Peer-review started: August 29, 2016
First decision: September 27, 2016
Revised: October 27, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: January 18, 2017
Processing time: 142 Days and 1 Hours
Over the last decade, the role of renin-angiotensin system (RAS) on the development of obesity and its comorbidities has been extensively addressed. Both circulating and local RAS components are up-regulated in obesity and involved in non-alcoholic fatty liver disease onset. Pharmacological manipulations of RAS are viable strategies to tackle metabolic impairments caused by the excessive body fat mass. Renin inhibitors rescue insulin resistance, but do not have marked effects on hepatic steatosis. However, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ARB) yield beneficial hepatic remodeling. ARBs elicit body mass loss and normalize insulin levels, tackling insulin resistance. Also, this drug class increases adiponectin levels, besides countering interleukin-6, tumoral necrosis factor-alpha, and transforming growth factor-beta 1. The latter is essential to prevent from liver fibrosis. When conjugated with peroxisome proliferator-activated receptor (PPAR)-alpha activation, ARB fully rescues fatty liver. These effects might be orchestrated by an indirect up-regulation of MAS receptor due to angiotensin II receptor type 1 (AT1R) blockade. These associations of ARB with PPAR activation and ACE2-angiotensin (ANG) (1-7)-MAS receptor axis deserve a better understanding. This editorial provides a brief overview of the current knowledge regarding AT1R blockade effects on sensitivity to insulin and hepatic structural alterations as well as the intersections of AT1R blockade with peroxisome proliferator-activated receptor activation and ACE2-ANG (1-7) - MAS receptor axis.
Core tip: Intrahepatic renin-angiotensin system activation contributes to insulin resistance and non-alcoholic fatty liver disease onset. ANG II interaction with angiotensin II receptor type 1 (AT1R) mediates pro-inflammatory and pro-fibrogenic responses, besides enhancing the oxidative stress, which makes the liver more prone to noxious liver diseases. AT1R blockers mitigate insulin resistance and fatty liver by enhancing beta-oxidation, reducing lipogenesis and controlling inflammation. The impact of the AT1R blockade on liver ACE2-angiotensin (1-7)-MAS receptor axis remains to be fully unraveled.
- Citation: Souza-Mello V. Hepatic structural enhancement and insulin resistance amelioration due to AT1 receptor blockade. World J Hepatol 2017; 9(2): 74-79
- URL: https://www.wjgnet.com/1948-5182/full/v9/i2/74.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i2.74
Liver injuries can result from virus infection, alcohol and/or drugs abuse, and autoimmune diseases[1]. However, the increase in high-energy dense food availability combined with a sedentary lifestyle brought up unprecedented obesity rates, with the consequent increase in its comorbidities (hypertension, type 2 diabetes, and dyslipidemias) prevalences[2]. The metabolic disturbances caused by obesity also impair liver structure and physiology, with increasing prevalence of non-alcoholic fatty liver disease (NAFLD) and greater susceptibility to more harmful types of liver diseases such as non-alcoholic steatohepatitis (NASH) and liver fibrosis[3,4].
Insulin resistance (IR) plays a central role in NAFLD pathogenesis[5]. Also, the low-grade inflammation observed in obese subjects and the increased adipocyte lipolysis are key factors for a more pronounced lipid droplet deposition within the hepatocytes[6]. Briefly, IR has opposite effects on adipose tissue and liver. On one hand, resistance to insulin action elicits enhanced lipolysis rate in the white adipose tissue as an attempt to compensate for the lack of glucose to be used as fuel by the adipocytes. Hence, increased free fatty acids (FFAs) are delivered to the liver[3,7]. On the other hand, insulin resistance impairs beta-oxidation within hepatocytes by reducing the expression of carnitine palmitoyltransferase 1 in the hepatic mitochondrion, besides reducing very low-density lipoprotein (VLDL) secretion. These conditions lead to unbalanced hepatic lipid metabolism as FFAs inflow surpasses fatty acid oxidation and lipoprotein exportation[8,9]. Therefore, excessive fatty acids are converted into triglycerides through the up-regulated lipogenic pathways, which accumulate as lipid droplets within hepatic parenchyma, characterizing the NAFLD[10].
Considering that NAFLD is currently considered as the hepatic manifestation of the metabolic syndrome and that this condition, despite benign at first, can initiate a harmful spectrum of liver diseases, treatments should target hepatic alterations, but also alleviate others comorbidities such as hypertension, inflammation, and insulin resistance[11,12]. Recently, the activation of a local renin-angiotensin system (RAS) in the liver has been linked to NAFLD onset and progression towards liver fibrosis[13]. In this way, the RAS emerges as a potential target to tackle hepatic alterations stemmed from obesity and other metabolic constraints imposed by increased body fat mass[14].
From a classical view, the circulating RAS is implicated in the systemic hemodynamic regulation. Briefly, under a reduced renal perfusion, renin is secreted by the juxtaglomerular apparatus. This enzyme converts angiotensinogen (produced by the liver) in angiotensin 1 (ANG I), which is converted into angiotensin 2 (ANG II) by the angiotensin-converting enzyme (ACE). ANG II has countless physiological effects such as the stimulation of aldosterone release, which promptly reestablishes the hemodynamic control by enhancing water and sodium retention in the kidneys[15,16].
ANG II exerts its main effects by interacting with two main receptors: Angiotensin II receptor type 1 (AT1R) or angiotensin II receptor type 2 (AT2R). AT1R has an important role in tissue repair and cell proliferation. However, when overexpressed, mediates pro-inflammatory and pro-atherogenic effects. Conversely, AT2R has anti-inflammatory effects, mainly by down-regulating tumoral necrosis factor-alpha (TNF-alpha) and nuclear factor-kappa B (NF-KB) pathways and by exerting anti-fibrogenic properties, besides reducing oxidative stress and cell proliferation[17,18]. Bearing this in mind, the angiotensin receptor blockers (ARBs) represents an evolution of the ACE inhibitors as they block exclusively the actions mediate by the interaction of ANG II with the AT1R[19,20]. Thus, important physiological effects stemmed from ANG II interaction with AT2R are maintained, leading to reduced atherogenesis, greater cardiac and endocrine pancreas functions, reduced glomerulosclerosis and fatty liver[21,22].
Recently, with the discovery of ACE2, another branch of RAS has been described. ACE2 converts ANG II to ANG (1-7) and cleaves ANG I into ANG (1-9), which is also converted to ANG (1-7) by ACE. ANG (1-7) exerts its physiological effects through the MAS receptor. It can be argued that [ACE2-ANG (1-7)-MAS axis] counters the (ACE - ANG II-AT1R axis) effects. So, ACE2/ACE balance is an important target to tackle metabolic diseases[23-25].
Lately, apart from this circulating RAS, many local RAS have been described in organs such as heart, pancreas, adipose tissue, skeletal muscle, and liver[17,26,27]. Animal models of obesity show raised circulating renin, angiotensinogen, and ANG II[28], besides higher expression of ACE and AT1R in the pancreas, which inhibit important steps of the insulin signaling cascade and contribute to IR and type 2 diabetes onset[29,30]. Intrahepatic activation of RAS favors NAFLD onset as it elicits greater triglycerides accumulation due to impaired beta-oxidation in conjunction with a significant fall in VLDL secretion. These conditions comply with the increase of de novo lipogenesis (the formation of fatty acids from excessive dietary carbohydrate)[27,31]. Concomitantly, the increased production of reactive oxygen species by mitochondria and the raised expression of pro-inflammatory cytokines contribute to the progression to NASH[22]. These effects are mainly mediated by higher expression of ACE, ANG II, and AT1R concomitant to reduced ACE2 tissue expression in the hepatocytes of obese mice[32].
Moreover, ANG II activates hepatic stellate cells (HSCs). Enhanced transforming growth factor-beta 1 (TGF-beta1) underlies this event, which implies a higher susceptibility to hepatic fibrosis, once HSCs acquire a myofibroblast phenotype[33,34]. These harmful effects of ANG II on liver structure and function are mediated predominantly by its interaction with the AT1R and results in collagen synthesis, pro-inflammatory cytokines release, stimulation of cell migration and proliferation[27,35]. These events altogether contribute to the second hit proposed by the two-hit theory, where inflammation and fibrogenesis play a decisive role in NAFLD progression to NASH[36].
Obese mice show higher hepatic steatosis rate coupled with insulin resistance, a pro-inflammatory adipokine profile, reduced hepatic beta-oxidation of fatty acids and enhanced lipogenesis[37]. Recently, it has been shown that a mouse model of NAFLD, even without obesity, presents with enhanced ACE/AT1R expression locally in the liver[38]. Rats with liver fibrosis present with favored ACE-ANGII-AT1R axis over ACE2-ANG (1-7)-MAS receptor axis, confirming that AT1R is involved with NAFLD progression to NASH and fibrosis[24,25]. These observations suggest that the local expression of AT1R is related to NASH onset and AT1R blockade, with the consequent ACE2 induction, emerging as a potential approach to prevent liver fibrosis and chronic inflammation.
The impact of pharmacological manipulations of the RAS system on insulin resistance and liver structure is a new field of study. Evidence from animal studies shows that aliskiren (a direct renin inhibitor) rescued insulin resistance and hepatic steatosis, though its effects are not more advantageous than ARBs[39,40].
Angiotensin-converting enzyme inhibitors (ACEi) inhibit ANGI to ANGII conversion and, therefore, enhances the availability of bradykinin[41]. This peptide yields cardiovascular protection by stimulating the release of important vasodilatators such as nitric oxide and prostacyclin[42]. Bradikinin reduces the hepatic expression of glucose-6 - phosphatase and phosphoenolpyruvate carboxykinase, inhibiting hepatic gluconeogenesis. Furthermore, isolated myocytes and adipocytes treated with bradykinin exhibited improved glucose uptake due to greater glucose transporter 4 translocation to the cell membrane[43]. These events show that by enhancing bradykinin availability, ACEi are able to mitigate insulin resistance and counter NAFLD. Even though ACEi represent a potent approach as it combines benefits from bradykinin and ANGII inhibition, ARBs preserve AT2R-mediated benefits and favor ACE2-ANG (1-7)-MAS receptor axis. These properties make ARBs an attractive option to treat metabolic impairments.
Olmesartan, a pure ARB, reduced body mass and hepatic triglyceride content, besides recovering the expression of hepatic antioxidant enzymes and sensitivity to insulin in rats[44,45]. The recovery of uncoupling protein 2 expression is put forward as the main mechanism that enhances hepatic lipid metabolization and antioxidant capacity after the blockade of AT1R[44]. Amelioration of IR after olmesartan treatment is also perceived in humans[46].
Irbesartan, another ARB, and an ACEi (perindopril) prevented obese Zucker rats from developing fatty liver in a recent study. Both treatments elicited a marked reduction in hepatic steatosis percentage, with no difference with the lean control group[47]. A remarkable reduction in hepatic expression of TNF-alpha, interleukine-6, and TGF-beta1 is produced by enhanced ACE2-ANG (1-7)-MAS receptor, leading to the alleviation of hepatic IR and, consequently, reducing fatty liver[25]. Furthermore, low TGF-beta1 expression complies with the marked reduction in liver fibrosis in obese animals treated with irbesartan[44]. In agreement to this, losartan, an ARB, led to anti-proliferative and anti-fibrogenic effects in ANG II stimulated HSCs in vitro. Once again, a marked reduction in TGF-beta1 expression and AT1R down-regulation explain these findings[48].
It was recently proposed a synergistic action between hepatic cholesterol metabolism and intrahepatic RAS activation in the physiopathology of NAFLD. In this context, chronic local RAS activation in the liver augments the extracellular matrix synthesis and disrupts LDL metabolism by impairing LDL receptor functioning. These alterations seem to rely on AT1R activation by ANG II. In agreement to this, telmisartan, an ARB that is also a partial peroxisome proliferator-activated receptor (PPAR)-gamma agonist, prevented from lipid deposition and overrode the translocation of SCAP/SREBP-2 complex from the endoplasmic reticulum to Golgi, blocking LDL receptor gene transcription in HepG2 cells[31].
Animal studies show that telmisartan rescues the sensitivity to insulin, markedly reduces hepatic steatosis and augments the numerical density of mitochondria per area of hepatic tissue in diet-induced obese mice[49]. These events rely on PPAR-alpha activation in the liver coupled with dual AT1R blockade/partial PPAR-gamma agonist properties, which determine enhanced adiponectin levels, favored beta-oxidation over lipogenesis and reduced HSCs activity[49,50].
Also, telmisartan limits hepatic fibrosis by enhancing mRNA levels of ACE2 and MAS receptor concomitant to reducing ACE, AT1R, collagen type II and TGF-beta1, besides blocking HSCs activation in bile duct-ligated rats[51]. However, some effects are stemmed from the partial PPAR-gamma agonist property, such as IR alleviation, reduced oxidative stress, and hepatic lipid deposition[52].
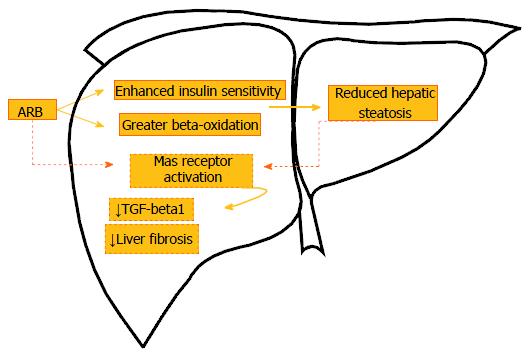
It is likely that the favored activity of the ACE2-ANG (1-7)-MAS receptor action under the AT1R blockade mediates the beneficial findings[25]. With regard to this, the infusion of ANG (1-7) in bile duct-ligated rats elicited fibrosis attenuation by the suppression of HSCs activity, while the use of MAS receptor antagonist confirmed these findings as the animals presented with a maximization of liver fibrosis, supported by higher expression of collagen and TGF-beta1[53]. Figure 1 illustrates the main pathways related to ARBs effects on the liver.
Increasing rates of obesity and NAFLD have drawn the attention of the scientific community to strategies to treat these metabolic diseases. Local RAS is up-regulated in the liver from obese individuals and in lean individuals with fatty liver. Among the pharmacological manipulations of RAS, AT1R blockade is considered the best approach as it favors AT2R effects and seems to activate indirectly the ACE2-ANG (1-7)-MAS receptor axis, with additional beneficial effects. The combination of AT1R blockers with oral ANG (1,7) treatment seems to be a promising approach to treating NAFLD and NASH and prevent liver fibrosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu SH, Shimada Y S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, Kim HK, Han JY, Cha DR. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008;74:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab. 2015;66 Suppl 2:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 447] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65:S57-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Gustafson B, Smith U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis. 2015;241:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015;26:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 8. | Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 426] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Magliano DC, Bargut TC, de Carvalho SN, Aguila MB, Mandarim-de-Lacerda CA, Souza-Mello V. Peroxisome proliferator-activated receptors-alpha and gamma are targets to treat offspring from maternal diet-induced obesity in mice. PLoS One. 2013;8:e64258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7:846-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (5)] |
| 11. | den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4482] [Article Influence: 224.1] [Reference Citation Analysis (0)] |
| 13. | Zhang W, Miao J, Li P, Wang Y, Zhang Y. Up-regulation of components of the renin-angiotensin system in liver fibrosis in the rat induced by CCL4. Res Vet Sci. 2013;95:54-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Day CP. Clinical spectrum and therapy of non-alcoholic steatohepatitis. Dig Dis. 2012;30 Suppl 1:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system--focusing on the vascular system. Peptides. 2011;32:2141-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77:I4-13. [PubMed] |
| 17. | Thatcher S, Yiannikouris F, Gupte M, Cassis L. The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol. 2009;302:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Pershadsingh HA. Dual Peroxisome Proliferator-Activated Receptor-alpha/gamma Agonists : In the Treatment of Type 2 Diabetes Mellitus and the Metabolic Syndrome. Treat Endocrinol. 2006;5:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Ostergren J. Renin-angiotensin-system blockade in the prevention of diabetes. Diabetes Res Clin Pract. 2007;76 Suppl 1:S13-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Gawrieh S, Chalasani N. Pharmacotherapy for Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Casillas-Ramirez A, Amine-Zaouali M, Massip-Salcedo M, Padrissa-Altés S, Bintanel-Morcillo M, Ramalho F, Serafín A, Rimola A, Arroyo V, Rodés J. Inhibition of angiotensin II action protects rat steatotic livers against ischemia-reperfusion injury. Crit Care Med. 2008;36:1256-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Moreira de Macêdo S, Guimarães TA, Feltenberger JD, Sousa Santos SH. The role of renin-angiotensin system modulation on treatment and prevention of liver diseases. Peptides. 2014;62:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Cao X, Yang F, Shi T, Yuan M, Xin Z, Xie R, Li S, Li H, Yang JK. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci Rep. 2016;6:21592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Cao X, Yang FY, Xin Z, Xie RR, Yang JK. The ACE2/Ang-(1-7)/Mas axis can inhibit hepatic insulin resistance. Mol Cell Endocrinol. 2014;393:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Skipworth JR, Szabadkai G, Olde Damink SW, Leung PS, Humphries SE, Montgomery HE. Review article: pancreatic renin-angiotensin systems in health and disease. Aliment Pharmacol Ther. 2011;34:840-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Frigolet ME, Torres N, Tovar AR. The renin-angiotensin system in adipose tissue and its metabolic consequences during obesity. J Nutr Biochem. 2013;24:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Zhang F, Liu C, Wang L, Cao X, Wang YY, Yang JK. Antioxidant effect of angiotensin (17) in the protection of pancreatic β cell function. Mol Med Rep. 2016;14:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 31. | Wu Y, Ma KL, Zhang Y, Wen Y, Wang GH, Hu ZB, Liu L, Lu J, Chen PP, Ruan XZ. Lipid disorder and intrahepatic renin-angiotensin system activation synergistically contribute to non-alcoholic fatty liver disease. Liver Int. 2016;36:1525-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, Hasegawa T, Tokusashi Y, Miyokawa N, Nakamura K. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Bataller R, Sancho-Bru P, Ginès P, Brenner DA. Liver fibrogenesis: a new role for the renin-angiotensin system. Antioxid Redox Signal. 2005;7:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Moreno M, Gonzalo T, Kok RJ, Sancho-Bru P, van Beuge M, Swart J, Prakash J, Temming K, Fondevila C, Beljaars L. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Moreno-Alvarez P, Sosa-Garrocho M, Briones-Orta MA, González-Espinosa C, Medina-Tamayo J, Molina-Jijón E, Pedraza-Chaverri J, Macías-Silva M. Angiotensin II increases mRNA levels of all TGF-beta isoforms in quiescent and activated rat hepatic stellate cells. Cell Biol Int. 2010;34:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3115] [Article Influence: 115.4] [Reference Citation Analysis (36)] |
| 37. | Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr. 2010;46:212-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 38. | Magliano DC, Penna-de-Carvalho A, Vazquez-Carrera M, Mandarim-de-Lacerda CA, Aguila MB. Short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system. Endocrine. 2015;50:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Lee KC, Hsieh YC, Yang YY, Chan CC, Huang YH, Lin HC. Aliskiren Reduces Hepatic steatosis and Epididymal Fat Mass and Increases Skeletal Muscle Insulin Sensitivity in High-Fat Diet-Fed Mice. Sci Rep. 2016;6:18899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Rabie EM, Heeba GH, Abouzied MM, Khalifa MM. Comparative effects of Aliskiren and Telmisartan in high fructose diet-induced metabolic syndrome in rats. Eur J Pharmacol. 2015;760:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Ceconi C, Francolini G, Olivares A, Comini L, Bachetti T, Ferrari R. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur J Pharmacol. 2007;577:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Morishita T, Tsutsui M, Shimokawa H, Tasaki H, Suda O, Kobayashi K, Horiuchi M, Okuda H, Tsuda Y, Yanagihara N. Long-term treatment with perindopril ameliorates dobutamine-induced myocardial ischemia in patients with coronary artery disease. Jpn J Pharmacol. 2002;88:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Barros CC, Haro A, Russo FJ, Schadock I, Almeida SS, Reis FC, Moraes MR, Haidar A, Hirata AE, Mori M. Bradykinin inhibits hepatic gluconeogenesis in obese mice. Lab Invest. 2012;92:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Montez P, Vázquez-Medina JP, Rodríguez R, Thorwald MA, Viscarra JA, Lam L, Peti-Peterdi J, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin resistant rats. Endocrinology. 2012;153:5746-5759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Enjoji M, Kotoh K, Kato M, Higuchi N, Kohjima M, Nakashima M, Nakamuta M. Therapeutic effect of ARBs on insulin resistance and liver injury in patients with NAFLD and chronic hepatitis C: a pilot study. Int J Mol Med. 2008;22:521-527. [PubMed] |
| 47. | Toblli JE, Muñoz MC, Cao G, Mella J, Pereyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity (Silver Spring). 2008;16:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Wei YH, Jun L, Qiang CJ. Effect of losartan, an angiotensin II antagonist, on hepatic fibrosis induced by CCl4 in rats. Dig Dis Sci. 2004;49:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Souza-Mello V, Gregório BM, Cardoso-de-Lemos FS, de Carvalho L, Aguila MB, Mandarim-de-Lacerda CA. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin Sci (Lond). 2010;119:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Kudo H, Yata Y, Takahara T, Kawai K, Nakayama Y, Kanayama M, Oya T, Morita S, Sasahara M, Mann DA. Telmisartan attenuates progression of steatohepatitis in mice: role of hepatic macrophage infiltration and effects on adipose tissue. Liver Int. 2009;29:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Yi ET, Liu RX, Wen Y, Yin CH. Telmisartan attenuates hepatic fibrosis in bile duct-ligated rats. Acta Pharmacol Sin. 2012;33:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 847] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 53. | Zhang LL, Huang S, Ma XX, Zhang WY, Wang D, Jin SY, Zhang YP, Li Y, Li X. Angiotensin(1-7) attenuated Angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radic Biol Med. 2016;97:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |