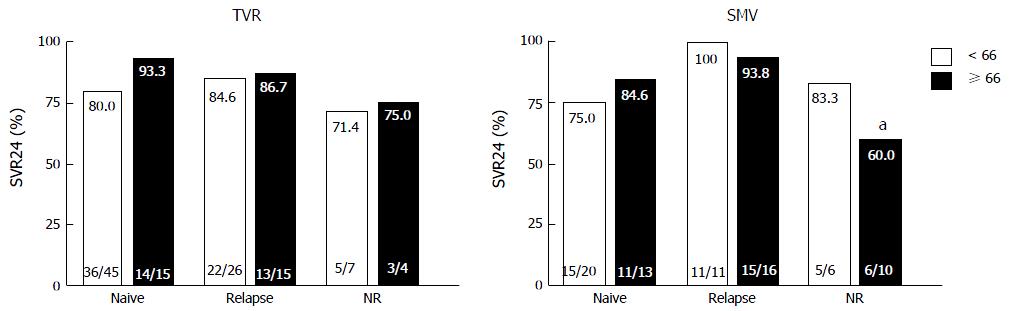
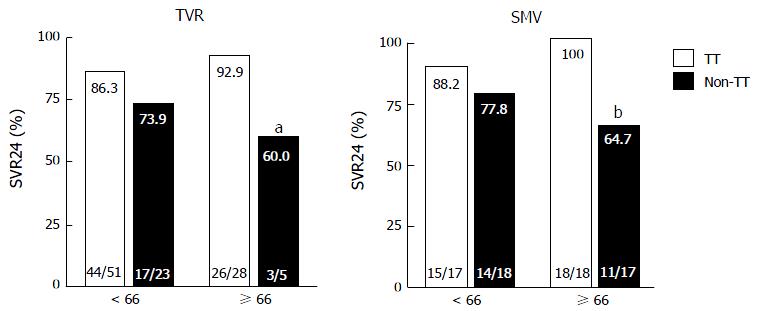
Figure 2 shows the virological responses according to prior treatment responses. In both the TVR and SMV groups, SVR did not significantly differ between the younger and older patients with the same treatment responses. In the older patients in the SMV group, SVR was significantly lower in the prior non-responders than the prior relapsers (60% vs 93.8%, P = 0.033). Figure 3 shows the virological responses according to IL28B (rs8099917) SNP status. In the TVR group, the SVR rate for the older patients with the IL28B TT-genotype was significantly higher than for the older patients with the IL28B TG/GG-genotypes (92.9% and 60%, P = 0.038). In the SMV group, the SVR rate for the older patients with the IL28B TT-genotype was also significantly higher than for the older patients with the IL28B TG/GG-genotypes (100% and 64.7%, P < 0.01).
Treatment tolerability was summarized in Tables 3 and 4. In the TVR group, adverse events resulted in treatment discontinuation in 16.7% (13/78 cases) and 11.8% (4/34 cases) of patients in the younger and older groups, respectively. Although a greater number of older patients in the TVR group was treated with the lower initial dose of TVR (1500 mg/d) than the younger patients (P < 0.01)[20], 9 patients (26.4%) discontinued TVR because of adverse events (four patients experienced skin rush, four patients experienced anemia, and one patient experienced renal dysfunction). However, the rate of discontinuation of TVR did not significantly differ between the younger and older patients (Table 3). The cumulative exposure to RBV for the whole 24-wk treatment period (as a percentage of the target dose) was significantly higher in the younger patients than in the older patients (79.3% ± 26.2% vs 62.7% ± 25.3%, P < 0.01), but the cumulative exposure to TVR was not significantly different between the younger and older patients (88.8% ± 22.8% vs 83.5% ± 25.5%, P = 0.103). Conversely, SMV was not discontinued in either the younger or older patients, although the rate of discontinuation of RBV was significantly higher in the older patients than the younger patients in the SMV group (58.9% vs 29.7%, P = 0.012) because of anemia. Adverse events resulted in treatment discontinuation in 8.1% (3/37 cases) and 7.6% (3/39 cases) of patients in the younger and older groups, respectively.
| Patients aged < 66 | Patients aged ≥ 66 | P value | |
| Initial doses (median, range) | |||
| PEG-IFN/BW (μg/kg per week) | 1.48 (0.98-2.00) | 1.49 (1.15-1.87) | 0.859 |
| TVR/BW (mg/kg per day) | 33.0 (19.2-64.3) | 29.2 (7.5-54.2) | 0.044 |
| TVR (2250 mg/1500 mg/others), n | 55/23/0 | 11/21/2 | < 0.001 |
| RBV/BW (mg/kg per day) | 11.4 (6.8-20.0) | 11.4 (5.7-28.0) | 0.103 |
| Dose reduction, n (%) | |||
| PEG-IFN | 7 (8.9) | 6 (17.6) | 0.209 |
| TVR | 19 (24.3) | 12 (35.3) | 0.256 |
| RBV | 40 (51.2) | 27 (79.4) | 0.006 |
| Discontinuation, n (%) | |||
| PEG-IFN | 13 (16.7) | 4 (11.8) | 0.580 |
| TVR | 12 (15.4) | 9 (26.5) | 0.192 |
| RBV | 12 (15.4) | 7 (20.6) | 0.585 |
| Adherence, mean ± SD (%) | |||
| PEG-IFN | 88.2 ± 25.7 | 90.1 ± 19.8 | 0.606 |
| TVR | 88.8 ± 22.8 | 83.5 ± 25.5 | 0.103 |
| RBV | 79.3 ± 26.2 | 62.7 ± 25.3 | < 0.001 |
| Patients aged < 66 | Patients aged ≥ 66 | P value | |
| Initial doses (median, range) | |||
| PEG-IFNα2a (180/90) (μg/wk) | 19/0 | 10/1 | 0.366 |
| PEG-IFNα2b (120/100/80/others) (μg/wk) | 2/16/5/1 | 0/25/5/1 | 0.422 |
| SMV/BW (mg/kg per day) | 1.6 (1.1-2.5) | 1.8 (1.3-2.7) | 0.011 |
| RBV/BW (mg/kg per day) | 11.6 (6.8-17.1) | 12.3 (6.0-20.6) | 0.166 |
| Dose reduction, n (%) | |||
| PEG-IFN | 5 (13.5) | 6 (15.3) | 1 |
| SMV | 0 | 0 | 1 |
| RBV | 3 (8.1) | 6 (15.3) | 0.481 |
| Discontinuation, n (%) | |||
| PEG-IFN | 5 (13.5) | 5 (12.8) | 1 |
| SMV | 2 (5.4) | 2 (5.1) | 1 |
| RBV | 11 (29.7) | 23 (58.9) | 0.012 |
| Adherence, mean ± SD (%) | |||
| PEG-IFN | 93.6 ± 16.8 | 92.3 ± 19.5 | 0.592 |
| SMV | 98.1 ± 7.2 | 93.9 ± 18.1 | 0.079 |
| RBV | 91.0 ± 16.1 | 86.8 ± 20.2 | 0.126 |
To identify pretreatment and treatment factors that contribute to SVR, univariate and multivariate analyses were performed in the TVR and SMV groups including the following variables: Gender, age, body mass index, baseline HCV viral load, serum ALT, hemoglobin, platelet counts, IL28B SNP, initial dose of TVR, TVR/BW (mg/kg per day), SMV/BW (mg/kg per day), dose reduction of treatments, and RVR (Tables 5 and 6). In the TVR group, the IL28B SNP significantly correlated with SVR according to the univariate analysis. A multivariate logistic regression analysis identified the IL28B TT-genotype (OR = 8.160; 95%CI: 1.593-41.804, P = 0.012) and the adherence of RBV (> 60%) (OR = 11.052; 95%CI: 1.160-105.273, P = 0.037) as independent factors associated with the SVR (Table 5). In the SMV group, the IL28B SNP and the absence of a dose reduction in PegIFN significantly correlated with SVR according to the univariate analysis. In the multivariate logistic regression analysis, the independent factors associated with the SVR were IL28B TT-genotype (OR = 9.677; 95%CI: 1.114-84.087, P = 0.040) and the absence of a dose reduction in PegIFN (OR = 6.557; 95%CI: 1.328-32.377, P = 0.021) (Table 6).
| Factors | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Age | 1.012 (0.955-1.072) | 0.689 | ||
| Gender (female) | 0.784 (0.262-2.342) | 0.663 | ||
| Body mass index (kg/m2) | 1.074 (0.875-1.318) | 0.495 | ||
| Prior treatment response (non-NR) | 3.850 (0.830-17.861) | 0.085 | ||
| Baseline HCV-RNA (log IU/mL) | 1.264 (0.457-3.495) | 0.652 | ||
| Baseline ALT (IU/mL) | 1.008 (0.998-1.017) | 0.105 | ||
| Baseline platelets (× 104/mm3) | 1.017 (0.906-1.142) | 0.775 | ||
| Baseline hemoglobin (g/dL) | 1.038 (0.736-1.464) | 0.830 | ||
| IL28B SNP (TT) | 6.700 (1.826-24.584) | 0.004 | 8.160 (1.593-41.804) | 0.012 |
| Initial dose of TVR (2250 mg/d) | 2.069 (0.670-6.553) | 0.204 | ||
| TVR/BW (mg/kg per day) | 0.938 (0.870-1.011) | 0.093 | ||
| RBV/BW (mg/kg per day) | 0.811 (0.617-1.066) | 0.133 | ||
| PEG-IFN dose reduction (none) | 2.134 (0.253-17.988) | 0.486 | ||
| TVR dose reduction (none) | 1.020 (0.281-3.703) | 0.976 | ||
| RBV dose reduction (none) | 1.548 (0.433-5.525) | 0.501 | ||
| Adherence of RBV (> 60%) | 6.873 (1.784-26.474) | 0.005 | 11.052 (1.160-105.273) | 0.037 |
| RVR (none) | 0.88 (0.123-1.216) | 0.104 | ||
| Factors | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Age | 0.998 (0.942-1.058) | 0.953 | ||
| Gender (female) | 0.330 (0.083-1.314) | 0.116 | ||
| Body mass index (kg/m2) | 1.164 (0.934-1.450) | 0.175 | ||
| Prior treatment response (non-NR) | 2.955 (0.811-10.764) | 0.101 | ||
| Baseline HCV-RNA (log IU/mL) | 0.767 (0.328-1.791) | 0.540 | ||
| Baseline ALT (IU/mL) | 0.998 (0.985-1.012) | 0.785 | ||
| Baseline platelets (× 104/mm3) | 1.082 (0.953-1.228) | 0.224 | ||
| Baseline hemoglobin (g/dL) | 1.257 (0.827-1.910) | 0.285 | ||
| IL28B SNP (TT) | 12.593 (1.516-104.576) | 0.019 | 9.677 (1.114-84.087) | 0.040 |
| SMV/BW (mg/kg per day) | 0.306 (0.054-1.742) | 0.182 | ||
| RBV/BW (mg/kg per day) | 1.085 (1.138-3.913) | 0.501 | ||
| PEG-IFN dose reduction (none) | 7.250 (1.712-30.700) | 0.007 | 6.557 (1.328-32.377) | 0.021 |
| RBV dose reduction (none) | 1.556 (0.470-5.160) | 0.470 | ||
| RVR (none) | 0.351 (0.075-1.637) | 0.183 | ||
In this study, we evaluated and compared the efficacy and safety of TVR- and SMV-based triple therapies in combination with PegIFN and RBV in elderly Japanese patients with chronic hepatitis C (CHC), specifically patients aged 66 years or older. The rate of SVR did not differ significantly between younger and older patients in either the TVR or the SMV groups. Among the older patients who were more difficult to treat, more patients carrying the IL28B TG/GG genotypes and prior non-responders were enrolled in the SMV group than the TVR group. However, the rate of SVR did not differ significantly between the TVR and SMV group, although the rates of RVR and relapse were significantly higher in the SMV group than the TVR group. When we performed univariate analyses of factors associated with SVR in all the enrolled patients, we did not find any significance in the type of treatment (TVR vs SMV) (OR = 1.115, 95%CI: 0.415-3.192, P = 0.787). Ogawa et al[21] reported that the rates of SVR were similar for patients with HCV genotype 1b who were treated with TVR- and SMV-based triple therapies, although patients treated with TVR-based triple therapy had more frequent severe adverse events than those treated with SMV-based triple therapy. In this study, the rate of adverse events that resulted in treatment discontinuation did not differ between the younger and older patients in either the TVR or the SMV group, although a higher frequency and severity of adverse events have been reported in patients treated with TVR-based triple therapy compared to patients treated with PegIFN and RBV dual therapy[10,11]. We found that both TVR- and SMV-based triple therapy were effective and tolerable among older patients aged 66 years or older.
In Japan, an estimated 1.5-2 million people are infected with HCV, and these patients are older than those infected in Europe and the United States[3,22]. However, previous studies describing the safety and efficacy of TVR- and SMV-based triple therapies, especially in elderly patients with CHC, are limited. One of the reasons may be that the inclusion criteria for clinical trials were usually set to a maximum age of 65 years[11,23]. Furusyo et al[4] reported that there were no differences in the efficacy, frequency and severity of adverse events between patients aged > 60 years and those aged ≤ 60 years who were treated with TVR-based triple therapy. Consistent with our study, they reported that a multivariate analysis revealed that the IL28B TT-genotype and the achievement of RVR were independent factors associated with SVR. Although the decrease in hemoglobin was significantly higher in patients aged > 60 years compared to younger patients aged ≤ 60 years, the rate of adverse events that resulted in treatment discontinuation was similar between the two groups[4]. Abe et al[23] also reported that in patients treated with TVR-based triple therapy, the SVR rate in patients aged > 65 years was similar to that of patients aged ≤ 65 years and that there was no notable increase of the rate of treatment discontinuation. In our study, the rate of adverse events that resulted in treatment discontinuation in the older patients was lower in the SMV group than in the TVR group, but the difference was not statistically significant. However, considering the risk of higher frequency and severity of adverse events associated with TVR-based triple therapy, we recommend the use of SMV rather than TVR.
The IL28B SNP genotype had a limited impact on the SVR rate with triple therapy in treatment-experienced patients[24], and the strength of the association between the IL28B genotype and the treatment outcome was attenuated in the triple therapy compared to the dual therapy[23,25]. In the present study, the IL28B SNP genotype displayed a striking influence on the outcome of both TVR- and SMV-based triple therapy, especially in older patients. In the older patients carrying the IL28B TT-genotype, the rates of SVR were 92.9% and 100% in the TVR and SMV groups, respectively. In contrast, in the older patients carrying the IL28B TG or GG-genotype, the rates of SVR were significantly decreased to 60% and 64.7% in the TVR and SMV groups (P = 0.038 and P < 0.01), respectively. Although the substitutions in the core aa70 of the HCV genotype 1b were reported to be important predictors of the efficacy of dual therapy and triple therapy[26,27], our study revealed that the substitutions in the HCV core aa70 were not associated with the achievement of SVR (data not shown). This discrepancy may be explained by the differences in the study population, as our study consisted of a relatively higher number of aged patients. We also measured serum CXCL10 in patients treated with TVR-based triple therapy because previous studies have reported that pretreatment serum CXCL10 concentrations were associated with early virological response and treatment efficacy in patients treated with this therapy[28,29]. However, we did not confirm the utility of pretreatment CXCL10 concentrations as a predictor of virological response in patients treated with TVR-based triple therapy.
The present study has a number of limitations. First, the sample size might have provided inadequate statistical power to detect definitive differences between the SVR and no-SVR data in both the older and younger patients. However, the best of our knowledge, this is the first study to compare the efficacy and safety of TVR- and SMV-based triple therapies for elderly patients aged 66 years or older. Second, we only investigated Japanese patients with the HCV genotype 1b. Among the Japanese population, the favorable IL28B SNP is found in the majority of the population (approximately 75%)[4]. Therefore, our results may not be generalizable to other racial cohorts. Third, the older patients who enrolled in the study did not have any severe baseline complications, such as renal and hematological diseases. Therefore, the conclusions drawn regarding the safety of triple therapies may be limited. However, we believe that our selection of older patients for the triple therapies was appropriate and acceptable. Therefore, our findings regarding the absence of severe adverse events, even in the older patients, are important.
Treatment for CHC has been changing worldwide[30,31], and IFN-free DAA combination therapies are now available in Japan. Although the majority of CHC patients are usually treated with IFN-free DAA combination therapies, PegIFN and RBV-based therapy may still have utility in a small number of patients who do not show a favorable effect after the treatment with IFN-free DAA therapies. Moreover, considering the effect of preventing HCC by an eradication of HCV, long-term prevention of HCC has been shown only through the use of IFN-based therapies thus far[32,33]. Therefore, we believe that the present study will provide useful information regarding antiviral treatment for older patients with CHC.
In conclusion, we found that both TVR- and SMV-based triple therapies can be successfully used to treat patients aged 66 years or older with genotype 1b CHC. The IL28B genotype indicates a potential to achieve SVR in these difficult-to-treat older patients.
In Japan, an estimated 1.5-2 million people are infected with hepatitis C virus (HCV), and these patients are older than those infected in Europe and the United States. However, previous studies describing the safety and efficacy of telaprevir (TVR)- and simeprevir (SMV)-based triple therapies, especially in elderly patients with chronic HCV infections, are limited.
The patients were categorized into two groups according to age as follows: a younger group of patients aged ≤ 65 years old and an older group of patients aged > 65 years old. The rate of sustained virological response (SVR) did not significantly differ between the younger and older patients in both the TVR and SMV groups. The rate of SVR did not significantly differ between the TVR and SMV group, although the rate of rapid virological response was significantly higher in the SMV group than the TVR group. The rate of adverse events resulted in treatment discontinuation did not differ between the younger and older patients in both TVR and SMV group, although a higher frequency and severity of adverse events has been reported in patients treated with TVR-based triple therapy compared to patients treated with pegylated interferon (PegIFN) and ribavirin (RBV) dual therapy.
In this study, the authors found that both TVR- and SMV-based triple therapies can be successfully used to treat patients aged 66 years or older with genotype 1b chronic hepatitis C (CHC). The interleukin 28B genotype indicates a potential to achieve SVR in these difficult-to-treat elderly patients.
Treatment for CHC has been changing worldwide, and interferon (IFN)-free direct-acting antiviral agents (DAA) combination therapies are now available in. Although the majority of CHC patients are usually treated with IFN-free DAA combination therapies, PegIFNα and RBV-based therapy may still have utility in a small number of patients who do not show a favorable effect after the treatment with IFN-free DAA therapies. Importantly, HCV mutants that are resistant to multiple IFN-free DAA therapies have been shown to be sensitive to IFN-based therapies. Moreover, considering the effect of preventing HCC by an eradication of HCV, long-term prevention of HCC has been shown only through the use of IFN-based therapies thus far. Therefore, they believe that the present study will still provide useful information regarding antiviral treatment for older patients with CHC.
TVR: An HCV non-structural 3/4A (NS3/4A) protease inhibitor; SMV: A second-generation oral HCV NS3/4A protease inhibitor with antiviral activity against HCV genotype 1, 2, 4, 5, and 6 infections.
The manuscript is well written and it is clear.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Conti B, Kawakami Y, Larrubia JR, Liang XS S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, Hollinger FB, Gitnick G, Knodell RG, Perrillo RP. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906-1911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 492] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1796] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 3. | Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh Y, Asahina Y, Tamori A, Hiramatsu N, Hayashi N. Management of hepatitis C; Report of the Consensus Meeting at the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:347-368. [PubMed] [Cited in This Article: ] |
| 4. | Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. J Hepatol. 2013;59:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Zeuzem S. Heterogeneous virologic response rates to interferon-based therapy in patients with chronic hepatitis C: who responds less well? Ann Intern Med. 2004;140:370-381. [PubMed] [Cited in This Article: ] |
| 7. | Honda T, Katano Y, Shimizu J, Ishizu Y, Doizaki M, Hayashi K, Ishigami M, Itoh A, Hirooka Y, Nakano I. Efficacy of peginterferon-alpha-2b plus ribavirin in patients aged 65 years and older with chronic hepatitis C. Liver Int. 2010;30:527-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Furusyo N, Ogawa E, Sudoh M, Murata M, Ihara T, Hayashi T, Ikezaki H, Hiramine S, Mukae H, Toyoda K. Raloxifene hydrochloride is an adjuvant antiviral treatment of postmenopausal women with chronic hepatitis C: a randomized trial. J Hepatol. 2012;57:1186-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1866] [Cited by in F6Publishing: 1835] [Article Influence: 141.2] [Reference Citation Analysis (0)] |
| 11. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1194] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 12. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 13. | Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [PubMed] [Cited in This Article: ] |
| 15. | Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Rito K, Komada Y, Seto C. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2932] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 18. | Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Ogura Y, Izumi N, Marumo F, Sato C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 752] [Cited by in F6Publishing: 727] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 19. | Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Someya T, Kobayashi M, Saitoh S, Watahiki S, Sato J. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon-ribavirin combination therapy. Intervirology. 2005;48:372-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Hara T, Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Kumada H. A pilot study of triple therapy with telaprevir, peginterferon and ribavirin for elderly patients with genotype 1 chronic hepatitis C. J Med Virol. 2013;85:1746-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Ogawa E, Furusyo N, Kajiwara E, Nomura H, Kawano A, Takahashi K, Dohmen K, Satoh T, Azuma K, Nakamuta M. Comparative effectiveness and safety study of triple therapy with simeprevir or telaprevir for non-cirrhotic patients with chronic hepatitis C virus genotype 1b infection. J Gastroenterol Hepatol. 2015;30:1759-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Yoshizawa H, Tanaka J, Miyakawa Y. National prevention of hepatocellular carcinoma in Japan based on epidemiology of hepatitis C virus infection in the general population. Intervirology. 2006;49:7-17. [PubMed] [Cited in This Article: ] |
| 23. | Abe H, Tsubota A, Shimada N, Atsukawa M, Kato K, Takaguchi K, Asano T, Chuganji Y, Sakamoto C, Toyoda H. Predictors of response to 24-week telaprevir-based triple therapy for treatment-naïve genotype 1b chronic hepatitis C patients. Gastroenterol Res Pract. 2014;2014:549709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Pol S, Aerssens J, Zeuzem S, Andreone P, Lawitz EJ, Roberts S, Younossi Z, Foster GR, Focaccia R, Horban A. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol. 2013;58:883-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Holmes JA, Desmond PV, Thompson AJ. Does IL28B genotyping still have a role in the era of direct-acting antiviral therapy for chronic hepatitis C infection? J Viral Hepat. 2012;19:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S. Amino acid substitution in HCV core/NS5A region and genetic variation near IL28B gene affect treatment efficacy to interferon plus ribavirin combination therapy. Intervirology. 2012;55:231-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Matsuura K, Watanabe T, Iijima S, Murakami S, Fujiwara K, Orito E, Iio E, Endo M, Kusakabe A, Shinkai N. Serum interferon-gamma-inducible protein-10 concentrations and IL28B genotype associated with responses to pegylated interferon plus ribavirin with and without telaprevir for chronic hepatitis C. Hepatol Res. 2014;44:1208-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Nishikawa H, Enomoto H, Nasu A, Aizawa N, Saito M, Tamori A, Kawada N, Kimura T, Osaki Y, Nishiguchi S. Clinical significance of pretreatment serum interferon-gamma-inducible protein 10 concentrations in chronic hepatitis C patients treated with telaprevir-based triple therapy. Hepatol Res. 2014;44:E397-E407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. 2015;385:1124-1135. [PubMed] [Cited in This Article: ] |
| 31. | Fujii H, Nishimura T, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Sumida Y, Mitsuyoshi H, Yokomizo C, Tanaka S. Comparison of peg-interferon, ribavirin plus telaprevir vs simeprevir by propensity score matching. World J Hepatol. 2015;7:2841-2848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, Boyer N, Asselah T, Martinot-Peignoux M, Maylin S. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 33. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [PubMed] [Cited in This Article: ] |