Published online Oct 28, 2017. doi: 10.4254/wjh.v9.i30.1166
Peer-review started: June 19, 2017
First decision: July 20, 2017
Revised: August 3, 2017
Accepted: September 16, 2017
Article in press: September 28, 2017
Published online: October 28, 2017
Spontaneous bacterial peritonitis (SBP) is the most common infection in end-stage liver disease patients. SBP is defined as an ascitic fluid infection with a polymorphonuclear leucocyte count ≥ 250/mm3 without an evident intra-abdominal surgically treatable source. Several mechanisms contribute to SBP occurrence, including translocation of gut bacteria and their products, reduced intestinal motility provoking bacterial overgrowth, alteration of the gut’s barrier function and local immune responses. Historically, Gram-negative enteric bacteria have been the main causative agents of SBP, thereby guiding the empirical therapeutic choice. However, over the last decade, a worryingly increasing prevalence of Gram-positive and multi-drug resistant (MDR) SBP has been seen. Recently, the microbiological spectrum of SBP seems to have changed in Europe due to a high prevalence of Gram-positive bacteria (48%-62%). The overall proportion of MDR bacteria is up to 22%-73% of cases. Consequently, empirical therapy based on third-generation cephalosporins or amoxicillin/clavulanic acid, can no longer be considered the standard of care, as these drugs are associated with poor outcomes. The aim of this review is to describe, with an epidemiological focus, the evidence behind this rise in Gram-positive and MDR SBP from 2000 to present, and illustrate potential targeted therapeutic strategies. An appropriate treatment protocol should include daptomycin plus ceftaroline and meropenem, with prompt stepdown to a narrower spectrum when cultures and sensitivity data are available in order to reduce both cost and potential antibiotic resistance development.
Core tip: Spontaneous bacterial peritonitis (SBP) is the most common infection in end-stage liver disease cirrhotic patients. Over the last decade, a worryingly increasing prevalence of Gram-positive and multi-drug resistant (MDR) SBP causative bacteria has been seen. Numerous driving factors have been proposed as associated with this epidemiological change. The aim of this review is to describe, with an epidemiological focus, the evidence behind this rise in Gram-positive and MDR SBP from 2000 to present, and illustrate potential targeted therapeutic strategies. Third-generation cephalosporins should be avoided in clinical settings with a high prevalence of MDR. An appropriate treatment protocol should include daptomycin plus ceftaroline and meropenem.
- Citation: Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, Passavanti MB, Aurilio C, Pace MC. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis. World J Hepatol 2017; 9(30): 1166-1175
- URL: https://www.wjgnet.com/1948-5182/full/v9/i30/1166.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i30.1166
The development of abdominal ascites is the most frequent complication in cirrhotic patients[1], and infected ascites, better known as spontaneous bacterial peritonitis (SBP), is the most common infection in these patients, together with urinary tract infections[2]. SBP is defined as a polymorphonuclear (PMN) leucocyte count ≥ 250/mm3, with or without positive ascitic culture and the absence of other sources of sepsis in the peritoneum or adjacent tissues[3]. SBP is a distinct clinical entity, as opposed to bacteriascites (positive ascitic culture with PMN < 250/mm3, not needing therapy in cases of no accompanying symptoms) and secondary bacterial peritonitis, which are usually polymicrobial and linked to the inflammation or perforation of an abdominal organ[3].
Several mechanisms contribute to the occurrence of SBP, including translocation of gut bacteria and their products, reduction of intestinal motility provoking bacterial overgrowth, alteration of the gut’s barrier function and local immune responses[4]. These premises explain why historically Gram-negative bacteria (GNB) have been the main causative agents of SBP, thereby guiding the empirical therapeutic choice[5]. However, over the last decade the prevalence of Gram-positive bacteria (GPB) and multidrug resistant (MDR) SBP has increased worryingly[6]. Important driving factors for this epidemiological change have been the extensive use of quinolones, as a prophylactic measure, and the increasing degree of instrumentalization of patients suffering from cirrhosis[7]. Consequently, empirical therapy based on third-generation cephalosporins (3GCs) or amoxicillin/clavulanic acid, especially within a healthcare setting, can no longer be considered the standard of care due to poor outcomes[8].
Bacterial infections are the primary cause of death in patients with end-stage liver disease (ESLD), and require timely and appropriate treatment[9]. Thus, the aim of this review is to describe, with an epidemiological focus, the evidence behind this rise in Gram-positive and MDR SBP from 2000 to present, and illustrate potential targeted therapeutic strategies.
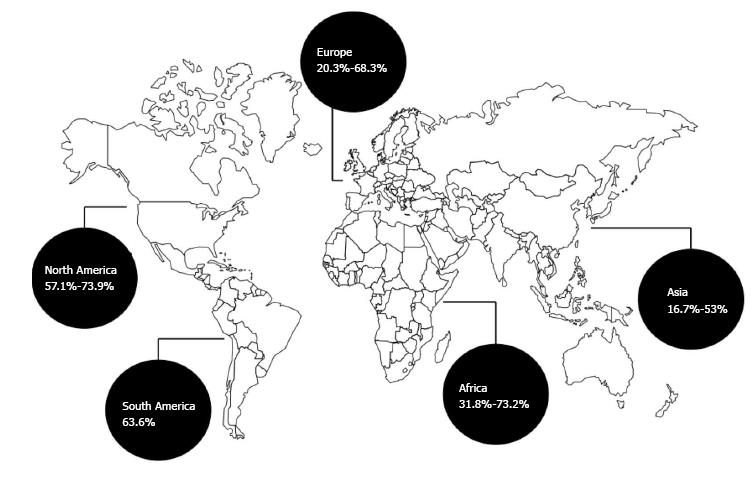
Globally, since 2000, there has been an increasing relevance of the role of GPB with respect to SBP (Figure 1). A description of this change follows, according to a geographical criterion.
Before 2000, GNB were, in consistency with the previous literature[10], the most prevalent etiologic cause of SBP in Asian cohorts of SBP patients. Then, changes occurred, but in a distinct fashion from country to country. In a retrospective study conducted in South Korea, which reviewed records of individuals diagnosed with SBP in 1995, 1998 and 1999, the rate of GPB was just 18.6% (44/237), with just 5 cases of infection by Staphylococcus aureus and 3 by Enterococcus spp., while the majority of GPB were represented by Streptococcus spp.[11]. These data were substantially confirmed by another South Korean retrospective study (episodes referring to the period from October 1998 to August 2003) that showed a proportion of GPB equal to 20.8% (22/106); no strains of S. aureus were detected, but there were 16 streptococci and 8 enterococci[12].
In a further South Korean retrospective study, which analysed cases from Janua ry 2002 to December 2004, the prevalence of GPB was also low; the bacterial isolates totalled 204 and S. aureus, Enterococcus spp. and Streptococcus spp. accounted for 3.9% (8/204), 3.9% (8/204) and 8.8% (18/204), respectively[13]. In addition, Heo et al[14] found a marginal proportion of GPB in their South Korean retrospective cohort (from June 2005 to May 2006), namely 16.7% (11/65). Interestingly, when keeping South Korean retrospective cohorts under consideration, the rate of GPB increases when data are split according to the onset of infection. Cheong et al[15] reviewing medical records from 1 January 2000 to 30 June 2007, found a relatively low number of GPB (22.9%, 54/236), but among nosocomial SBP (N-SBP; which occurred > 48 h after hospital admission), this rate was equal to 29.3% (37/126). At any rate, South Korea does not seem to be impacted by a remarkable increment of GPB. A recently published retrospective study, referring to a 10-year period (from 2005 to 2014) and comparing cases of culture-positive SBP with cases of culture-negative SBP, showed a rate of GPB equal to 25.5 (66/259), with a low number of S. aureus (2.7%, 7/259) and Enterococcus spp. (3.5%, 9/259)[16].
On the contrary, in China, the epidemiological shift towards GPB has been more apparent. In a retrospective study of 98 patients, 48 from 1996 to 2002, and 50 from 2003 to 2009, the proportion of GPB passed from 27% (13/48) to 53% (26/49); the rate of staphylococci also increased from 14% (7/48) to 37% (18/49), but with only 1 case of methicillin-resistant S. aureus (MRSA)[17]. More importantly, in-hospital mortality was greater among GPB-SBP than GNB-SBP cases (26% vs 11%, namely 7 deaths vs 2 deaths), although the result was not significant (P = 0.20)[17]. In a subsequent retrospective study conducted in China, which reviewed medical records from 2011 to 2013, Li et al[18] found a less prominent rate of GPB, equal to 27.8% (85/306), overlapping between nosocomial (27.3%, 27/99) and non-nosocomial episodes (28.0%, 58/207). Nonetheless, a worrisome percentage of MRSA stood out from this study: 37.5% (6/16) among non-nosocomial infections and, even worse, 85.7% (6/7) among nosocomial cases[18].
More recently in a Chinese study, performed to compare the microbiological profiles of N-SBP and community acquired SBP (CA-SBP), 575 strains were isolated from January 2014 to December 2014. In the CA-SBP cases, the most frequently isolated pathogens were Escherichia coli (E. coli) (27.4%), coagulase-negative staphylococci (22%), Klebsiella pneumoniae (13.7%), Enterococcus spp. (9%) and Streptococcus (8.2%). In the N-SBP, the most frequently isolated pathogens were E. coli (25.9%), coagulase-negative staphylococci (23.4%), K. pneumoniae (2.5%), Enterococcus spp. (16.6%) and Streptococcus (6.2%). In the statistical analysis, there were no significant differences in the distributions of GPB between the CA and N-SBP. In contrast, compared with the CA-SBP, the distribution of enterococci was increased in the N-SBP (9.0% vs 16.6%, P < 0.05)[19].
Different results have come from studies in other Asian countries. In Iran, a prospective study (from November 2005 to December 2007) showed a proportion of GPB equal to 27.3% (12/44)[20]. A similar result (28.6%, 90/314) was found in another study conducted in Iran (from April 2005 to September 2011)[21]. A small cohort from Pakistan showed a relatively low percentage of GPB: 25% (3/12) in a 2007 prevalence study[22].
In an Egyptian prospective cohort, the burden of GPB turned out to be as high as 73.2%, namely 30 out of 41 episodes, including 10 cases by Listeria monocytogenes[23]. In contrast, a retrospective study conducted in Nigeria, which reviewed medical records from August 2009 to July 2010, showed a much smaller proportion of GBP, which although not marginal was equal to 31.8% (7/22)[24].
In a Brazilian retrospective study referring to a 5-year period (from November 2001 to November 2006), a significant rate of GPB emerged despite the lack of a complete microbiological profile of 63 cases [Streptococcus spp. 23.8% (15/63), S. aureus 7.9% (5/63)][25]. A more recent and prospective multicentre study conducted in Argentina, from March 2011 to April 2012, showed a clear predominance of GPB over GNB [21/33 (63.6%)]; of note, the study, which aimed at investigating the potential association between proton pump inhibitors (PPIs) and SPB, showed no significant difference with regard to PPIs’ consumption and duration between patients with and without SBP (as well as with and without other infections) nor with regard to the type of bacteria[26].
A high number of GPB was found in a United States retrospective study, referring to medical records from July 2009 and November 2010: 80% (8/10), including two vancomycin-resistant enterococci (VRE)[27]. The high impact of GPB in SBP in North America has been further confirmed in a Canadian retrospective cohort that reviewed cases from February 2003 to May 2011; the data indicated that 57.1% (44/77) and 34.1% of these strains (15/44) were resistant to 3GCs (acquired or intrinsic resistance)[28].
In a prospective French study conducted from January 1996 to March 2001, GPB accounted for 68.3% (125/ 183) of ascitic fluid infections[29]. GPB cases were mainly explained by enterococci (43/125), streptococci (43/125) and S. aureus (36/125); the large majority of the latter (94.4%, 34/36) were MRSA[29]. In that study, the multivariate analysis showed that an infection provoked by S. aureus (while taking into account cases of bacteraemia) was independently linked to a higher mortality rate in cirrhotic patients (OR = 2.845, 95%CI: 1.421-5.695, P = 0.031)[29]. In France, Gram-positive cocci are today the predominant ascitic fluid microbes, with isolates ranging from 47.4%[30] to 56.1% of cases[31]. Data from a Spanish study, conducted from April 1998 to April 2000, demonstrated a low proportion of GPB (20.3%, 11/54)[32].
In a retrospective study by Ariza et al[33], reviewing medical records related to a subsequent period from 2001 to 2009, GPB rate was again relevant [35.8% (88/246)]. Surprisingly, the lowest percentage was among nosocomial infections (27.3%, 18/66) in comparison with community-acquired (36.5%, 18/85) and healthcare-related infections (41.1%, 39/95); however, the highest rate of MDR-GPB was found among nosocomial cases (27.8%, 5/18)[33]. In Italy, interesting data stem from a recently published randomized clinical trial (RCT), conducted from 2011 to 2014. The aim of that RCT was to compare ceftazidime to the combination of daptomycin plus meropenem, applied as an empirical treatment of N-SBP (in this case, defined if it occurred > 72 h after hospital admission); in particular, 62.5% (10/16) of culture-positive cases were due to GPB (8 enterococci)[34]. Of note, the broad-spectrum regimen proved to be significantly more effective with regard to the primary outcome, namely the resolution of SBP after 7 d of treatment (86.7% vs 25%; P < 0.001); that finding did not come as a surprise, in the light of the total rate of MDR bacteria [37.5% (6/16)][34].
In Germany, the growing number of GPB was already a touted issue, more than a decade ago. In a prospective cohort from 2002 to August 2006, Umgelter et al[35] found a GPB rate equal to 45.4% (20/44, 10 E. faecium). Again, in Germany, a retrospective cohort covering a 12-year period (from January 2001 to November 2011) found a predominance of GPB (53.7%, 65/121), where Enterococcus spp. (28 out of 65 GPB) played a highly relevant role[36]. In the multivariate analysis, use of antibiotics (OR = 3.875, 95%CI: 1.189-12.631, P = 0.025) and nosocomial infection (OR = 3.287, 95%CI: 1.311-8.243, P = 0.011) were the independent predictors of enterococcal infections, which were associated with higher mortality (12% probability of 90-d survival vs 50% in non-enterococcal cases, P = 0.022 by log-rank test) in case of treatment with a 3GC or a quinolone[36]. Also, in a more recent German prospective cohort, followed from March 2012 to February 2016, and focusing only on nosocomial and healthcare-related SBP, GPB were relevant [Staphylococcus spp., Enterococcus spp. and Streptococcus spp. accounted for 40% of cases (20/50)][37].
Greece was one the first countries to warn about the increasing importance of GPB-SBP. Cholongitas et al[38], in a retrospective evaluation, observed that the rate of GPB went from 25% (5/20) to 59.1% (13/22%) in two subsequent periods of time, from 1998 to 1999 and from 2000 to 2002, respectively. This trend in Greece was confirmed by another retrospective study, including cases from 2008 to May 2011, with 26 episodes out of 47 (55%) due to GPB, most of all streptococci (10 isolates), followed by 6 E. faecalis, 3 E. faecium and 2 S. aureus; neither VRE nor MRSA were detected[6].
In Denmark, a retrospective review of medical records from 2000 to 2006 showed a proportion of Gram-positive cocci, without considering other GPB, equal to 45.9% (86/187)[39].
Although some authors have previously considered the isolation of coagulase-negative staphylococci within ascitic culture as skin contamination[15,40,41], today the clinical significance of such a finding appears relevant in both nosocomial[19,34] and community acquired infections[19]. More than 40 years ago, MacGregor and Beaty[42] proposed guidelines to differentiate contamination from significant positive blood cultures in bacteraemic patients; nowadays guidelines, however, are still lacking in their ability to differentiate contamination from significant positive ascitic cultures.
In our opinion, the absence of recommendations based on solid evidence does not justify concluding isolation of coagulase-negative staphylococci as contamination[15]. Future studies are required to establish the hypothetical difference between the contaminants or pathogens.
The current guidelines rely on outdated epidemiology[43-45] and take into account neither the increasing prevalence of GPB nor the emerging phenomenon of MDR bacteria as aetiological agents of SBP[46]. Opinion leaders recommend 3GCs[47] or piperacillin/tazobactam, meropenem ± glycopeptide[1] for patients at risk of MDR SBP. The role of piperacillin/tazobactam in the treatment of life-threatening infections due to extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae is a controversial issue[47-49]; moreover, meropenem is active against ESBL-producing Enterobacteriaceae but is weakly active against Gram-positive cocci[50,51]. Glycopeptides are active against Gram-positive cocci, as well as MDR, but their use is not advisable because of their nephrotoxicity. Acute kidney injury is higher in ESLD patients, it could be related to hemodynamic instability and/or hepatorenal syndrome[52]. Furthermore, the minimum inhibitory concentration (MIC) of vancomycin appears to be shifting upwards in some institutions, a phenomenon known as MIC creep; and, where the MIC increase occurs, treatment failure is common[53,54]. Teicoplanin MIC creep has also been described; but, regardless, when it is administered intravenously it does not achieve therapeutic concentration in the ascitic fluid[55].
Antibiotics active against VRE are linezolid, tigecycline, and daptomycin. Linezolid is not recommended in the majority of ESLD and SBP patients because of high frequency thrombocytopenia[56]. A tigecycline dose adjustment is requested in patients with severe hepatic impairment[57,58]. Daptomycin is a lipopeptide active against MDR GPB, including drug-resistant and drug-susceptible S. aureus and VRE[59]. Decreased susceptibility to daptomycin has been reported in drug-resistant S. aureus; it is frequently accompanied by a paradoxical decrease in beta-lactam resistance, a process known as the “see-saw” effect. Despite the observed discordance in resistance phenotypes, the combination of daptomycin/beta-lactams has been proven clinically effective for the prevention and treatment of infections due to daptomycin-resistant S. aureus strains[60,61]. Therefore, daptomycin monotherapy should not be used for the treatment of SBP due to MRSA, unless the isolate is likely to be fully susceptible[62]. The combination of daptomycin plus ceftaroline is highly active against MRSA, the potent bactericidal activity appears to be sufficiently robust to allow rapid de-escalation to single ceftaroline with daptomycin sparing[63]. Furthermore, ceftaroline in combination with daptomycin restores daptomycin activity against daptomycin-resistant VRE strains[64]. Aminoglycoside antibiotics, especially gentamicin, are used in combination with ampicillin for the treatment of enterococcal systemic infections[65]. Despite rigorous patient monitoring, nephrotoxicity appears in 10%-25% of therapeutic courses[66]. Therefore, their use is not advisable in cirrhotic patients. In recent years, an alternative treatment with ampicillin plus ceftriaxone has proved to be safer than gentamicin in combination with ampicillin[67]. In ESLD patients, the combination of ampicillin plus ceftriaxone should be used for SBP due to enterococci, regardless of aminoglycoside resistance-level status.
The 20th century has been characterized by the dramatic effect of the large-scale use of antibiotics after their discovery, saving millions of lives[68]. Unfortunately, natural selection and misuse of antibiotics, both in human beings and in animals, have led to the development of difficult-to-treat infections by MDR bacteria, also known as superbugs, the nightmare of the new century[69]. Research efforts by pharmaceutical companies are not keeping pace with the worldwide spread of superbugs and this has prompted new strategies to optimize existing resources, such as the reviving of old antibiotics[70], the implementation of antimicrobial stewardship programs[71,72], and the judicious use of new anti-infective agents[73]. However, the epidemiology of bacterial infections has a huge inter-centre variability and the therapeutic approach should be inspired by the principle of “one size does not fit all”, which obviously also applies to SBP[74]. In other words, the current challenge is to accurately identify patients with SBP for whom empirical broad-spectrum therapy would be appropriate, with special attention to MDR-GPB in contexts where their prevalence is relevant[74].
Some risk factors are well established. The setting of acquisition (nosocomial or healthcare-related vs community-acquired) and the history of exposition to antibiotics, such as beta-lactams and/or quinolones, are probably the main ones[75,76]. Exposure to quinolones, largely used to prevent SBP in cirrhotic patients, is a significant risk factor for MRSA infections[77,78]. Moreover, antibiotics administered within the past 30 d before SBP diagnosis and a lower sepsis-related organ failure assessment (commonly known as SOFA) score proved to be significantly associated with SBP by GPB in a cohort of 77 patients[79]. The impact of MDR-GPB on SBP patient mortality is not well investigated; recently, we performed a systematic review aimed at summarizing the evidence from the literature concerning the epidemiology of nosocomial cases of SBP, in order to highlight the importance of MDR bacteria outcome; of the initial 2556 manuscripts retrieved, only 9 were included in the qualitative analysis, and a quantitative analysis on mortality was not possible[80].
Risk factors could be integrated into predictive models of mortality in individuals with SBP so as to further help identify patients in need of more aggressive therapeutic strategies from the very start of the infective process[81].
GPB are increasingly important as causative agents of SBP. In some contexts, they even supersede GNB as the main cause of this infection (Table 1 describes the main features of included studies). In parallel with this phenomenon, physicians have to face the rise of superbugs, both among GNBs and GPBs. In presence of particularly worrisome epidemiological data and other risk factors for superbug infections, a broad-spectrum empirical approach is required, encompassing antibiotics with well-established activity against pathogens, such MRSA and VRE, pending the results of microbiological tests that would allow a de-escalation strategy whenever possible.
| Ref. | Journal | Publication year | Observation time span | Study design | Country, clinical setting | Proportion of infections by GPB (%) |
| Asia - South Korea | ||||||
| Park et al[11] | J Gastroenterol Hepatol | 2003 | 1995, 1998, 1999 | RC, single centre | South Korea, University Hospital | 44/237 (18.6) |
| Song et al[12] | J Korean Med Sci | 2006 | 1998 (October) - 2003 (August) | RC, single centre | South Korea, University | 22/106 (20.8) |
| Hospital | ||||||
| Cho et al[13] | Scand J Infect Dis | 2007 | 2002-2004 | RC, single centre | South Korea, University | 34/204 (16.6) |
| Hospital | ||||||
| Heo et al[14] | Gut Liver | 2009 | 1998 (June) - 2003 (May) | RC, multicentre | South Korea | 11/65 (16.7) |
| Cheong et al[15] | Clin Infect Dis | 2009 | 2000 (January) - 2007 (June) | RC, single centre | South Korea, University | 54/236 (22.9) |
| Hospital | ||||||
| Na et al[16] | Scand J Infect Dis | 2017 | 2005-2014 | RC, single centre | South Korea, University | 66/259 (25.5) |
| Hospital | ||||||
| Asia - China | ||||||
| Gou et al[17] | Saudi Med J | 2010 | 1996-2009 | RC, single centre | China, University Hospital | 39/97 (42.2) |
| Li et al[18] | World J Gastroenterol | 2015 | 2011-2013 | RC, single centre | China, University Hospital | 85/306 (27.8) |
| Shi et al[19] | Sci Rep | 2017 | 2014 | RC, single centre | China, Tertiary Hospital | 293/575 (50.9) |
| Asia - Other countries | ||||||
| Kamani et al[20] | BMC Gastroenterol | 2008 | 2005 (November) - 2007 (December) | PC, single centre | Iran, University Hospital | 12/44 (27.3) |
| Sheikhbahaei etal[21] | Int J Hepatol | 2014 | 2005 (April) - 2011 (September) | PC, single centre | Iran, University Hospital | 90/314 (28.6) |
| Zaman et al[22] | J Ayub Med Coll Abbottabad | 2011 | 2007 | PC, single centre | Pakistan, University Hospital | 3/12 (25) |
| Africa | ||||||
| El Sayed Zaki etal[23] | J Infect Public Health | 2011 | Not provided | PC, single centre | Egypt, University Hospital | 30/41 (73.2) |
| Oladimeji et al[24] | Pan Afr Med J | 2013 | 2009 (August) - 2010 (July) | RC, single centre | Nigeria, University Hospital | 7/22 (31.8) |
| South America | ||||||
| Reginato et al[25] | Sao Paolo Med J | 2011 | 2001 (November) - 2006 (November) | RC, single centre | Brazil, Tertiary Hospital | 20/63 (31.7) |
| Terg et al[26] | J Hepatol | 2015 | 2011 (March) - 2012 (April) | PC, multicentre | Argentina | 21/33 (63.6) |
| North America | ||||||
| Tandon et al[27] | Clin Gastroenterol Hepatol | 2012 | 2009 (July) - 2010 (November) | RC, single centre | United States, University Hospital | 8/10 (80) |
| Chaulk et al[28] | Can J Gastroenterol Hepatol | 2014 | 2003 (February) - 2010 (May) | RC, single centre | Canada, Tertiary Hospital | 44/77 (57.1) |
| Europe | ||||||
| Campillo et al[29] | Clin Infect Dis | 2002 | 1996 (January) - 2001 (March) | PC, single centre | France, Tertiary Hospital | 125/183 (68.3) |
| Piroth et al[31] | BMC Infect Dis | 2014 | 2010-2011 | PC, multicentre | France, University Hospitals | 32/57 (56.1) |
| Thévenot et al[30] | Am J Gastroenterol | 2016 | 2014 (March) – 2015 (August) | PC, multicentre | France | 40/84 (47.4) |
| Fernández et al[32] | Hepatology | 2002 | 1998 (April) - | PC, single centre | Spain, University Hospital | 11/54 (20.3) |
| 2000 (April) | ||||||
| Ariza et al[33] | J Hepatol | 2012 | 2001-2009 | RC, single centre | Spain, University Hospital | 88/246 (35.8) |
| Piano et al[34] | Hepatology | 2016 | 2011-2014 | RCT, multicentre | Italy | 10/16 (62.5) |
| Umgelter et al[35] | Infection | 2009 | 2002 (January) - 2006 (August) | PC, single centre | Germany, University Hospital | 20/44 (45.4) |
| Reuken et al[36] | Aliment Pharmacol Ther | 2009 | 2002 (January) - 2011 (November) | RC, single centre | Germany, Tertiary Hospital | 65/121 (53.7) |
| Lutz et al[37] | Eur J Clin Invest | 2017 | 2012 (March) - 2016 (February) | PC, single centre | Germany, University Hospital | 20/50 (40) |
| Cholongitas etal[38] | Liver Int | 2005 | 1998-200 | RC, single centre | Greece, University Hospital | 18/42 (42.9) |
| Novocic et al[39] | Scand J Gastroenterol | 2012 | 2000-2006 | RC, multicentre | Denmark, University Hospitals | 86/187 (45.9) |
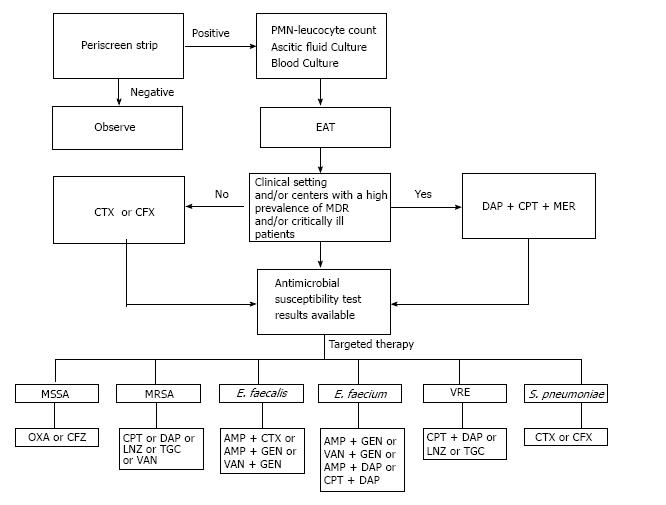
On the basis of the current literature, we propose a treatment algorithm for SBP due to GPB (Figure 2). If an ESLD patient with ascites is “symptomatic” for SBP (temperature above 38 °C or below 36.5 °C, chills, abdominal tenderness, arterial hypotension, developing or worsening hepatic encephalopathy, gastrointestinal bleeding within the previous 15 d) it is necessary to perform a Periscreen strip on the ascitic fluid[30]. If the Periscreen strip is positive the patient requires immediate hospitalization with comparison of this result with cytology and immediate microbiological cultures. A culture of ascitic fluid and blood should systematically be carried out at the bedside[34]. Empiric antibacterial therapy (EAT) should be initiated after obtaining appropriate cultures. 3GCs should not be used in clinical settings and/or centres with a high prevalence of MDR bacteria. ESLD patients with SBP in clinical settings and/or centres with a high prevalence of VRE, MRSA and ESBL should immediately receive broad-spectrum EAT[82]. An appropriate treatment protocol should include daptomycin plus ceftaroline and meropenem[83]. When the culture is positive and susceptibility data are available, an antibiotic with a narrower spectrum should be promptly initiated (early de-escalation strategy); this strategy limits the selection of antibiotic resistances and saves on costs[83].
The authors are deeply grateful to Miss Lucy Hedley (MPharm, MFRPSI, MRPharmS, PGDipGPP, IPresc), Senior Clinical Pharmacist - HIV and Infectious Diseases at University College London Hospitals NHS Foundation Trust (London, Greater London, Great Britain) for her valuable pro bono help in revising the manuscript in order to improve and polish language. The authors are also grateful to Dr. Luisa Maria Roberta Tedesco, Department of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy for providing, pro bono, excellent bibliographic service and assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bock CT, Chiu KW, Enomoto H S- Editor: Kong JX L- Editor: Filipodia E- Editor: Lu YJ
| 1. | Solà E, Solé C, Ginès P. Management of uninfected and infected ascites in cirrhosis. Liver Int. 2016;36 Suppl 1:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 580] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 3. | Mowat C, Stanley AJ. Review article: spontaneous bacterial peritonitis--diagnosis, treatment and prevention. Aliment Pharmacol Ther. 2001;15:1851-1859. [PubMed] [Cited in This Article: ] |
| 4. | Pericleous M, Sarnowski A, Moore A, Fijten R, Zaman M. The clinical management of abdominal ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: a review of current guidelines and recommendations. Eur J Gastroenterol Hepatol. 2016;28:e10-e18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Fagiuoli S, Colli A, Bruno R, Burra P, Craxì A, Gaeta GB, Grossi P, Mondelli MU, Puoti M, Sagnelli E. Management of infections in cirrhotic patients: report of a consensus conference. Dig Liver Dis. 2014;46:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, Pectasides D. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33:975-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Acevedo J. Multiresistant bacterial infections in liver cirrhosis: Clinical impact and new empirical antibiotic treatment policies. World J Hepatol. 2015;7:916-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Merli M, Lucidi C, Di Gregorio V, Falcone M, Giannelli V, Lattanzi B, Giusto M, Ceccarelli G, Farcomeni A, Riggio O. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PLoS One. 2015;10:e0127448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 10. | Garcia-Tsao G. Spontaneous bacterial peritonitis. Gastroenterol Clin North Am. 1992;21:257-275. [PubMed] [Cited in This Article: ] |
| 11. | Park YH, Lee HC, Song HG, Jung S, Ryu SH, Shin JW, Chung YH, Lee YS, Suh DJ. Recent increase in antibiotic-resistant microorganisms in patients with spontaneous bacterial peritonitis adversely affects the clinical outcome in Korea. J Gastroenterol Hepatol. 2003;18:927-933. [PubMed] [Cited in This Article: ] |
| 12. | Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ, Cheong HJ. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community acquired. J Korean Med Sci. 2006;21:666-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Cho JH, Park KH, Kim SH, Bang JH, Park WB, Kim HB, Kim NJ, Oh MD, Lee HS, Choe KW. Bacteremia is a prognostic factor for poor outcome in spontaneous bacterial peritonitis. Scand J Infect Dis. 2007;39:697-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Heo J, Seo YS, Yim HJ, Hahn T, Park SH, Ahn SH, Park JY, Park JY, Kim MY, Park SK. Clinical features and prognosis of spontaneous bacterial peritonitis in korean patients with liver cirrhosis: a multicenter retrospective study. Gut Liver. 2009;3:197-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Na SH, Kim EJ, Nam EY, Song KH, Choe PG, Park WB, Bang JH, Kim ES, Park SW, Kim HB. Comparison of clinical characteristics and outcomes of spontaneous bacterial peritonitis and culture negative neutrocytic ascites. Scand J Gastroenterol. 2017;52:199-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Gou YZ, Liu B, Pan L, Yu HT, Wang JP, Wang DC. Pathogens of spontaneous bacterial peritonitis change in northern China. Saudi Med J. 2010;31:1152-1156. [PubMed] [Cited in This Article: ] |
| 18. | Li YT, Yu CB, Huang JR, Qin ZJ, Li LJ. Pathogen profile and drug resistance analysis of spontaneous peritonitis in cirrhotic patients. World J Gastroenterol. 2015;21:10409-10417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 19. | Shi L, Wu D, Wei L, Liu S, Zhao P, Tu B, Xie Y, Liu Y, Wang X, Liu L. Nosocomial and Community-Acquired Spontaneous Bacterial Peritonitis in patients with liver cirrhosis in China: Comparative Microbiology and Therapeutic Implications. Sci Rep. 2017;7:46025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kamani L, Mumtaz K, Ahmed US, Ali AW, Jafri W. Outcomes in culture positive and culture negative ascitic fluid infection in patients with viral cirrhosis: cohort study. BMC Gastroenterol. 2008;8:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Sheikhbahaei S, Abdollahi A, Hafezi-Nejad N, Zare E. Patterns of antimicrobial resistance in the causative organisms of spontaneous bacterial peritonitis: a single centre, six-year experience of 1981 samples. Int J Hepatol. 2014;2014:917856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zaman A, Kareem R, Mahmood R, Hameed K, Khan EM. Frequency of microbial spectrum of spontaneous bacterial peritonitis in established cirrhosis liver. J Ayub Med Coll Abbottabad. 2011;23:15-17. [PubMed] [Cited in This Article: ] |
| 23. | El Sayed Zaki M, El Shabrawy WO, El-Eshmawy MM, Aly Eletreby S. The high prevalence of Listeria monocytogenes peritonitis in cirrhotic patients of an Egyptian Medical Center. J Infect Public Health. 2011;4:211-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Oladimeji AA, Temi AP, Adekunle AE, Taiwo RH, Ayokunle DS. Prevalence of spontaneous bacterial peritonitis in liver cirrhosis with ascites. Pan Afr Med J. 2013;15:128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Reginato TJ, Oliveira MJ, Moreira LC, Lamanna A, Acencio MM, Antonangelo L. Characteristics of ascitic fluid from patients with suspected spontaneous bacterial peritonitis in emergency units at a tertiary hospital. Sao Paulo Med J. 2011;129:315-319. [PubMed] [Cited in This Article: ] |
| 26. | Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, Niveyro C, Benavides J, Marino M, Colombato L. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62:1056-1060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Chaulk J, Carbonneau M, Qamar H, Keough A, Chang HJ, Ma M, Kumar D, Tandon P. Third-generation cephalosporin-resistant spontaneous bacterial peritonitis: a single-centre experience and summary of existing studies. Can J Gastroenterol Hepatol. 2014;28:83-88. [PubMed] [Cited in This Article: ] |
| 29. | Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Thévenot T, Briot C, Macé V, Lison H, Elkrief L, Heurgué-Berlot A, Bureau C, Jézéquel C, Riachi G, Louvet A. The Periscreen Strip Is Highly Efficient for the Exclusion of Spontaneous Bacterial Peritonitis in Cirrhotic Outpatients. Am J Gastroenterol. 2016;111:1402-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Piroth L, Pechinot A, Di Martino V, Hansmann Y, Putot A, Patry I, Hadou T, Jaulhac B, Chirouze C, Rabaud C. Evolving epidemiology and antimicrobial resistance in spontaneous bacterial peritonitis: a two-year observational study. BMC Infect Dis. 2014;14:287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 661] [Cited by in F6Publishing: 596] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 33. | Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, Cavallin M, Gola E, Sticca A, Loregian A. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: Results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 35. | Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009;37:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Reuken PA, Pletz MW, Baier M, Pfister W, Stallmach A, Bruns T. Emergence of spontaneous bacterial peritonitis due to enterococci - risk factors and outcome in a 12-year retrospective study. Aliment Pharmacol Ther. 2012;35:1199-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Lutz P, Nischalke HD, Krämer B, Goeser F, Kaczmarek DJ, Schlabe S, Parcina M, Nattermann J, Hoerauf A, Strassburg CP. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017;47:44-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Cholongitas E, Papatheodoridis GV, Lahanas A, Xanthaki A, Kontou-Kastellanou C, Archimandritis AJ. Increasing frequency of Gram-positive bacteria in spontaneous bacterial peritonitis. Liver Int. 2005;25:57-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 39. | Novovic S, Semb S, Olsen H, Moser C, Knudsen JD, Homann C. First-line treatment with cephalosporins in spontaneous bacterial peritonitis provides poor antibiotic coverage. Scand J Gastroenterol. 2012;47:212-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Sewell CM, Clarridge JE, Young EJ, Guthrie RK. Clinical significance of coagulase-negative staphylococci. J Clin Microbiol. 1982;16:236-239. [PubMed] [Cited in This Article: ] |
| 41. | Kim SU, Chon YE, Lee CK, Park JY, Kim DY, Han KH, Chon CY, Kim S, Jung KS, Ahn SH. Spontaneous bacterial peritonitis in patients with hepatitis B virus-related liver cirrhosis: community-acquired versus nosocomial. Yonsei Med J. 2012;53:328-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | MacGregor RR, Beaty HN. Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med. 1972;130:84-87. [PubMed] [Cited in This Article: ] |
| 43. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1125] [Cited by in F6Publishing: 1070] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 44. | Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 488] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 45. | Esposito S, Leone S, Carosi G. Analysis of current guidelines for intra-abdominal infections. J Chemother. 2009;21 Suppl 1:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Fiore M. Letter: the emergence of multi-drug resistant spontaneous bacterial peritonitis: a new challenge for the hepatologist? Aliment Pharmacol Ther. 2016;43:944-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis--bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41:1116-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Tamma PD, Rodriguez-Bano J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin Infect Dis. 2017;64:972-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 49. | Retamar P, López-Cerero L, Muniain MA, Pascual Á, Rodríguez-Baño J; ESBL-REIPI/GEIH Group. Impact of the MIC of piperacillin-tazobactam on the outcome of patients with bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2013;57:3402-3404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Edwards JR. Meropenem: a microbiological overview. J Antimicrob Chemother. 1995;36 Suppl A:1-17. [PubMed] [Cited in This Article: ] |
| 51. | Leone S, Bisi L, Rossi M, Gori A. Comment on “Management of infections in cirrhotic patients: report of a consensus conference” S Fagiuoli et al. [Dig liver dis 2014;46:204-212]. Dig Liver Dis. 2014;46:573-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014;7:457-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Gould IM. Treatment of bacteraemia: meticillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA). Int J Antimicrob Agents. 2013;42 Suppl:S17-S21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Ippolito G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Methicillin-resistant Staphylococcus aureus: the superbug. Int J Infect Dis. 2010;14 Suppl 4:S7-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 55. | Stamatiadis D, Papaioannou MG, Giamarellos-Bourboulis EJ, Marinaki S, Giamarellou H, Stathakis CP. Pharmacokinetics of teicoplanin in patients undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 2003;23:127-131. [PubMed] [Cited in This Article: ] |
| 56. | Zhang YM, Yu W, Zhou N, Li JZ, Xu LC, Xie ZY, Lu YF, Li LJ. High frequency of thrombocytopenia in patients with acute-on-chronic liver failure treated with linezolid. Hepatobiliary Pancreat Dis Int. 2015;14:287-292. [PubMed] [Cited in This Article: ] |
| 57. | Leone S, Rossi M, Bisi L, Gori A, Esposito S. Letter: antibiotic dose adjustment in patients with advanced liver disease. Aliment Pharmacol Ther. 2013;38:561-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Noviello S, Ianniello F, Leone S, Fiore M, Esposito S. In vitro activity of tigecycline: MICs, MBCs, time-kill curves and post-antibiotic effect. J Chemother. 2008;20:577-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Eisenstein BI, Oleson FB Jr, Baltz RH. Daptomycin: from the mountain to the clinic, with essential help from Francis Tally, MD. Clin Infect Dis. 2010;50 Suppl 1:S10-S15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 60. | Renzoni A, Kelley WL, Rosato RR, Martinez MP, Roch M, Fatouraei M, Haeusser DP, Margolin W, Fenn S, Turner RD. Molecular Bases Determining Daptomycin Resistance-Mediated Resensitization to β-Lactams (Seesaw Effect) in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2016;61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Leone S, Noviello S, Boccia G, De Caro F, Esposito S. Methicillin-resistant Staphylococcus aureus infections: role of daptomycin/β-lactams combination. Infez Med. 2015;23:99-104. [PubMed] [Cited in This Article: ] |
| 62. | Fiore M, Andreana L. The Possible Role of Anti-Methicillin-Resistant Staphylococcus Aureus Antimicrobial Agents in Spontaneous Bacterial Peritonitis. Infect Dis Rep. 2015;7:6286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Barber KE, Werth BJ, Rybak MJ. The combination of ceftaroline plus daptomycin allows for therapeutic de-escalation and daptomycin sparing against MRSA. J Antimicrob Chemother. 2015;70:505-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 2014;58:1494-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Leone S, Noviello S, Esposito S. Combination antibiotic therapy for the treatment of infective endocarditis due to enterococci. Infection. 2016;44:273-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 396] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 67. | Pericas JM, Cervera C, del Rio A, Moreno A, Garcia de la Maria C, Almela M, Falces C, Ninot S, Castañeda X, Armero Y. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect. 2014;20:O1075-O1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675-685. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N Engl J Med. 2009;360:439-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 689] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 70. | Theuretzbacher U, Van Bambeke F, Cantón R, Giske CG, Mouton JW, Nation RL, Paul M, Turnidge JD, Kahlmeter G. Reviving old antibiotics. J Antimicrob Chemother. 2015;70:2177-2181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, Srinivasan A, Dellit TH, Falck-Ytter YT, Fishman NO. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51-e77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1887] [Cited by in F6Publishing: 1763] [Article Influence: 220.4] [Reference Citation Analysis (0)] |
| 72. | Leone S, Stefani S, Venditti M, Grossi P, Colizza S, De Gasperi A, Scaglione F, Sganga G, Esposito S; Italian Intra-abdominal Infections Working Group. Intra-abdominal infections: model of antibiotic stewardship in an era with limited antimicrobial options. Int J Antimicrob Agents. 2011;38:271-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 73. | Gentile I, Maraolo AE, Borgia G. What is the role of the new β-lactam/β-lactamase inhibitors ceftolozane/tazobactam and ceftazidime/avibactam? Expert Rev Anti Infect Ther. 2016;14:875-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Ison MG. Empiric treatment of nosocomial spontaneous bacterial peritonitis: One size does not fit all. Hepatology. 2016;63:1083-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Fernandez J, Arroyo V. Bacterial Infections in Cirrhosis: A Growing Problem with Significant Implications. Clin Liver Dis. 2013;2:102-105. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Esposito S, Capuano A, Noviello S, Mazzeo F, Ianniello F, Filippelli A, Rossi F, Leone S. Modification of patients’ endogenous bacterial flora during hospitalization in a large teaching hospital in Naples. J Chemother. 2003;15:568-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Weber SG, Gold HS, Hooper DC, Karchmer AW, Carmeli Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9:1415-1422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 248] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 78. | Couderc C, Jolivet S, Thiébaut AC, Ligier C, Remy L, Alvarez AS, Lawrence C, Salomon J, Herrmann JL, Guillemot D; Antibiotic Use and Staphylococcus aureus Resistant to Antibiotics (ASAR) Study Group. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis. 2014;59:206-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Kim JH, Jeon YD, Jung IY, Ahn MY, Ahn HW, Ahn JY, Ku NS, Han SH, Choi JY, Ahn SH. Predictive Factors of Spontaneous Bacterial Peritonitis Caused by Gram-Positive Bacteria in Patients With Cirrhosis. Medicine (Baltimore). 2016;95:e3489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, Passavanti MB, Aurilio C, Pace MC. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: A systematic review. World J Gastroenterol. 2017;23:4654-4660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 81. | Poca M, Alvarado-Tapias E, Concepción M, Pérez-Cameo C, Cañete N, Gich I, Romero C, Casas M, Román E, Castells L. Predictive model of mortality in patients with spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2016;44:629-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Fiore M, Andreana L, Leone S. Treatment of spontaneous bacterial peritonitis: beyond the current international guidelines. Liver Int. 2016;36:918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Fiore M. Spontaneous bacterial peritonitis due to multidrug resistant bacteria: are the current guidelines outdated? Eur J Gastroenterol Hepatol. 2016;28:731. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Fiore M. Nosocomial spontaneous bacterial peritonitis: discussing a specific infection treatment algorithm. Liver Int. 2016;36:1074-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |