Published online Aug 8, 2016. doi: 10.4254/wjh.v8.i22.933
Peer-review started: March 4, 2016
First decision: April 15, 2016
Revised: June 22, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 8, 2016
Processing time: 153 Days and 18.7 Hours
AIM: To examine the effects of the endothelin type A receptor antagonist ambrisentan on hepatic steatosis and fibrosis in a steatohepatitis mouse model.
METHODS: Fatty liver shionogi (FLS) FLS-ob/ob mice (male, 12 wk old) received ambrisentan (2.5 mg/kg orally per day; n = 8) or water as a control (n = 5) for 4 wk. Factors were compared between the two groups, including steatosis, fibrosis, inflammation, and endothelin-related gene expression in the liver.
RESULTS: In the ambrisentan group, hepatic hydroxyproline content was significantly lower than in the control group (18.0 μg/g ± 6.1 μg/g vs 33.9 μg/g ± 13.5 μg/g liver, respectively, P = 0.014). Hepatic fibrosis estimated by Sirius red staining and areas positive for α-smooth muscle actin, indicative of activated hepatic stellate cells, were also significantly lower in the ambrisentan group (0.46% ± 0.18% vs 1.11% ± 0.28%, respectively, P = 0.0003; and 0.12% ± 0.08% vs 0.25% ± 0.11%, respectively, P = 0.047). Moreover, hepatic RNA expression levels of procollagen-1 and tissue inhibitor of metalloproteinase-1 (TIMP-1) were significantly lower by 60% and 45%, respectively, in the ambrisentan group. Inflammation, steatosis, and endothelin-related mRNA expression in the liver were not significantly different between the groups.
CONCLUSION: Ambrisentan attenuated the progression of hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing procollagen-1 and TIMP-1 gene expression. Ambrisentan did not affect inflammation or steatosis.
Core tip: Endothelin (ET) can activate hepatic stellate cells, leading to the progression of hepatic fibrosis. Furthermore, ET-1 may increase the inflow of free fatty acids from the fat tissue into the liver and exacerbate hepatic steatosis. Therefore, ET-1 antagonism may be a novel target for steatohepatitis. The present study showed that ambrisentan, an ET type A receptor antagonist, attenuated hepatic fibrosis by inhibiting hepatic stellate cell activation, without affecting hepatic steatosis, in a non-alcoholic steatohepatitis mouse model.
- Citation: Okamoto T, Koda M, Miyoshi K, Onoyama T, Kishina M, Matono T, Sugihara T, Hosho K, Okano J, Isomoto H, Murawaki Y. Antifibrotic effects of ambrisentan, an endothelin-A receptor antagonist, in a non-alcoholic steatohepatitis mouse model. World J Hepatol 2016; 8(22): 933-941
- URL: https://www.wjgnet.com/1948-5182/full/v8/i22/933.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i22.933
Non-alcoholic steatohepatitis (NASH) is characterized by hepatic fat deposition, inflammation, and differing degrees of fibrosis[1]. In the pathophysiology of NASH, the deposition of fat in liver cells, which occurs in association with obesity and insulin resistance, is a benign process in most patients but is followed by inflammation and fibrosis in the liver in response to multiple insults, such as oxidative stress and various adipokines or cytokines acting in parallel[2]. In NASH, the serum endothelin-1 (ET-1) level is elevated and is correlated with hepatic fibrosis severity[3]. The development of hepatic fibrosis is mediated to a large extent by the activation of hepatic stellate cells (HSCs). ET-1 is released from sinusoidal endothelial cells and HSCs, which serves to activate the HSCs and accelerate collagen fiber synthesis in them[4]. Furthermore, ET-1 acts as a mediator and is elevated in conditions such as insulin resistance, hyperglycemia, oxidative stress, and endothelial cell dysfunction[5,6]. ET-1 also increases vascular superoxide production and promotes cell proliferation by inducing reactive oxygen species[7].
Ambrisentan is a selective ET type A receptor (ETAR) antagonist approved for the treatment of patients with pulmonary arterial hypertension[8]. ETAR antagonists improve liver fibrosis in cirrhotic rats[9], but their effects on NASH are unknown. Fatty liver shionogi (FLS)-ob/ob mice are characterized by hyperphagia, obesity, hyperlipidemia, and diabetes mellitus[10]. As described in our previous study using these mice[11], FLS-ob/ob mice are generated by transferring the Lepob gene into the FLS mouse genome, causing FLS mice to spontaneously develop chronic hepatic steatosis but not obesity. The resultant FLS-ob/ob mice show severe steatosis, hepatocellular ballooning, and advanced hepatic fibrosis histologically. They also display increased oxidative stress, elevated production of inflammatory and profibrotic cytokines, and increased apoptosis of hepatocytes, and eventually develop cirrhosis and liver tumors[12,13]. For these reasons, FLS-ob/ob are considered to be animal model the most closely represents human metabolic syndrome-related NASH. Against this background, this study investigated the therapeutic effects of ambrisentan on hepatic steatosis and fibrosis in NASH using FLS-ob/ob male mice.
A total of 13 male FLS-ob/ob mice (age, 8 wk; body weight, 42.88 g ± 1.74 g) were obtained from Shionogi Research Laboratories (Shiga, Japan) and housed in a controlled environment (24 °C ± 2 °C; 12:12-h light:Dark cycle). Mice were provided ad libitum water and a standard powdered diet (CE-2, 4.6% fat; CLEA Japan, Tokyo, Japan). To maintain dietary intake in both groups at an equal level, food consumption and body weight were monitored throughout observation. All experiments were performed in accordance with the Animal Experimentation Guidelines of Tottori University (Yonago, Japan). The study was reviewed and approved by the ethics committee of Tottori University. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Tottori University (approval number, 14-Y-8) and the animal protocol was designed to minimize pain and discomfort to the animals.
At the age of 12 wk, male FLS-ob/ob mice were randomly assigned to the ambrisentan (n = 8) or control (n = 5) group. Intragastric gavage administration was carried out in conscious animals with an appropriately sized gastric tube. Ambrisentan (2.5 mg/kg per day; ADooQ BioScience, Irvine, CA) was orally administered every afternoon for 4 wk as a bolus through a gastric tube. Water was administered to the control group. At week 4, animals were fasted for 4 h and tail vein blood was drawn and subjected to blood glucose determination. Animals were killed by pentobarbital anesthesia injection (Dainippon Sumitomo Pharma, Osaka, Japan) after 4 wk and blood was collected from the right ventricle. Plasma samples were frozen and stored at -80 °C Liver and visceral fat were then weighed, snap-frozen in liquid nitrogen, and stored at -80 °C. Additional liver specimens were fixed in 10% buffered formalin (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and embedded in paraffin (Wako Pure Chemical Industries, Ltd.) for histological analysis.
Snap-frozen liver samples (50 mg) were homogenized and extracted using chloroform-methanol (2:1 v/v; Wako Pure Chemical Industries, Ltd.). The organic phase was then dried and resuspended in 2-propanol containing 10% Triton X-100. Total cholesterol and triglyceride contents were measured with the Cholesterol E-test (Wako Pure Chemical Industries, Ltd.) and Triglyceride E-test (Wako Pure Chemical Industries, Ltd.), respectively.
Blood samples were immediately separated by centrifugation at 2000 g for 15 min at 4 °C and stored at -80 °C until further use. Serum samples were analyzed to determine the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Hepatic tissue (400 mg wet weight) was hydrolyzed in 4 mL of 6 mol/L HCl at 105 °C overnight. The hydrolysate was then thoroughly evaporated under vacuum. The sediment was resuspended in distilled water, decolorized with activated charcoal, and filtered; the filtrate was then acidified to pH 5.0 and evaporated under vacuum. The sediment was resuspended in distilled water, mixed with 2 mL of isopropanol, and then incubated with 1 mL of 7% chloramine-T for 5 min at room temperature. After addition of Ehrlich's solution (2 mL; 1.76 g p-dimethylaminobenzaldehyde dissolved in 4.08 mL 60% perchloric acid and 95.5 mL of isopropanol), the mixture was incubated at 60 °C for 10 min. The absorbance of the cooled mixture was measured at 562 nm.
As in our previous study[11], formalin-fixed, paraffin-embedded liver sections (4-μm-thick) were stained with picrosirius red (Chroma-Gesellschaft Schmid GmbH and Co., Munster, Germany) and counterstained with fast green (Chroma-Gesellschaft Schmid GmbH and Co.). The areas of hepatic fibrosis were subsequently measured in 10 randomly selected fields in each specimen (magnification, × 400) using WinROOF ver.5.71 software and the Olympus BX51N-34 microscope.
Following the staining of neutral lipids in frozen-fixed, cryostat-embedded liver sections (4-mm-thick) with oil red O (Sigma-Aldrich, St. Louis, MO), areas of hepatic steatosis were measured using WinROOF version 5.71 software (Mitani Corporation, Tokyo, Japan) in 10 randomly selected fields (magnification, × 400; Olympus BX51N-34; Olympus Corporation, Tokyo, Japan) per specimen[11].
Immunostaining for α-smooth muscle actin (α-SMA) was used for the detection and counting of activated HSCs. As described previously[11], α-SMA was detected by staining with mouse monoclonal anti-α-SMA antibody (cat. No. MS-113-R7; Thermo Fisher Scientific, Fremont, CA) without dilution. Goat anti-mouse Ig from the Histofine Mouse Stain kit (cat. No. 414322; Nichirei Biosciences, Inc., Tokyo, Japan) was used without dilution as the secondary antibody. HSCs activation was assessed by using WinROOF ver.5.71 software to measure the areas of α-SMA staining in 10 randomly selected fields (magnification × 400; Olympus BX51N-34) per specimen.
F4/80, a mature mouse cell surface glycoprotein expressed at high levels on Kupffer cells, was immunohistochemically stained using a rat monoclonal anti-mouse F4/80 antibody (cat. No. ab6640; Abcam, Tokyo, Japan) diluted to 1:100 with 0.01 mol/L PBS according to the manufacturer’s instructions. Goat anti-rat secondary antibody from the Histofine Simple Stain Mouse MAX-PO (Rat) kit (cat. No. 414311; Nichirei Biosciences, Inc.) was used without dilution. Immunopositive cells were analyzed in 10 intralobular ocular fields (magnification, × 400; Olympus BX41N-34) per specimen[11].
Immunohistochemical staining for 8-hydroxy-2-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage, was used to assess oxidative stress[11]. A monoclonal mouse anti-8-OHdG antibody (cat. No. MOG-020P; Nikken SEIL, Shizuoka, Japan) diluted in 200 μL distilled water was used, following the manufacturer’s instructions. Goat anti-mouse Ig from the Histofine Mouse Stain kit served as the secondary antibody without dilution. WinROOF ver.5.71 software was used to analyzed immunopositive cells using 10 intralobular ocular fields (magnification × 400; Olympus BX41N-34) per specimen, and values are expressed as the ratios (%) of fields. Also, 4-hydroxynonenal (4-HNE) was semi-quantified via immunohistochemical staining using a monoclonal mouse anti-4-HNE antibody (cat. no. MHN-020P; Nikken SEIL) diluted in 200 μL distilled water following the manufacturer’s instructions. Goat anti-mouse Ig from the Histofine Mouse Stain kit was used as the secondary antibody without dilution. Ten randomly selected fields (magnification, × 400) in each 4-HNE-stained specimen were classified into immunopositive grades 1, 2, 3 and 4 (0%-10%, 11%-20%, 21%-30%, and > 30%, respectively) and the mean values of 10 fields were calculated.
As described previously[11], total RNA was extracted from homogenized hepatic tissue samples using the RNeasy Lipid Tissue Mini kit (Qiagen, Hilden, Germany). Absorbance at 260 nm was measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific), to determine RNA concentrations and RNA quality was confirmed by electrophoresis on ethidium bromide-stained 1% agarose gels. Total RNA (2 μg) was reverse transcribed in a final volume of 11.5 μL containing 4 μL of 5 × standard buffer, 2 μL of 0.1 mol/L dithiothreitol, 1 μL of SuperScript II RNase H reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA), 2 μL of 10 mol/L MdNTP (Promega, Madison, WI), 1 μL of 50 pmol/μL Random Primer (Promega), 0.5 μL of 100 pmol/μL Oligo (dT)15 Primer (Promega), and 1 μL of 40 U/μL ribonuclease inhibitor (Wako Pure Chemical Industries, Ltd.). Mixtures were incubated at 37 °C for 60 min and 95 °C for 5 min, and were then cooled to 4 °C for 5 min using a MyCycler Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA).
Quantitative real-time PCR assays (7900HT Fast Real-time PCR system; Applied Biosystems, Carlsbad, CA) proceeded as described previously[11]. The assays were used a final volume of 10 mL containing 250 nmol/L Universal ProbeLibrary probe (Roche, Basel, Switzerland), 900 nmol/L forward primer, 900 nmol/L reverse primer, 5 mL EXPRESS qPCR Supermix with Premixed Rox (Invitrogen), and 2 mL cDNA. mRNA level of transforming growth factor-β1 (TGF-β1; GenBank: NM_011577), procollagen-type I (GenBank: U08020), connective tissue growth factor (CTGF; GenBank: NM_010217), tumor necrosis factor-α (TNF-α; GenBank: NM_013693), monocyte chemoattractant protein-1 (MCP-1; GenBank: NM_100127112), tissue inhibitor of metalloproteinases-1 (TIMP-1; GenBank: NM_011593), peroxisome proliferator-activated receptor (PPAR-α; GenBank: NM_007988.3), sterol regulatory element-binding protein 1c (SREBP1c; GenBank: NM_011480), microsomal triglyceride transfer protein (MTP; GenBank: NM_008642), endothelin-1 (ET-1; GenBank: NM_010204), endothelin-converting enzyme (ECE; GenBank: NM_199307), endothelin-1 type A receptor (ET-1A; GenBank: NM_010332), and endothelin-1 type B receptor (ET-1B; GenBank: U32329) were assessed using the 7900HT Fast Real-Time PCR System with SDS2.3 software (Applied Biosystems) and with β-actin (GenBank: NM_007393) as an internal standard. Thermal cycle conditions were 95 °C for 20 s, followed by 45 cycles of 1 s at 95 °C and 20 s at 60 °C. The relative mRNA expression levels were calculated using the 2-ΔΔCT method.
Differences between groups were statistically analyzed using unpaired Student’s t-tests. All statistical analysis was performed using StatFlex ver.6.0 for Windows software (Artech Co. Ltd., Osaka, Japan). All data are expressed as means ± SD, with P values less than 0.05 considered to indicate significant differences.
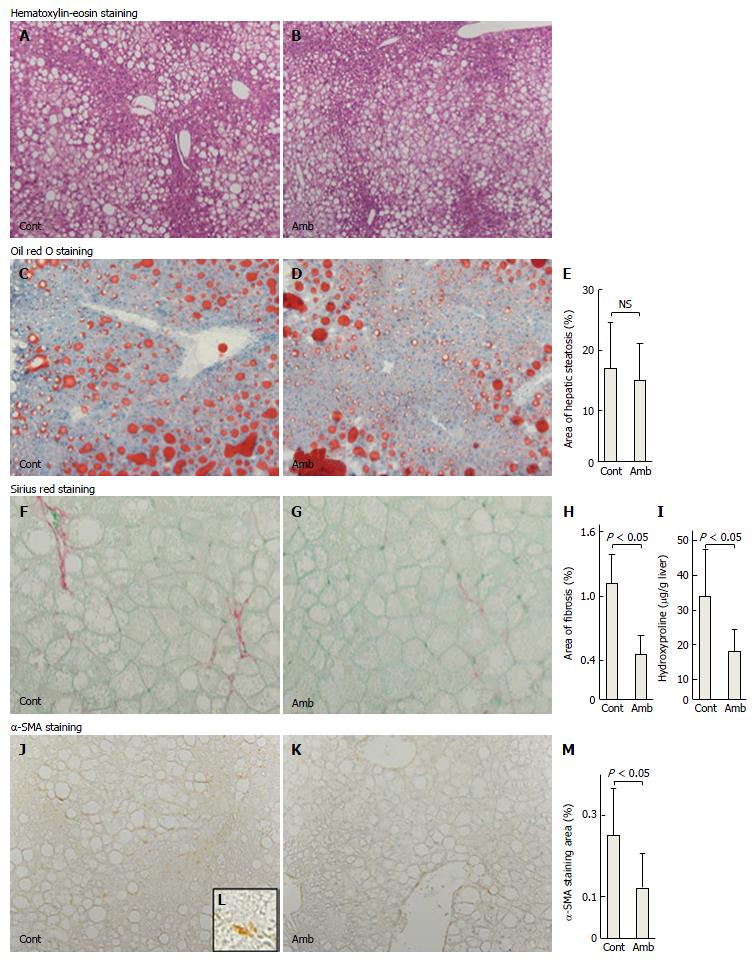
As shown in Table 1, the two groups of mice did not differ in terms of food consumption, bodyweight, liver weight, liver-to-bodyweight ratio, visceral fat weight, or levels of serum AST and ALT. There was no difference in hepatic histology with hematoxylin-eosin staining between the two groups (Figure 1A and B).
| Parameters | Control group (n = 5) | Ambrisentan group (n = 8) | P value |
| Body weight (g) | 47.3 ± 3.6 | 47.0 ± 4.6 | 0.27 |
| Liver weight (g) | 5.4 ± 1.2 | 5.1 ± 1.1 | 0.75 |
| Liver/body weight ratio | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.68 |
| Visceral fat weight (g) | 2.5 ± 0.3 | 2.7 ± 0.3 | 0.32 |
| Weekly dietary intake (g) | 31.7 ± 9.3 | 29.4 ± 9.0 | 0.66 |
| Serum AST (U/L) | 143 ± 20 | 155 ± 43 | 0.59 |
| Serum ALT (U/L) | 120 ± 52 | 151 ± 65 | 0.38 |
| Hepatic cholesterol (mg/dL) | 24.5 ± 1.56 | 27.2 ± 2.58 | 0.06 |
| Hepatic triglyceride (mg/dL) | 1152 ± 500 | 929 ± 210 | 0.28 |
To assess the effects of ambrisentan on lipid metabolism, we determined the hepatic steatosis area, hepatic lipid contents, and gene expression of hepatic lipogenesis, lipolysis, and lipid transporter genes. Oil red O staining showed no differences in area of hepatic steatosis between the groups (ambrisentan vs control; 15.0% ± 6.0% vs 17.0% ± 7.7%; P = 0.614; Figure 1C-E). Steatosis-related mRNA expression levels (PPAR-α, SREBP-1c, FAS, and MTP) were not different between the two groups (Table 2). Hepatic total cholesterol and triglyceride contents also revealed no differences between the two groups (Table 1). These findings suggested that ambrisentan did not affect lipid metabolism and accumulation in the liver of FLS-ob/ob mice.
| mRNA | Control group (n = 5) | Ambrisentan group (n = 8) | P value |
| Procollagen-1 | 1.76 ± 0.58 | 1.06 ± 0.43 | 0.024 |
| TGF-β1 | 1.60 ± 0.80 | 1.14 ± 0.17 | 0.13 |
| CTGF | 1.43 ± 0.49 | 1.52 ± 0.40 | 0.36 |
| TIMP-1 | 2.98 ± 1.58 | 1.34 ± 0.61 | 0.02 |
| TNF-α | 2.37 ± 2.65 | 2.37 ± 3.02 | 1 |
| MCP-1 | 10.20 ± 10.06 | 8.14 ± 8.90 | 0.39 |
| SREBP1c | 0.69 ± 0.19 | 0.80 ± 0.17 | 0.29 |
| FAS | 0.76 ± 0.34 | 0.87 ± 0.46 | 0.67 |
| PPAR-α | 0.81 ± 0.16 | 0.98 ± 0.27 | 0.24 |
| MTP | 0.95 ± 0.09 | 0.99 ± 0.09 | 0.45 |
| ET-1 | 1.40 ± 0.57 | 1.47 ± 0.50 | 0.82 |
| ECE | 1.02 ± 0.13 | 1.23 ± 0.23 | 0.09 |
| ETAR | 3.74 ± 3.35 | 2.55 ± 1.56 | 0.4 |
| ETBR | 2.07 ± 0.76 | 1.87 ± 0.49 | 0.59 |
To assess whether ambrisentan attenuated hepatic fibrosis, we determined the antifibrotic effects of ambrisentan in the FLS-ob/ob mice. Sirius red staining showed that the area of fibrosis was decreased by ambrisentan compared with the control (0.46% ± 0.18% vs 1.11% ± 0.28%, respectively, P = 0.0003; Figure 1F-H). Hepatic hydroxyproline (Hyp) content was significantly reduced by ambrisentan compared with the control (18.0 μg/g ± 6.1 μg/g liver vs 33.9 μg/g ± 13.5 μg/g liver, respectively, P = 0.014; Figure 1I). Moreover, the area of positive α-SMA immunostaining was significantly reduced by ambrisentan (0.12% ± 0.08% vs 0.25% ± 0.11%, respectively P = 0.047; Figure 1J-M).
In relation to extracellular matrix metabolism in the liver, as shown in Table 2, ambrisentan reduced the mRNA expression levels of procollagen-1 by 60% and TIMP-1 by 45% but the mRNA expression of TGF-β1 and CTGF did not differ between the two groups.
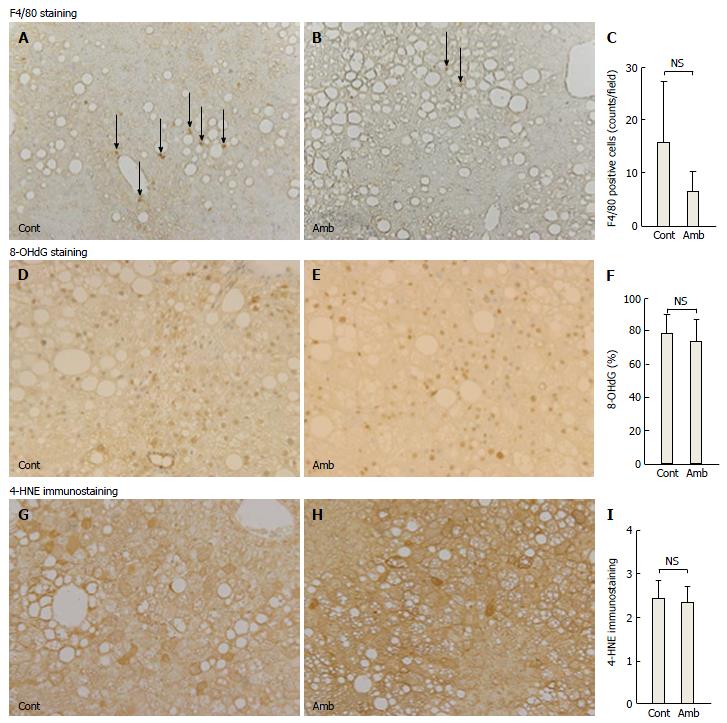
The process of hepatic fibrosis is driven primarily by inflammation in response to liver damage. There were fewer F4/80-positive cells in the ambrisentan group than in the control group, but not significantly so (6.5 ± 3.9 vs 15.2 ± 11.5, respectively, P = 0.055; Figure 2A-C). Levels of inflammation-related mRNA (TNF-α and MCP-1) did not differ between the two groups (Table 2).
Oxidative stress is involved in the development of NASH. We determined oxidative stress by two methods: 8-OHdG as an index of DNA damage and 4-HNE as an index of lipid peroxidation. Ambrisentan did not affect the ratio of 8-OHdG-positive cells in the liver compared with the control (73.8% ± 12.4% vs 78.2% ± 11.5%, respectively, P = 0.538; Figure 2D-F) and did not alter the immunostaining grade for liver 4-HNE (2.36 ± 0.37 vs 2.35 ± 0.41, respectively, P = 0.958; Figure 2G-I).
Finally, we measured ET-related gene expression in FLS-ob/ob mice. The levels of ET-related mRNAs (ET-1, ECE, ETAR, and ETBR) were not different between the two groups (Table 2).
This study had two important findings. First, ambrisentan did not affect lipid metabolism. Second, it significantly attenuated the progression of hepatic fibrosis. Thus, ET-1 antagonism reduced hepatic fibrosis without improving hepatic steatosis. Ambrisentan did not reduce body weight, blood glucose levels, or hepatic steatosis compared with the control group. ET-1 is reported to increase lipolysis in human and bovine adipocytes[14]. Therefore, ET-1 may increase the inflow of free fatty acids from the fat tissue into the liver and exacerbate hepatic steatosis. ET-1 reduced the cholesterol efflux in macrophages, resulting in exacerbation of lipid accumulation in macrophages[15]. However, the present study showed that ambrisentan did not affect lipid accumulation in hepatocytes or the contents of hepatic cholesterol and triglyceride. Furthermore, the expression levels of lipid metabolism-related genes-such as SREBP-1c and FAS, which are involved in hepatic lipogenesis[16], PPAR-α, which is involved in β-oxidation of fatty acids, and MTP, which transports triglyceride to very low-density lipoprotein-were not affected by ambrisentan. From these findings, our in vivo experiments using FLS-ob/ob mice indicated that ETAR antagonism was not involved in hepatic lipid metabolism. Hyperleptinemia is reported to regulate the sensitivity of ET-1 for steatosis in NASH cirrhotic rats[16]. Because the FLS-ob/ob mice used in our study are leptin deficient[12], FLS-ob/ob mice may have low sensitivity for ET-1 in steatosis, and ET-1 may be less involved in hepatic steatosis in these mice.
Second, we investigated the effect of ambrisentan on hepatic fibrosis. The present study showed that ETAR antagonism reduced the hepatic Hyp content and the area of hepatic fibrosis through the inhibition of HSC activation. Several studies have implicated ET-1 in fibrogenesis of the kidney, cardiovascular system, and liver[2,9,17,18]. HSCs express ETAR and ET type B receptors. ET-1 is secreted from HSCs and acts in HSCs and other cells in an autocrine and paracrine manner. Our previous in vitro experiments showed that ET-1 increased fibrogenic gene expression via ETAR[17]. Furthermore, Cho et al[19] reported that an oral ETAR antagonist attenuated collagen synthesis in rat liver fibrosis due to cholestasis. The present study confirmed that the ETAR antagonist also inhibited hepatic fibrosis in a mouse NASH model. HSCs are activated by several factors and stimulants and produce extracellular matrix proteins. Rocky et al[9] and Pinzani et al[20] showed that ET-1 increased DNA synthesis and cell growth via ETAR in cultured HSCs. Our study showed that ETAR antagonism reduced HSC activation. Therefore, in the NASH model, ET-1 is involved in the activation of HSCs via ETAR. HSCs are activated by cytokines, oxidative stress, and inflammation. However, ambrisentan did not affect oxidative stress, as assessed by 8-OHdG and 4-HNE, or the inflammatory reaction, as assessed by TNF-α and MCP-1 gene expression or F4/80-positive cells. Therefore, ET-1 may directly activate HSCs.
ET-1 stimulates extracellular matrix protein production by HSCs. In an HSC culture study, ET-1 increased the production of procollagen-1 and TGF-β1 via ETAR[17]. However, although the present study indicated that ETAR antagonism attenuated the gene expression of procollagen-1, it did not influence the gene expression of TGF-β1 and CTGF, which is downstream of TGF-β1. This discrepancy may be attributable to the model of liver injury. A previous report[9] showed that ET antagonism reduced TGF-β1 mRNA levels in the carbon tetrachloride model, but its levels were not altered in cholestatic-induced liver injury. Such data showed that the effects of ET-1 antagonism in TGF-β1 may depend on the liver injury model. Therefore, ET-1 might not play a major role in TGF-β1 expression in mild liver injury models such as cholestasis or steatohepatitis.
The present study showed that ETAR antagonism reduced TIMP-1 gene expression. TIMP-1 is a high-affinity inhibitor of many matrix metalloproteinases and suppresses matrix degradation, resulting in the progression of liver fibrosis. ET-1 is reported to increase TIMP-1 mRNA in fibroblasts[21]. In our study, ETAR antagonism attenuated TIMP-1 expression and might improve hepatic fibrosis by increasing fibrolysis. From these results, it appears that ambrisentan improved hepatic fibrosis by inhibiting HSC activation and suppressing procollagen-1 and TIMP-1 gene expression.
The present study has some limitations. First, it involved a small number of mice and a relatively short duration of ambrisentan treatment. We included only 8 ambrisentan-treated mice and 5 controls and the study duration was only 4 wk. Therefore, examination of a larger number of mice and a longer administration period is required to validate these results. Second, our experiments did not include non-NASH mice arms because we could not obtain DS mice, the original wild-type of FLS-ob/ob mice. Therefore, further study is needed using another NASH mouse model.
In conclusion, ambrisentan attenuated the progression of hepatic fibrosis by suppressing the activation of HSCs and reducing procollagen-1 and TIMP-1 expression.
In non-alcoholic steatohepatitis (NASH), the serum endothelin-1 (ET-1) level is elevated and is correlated with hepatic fibrosis severity. The development of hepatic fibrosis is mediated to a large extent by the activation of hepatic stellate cells (HSCs). ET-1 serves to activate the HSCs and accelerates collagen fiber synthesis in them. Furthermore, ET-1 acts as a mediator and is elevated in conditions such as insulin resistance, hyperglycemia, oxidative stress, and endothelial cell dysfunction.
Ambrisentan, a selective ET type A receptor (ETAR) antagonist improves liver fibrosis in cirrhotic rats, but their effects on NASH are unknown. ET-1 may become a novel target for the treatment of NASH.
The present study has shown ambrisentan improved hepatic fibrosis by inhibiting HSC activation and suppressing procollagen-1 and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression, but did not affect hepatic steatosis. The combination therapy of ambrisentan with other drugs for lipid accumulation may be more effective for NASH.
NASH: Nonalcoholic steatohepatitis is characterized by hepatic fat deposition, inflammation, and differing degrees of fibrosis.
This is an interesting study. The authors report that “ambrisentan” attenuates the progression of hepatic fibrosis by inhibiting the activation of HSCs and reducing procollagen-1 and TIMP-1 gene expression. According to them it did not affect inflammation and steatosis. No doubt these results are interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Murdaca G, Ohkoshi S, Sanal MG, Tasci I S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Pascale A, Pais R, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis. 2010;19:415-423. [PubMed] |
| 2. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1811] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 3. | Degertekin B, Ozenirler S, Elbeg S, Akyol G. The serum endothelin-1 level in steatosis and NASH, and its relation with severity of liver fibrosis. Dig Dis Sci. 2007;52:2622-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Mallat A, Préaux AM, Serradeil-Le Gal C, Raufaste D, Gallois C, Brenner DA, Bradham C, Maclouf J, Iourgenko V, Fouassier L. Growth inhibitory properties of endothelin-1 in activated human hepatic stellate cells: a cyclic adenosine monophosphate-mediated pathway. Inhibition of both extracellular signal-regulated kinase and c-Jun kinase and upregulation of endothelin B receptors. J Clin Invest. 1996;98:2771-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 5. | Ottosson-Seeberger A, Lundberg JM, Alvestrand A, Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol Scand. 1997;161:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Shaw SG, Boden PJ. Insulin resistance, obesity and the metabolic syndrome. Is there a therapeutic role for endothelin-1 antagonists? Curr Vasc Pharmacol. 2005;3:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1058-L1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 747] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 8. | Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010-3019. [PubMed] |
| 9. | Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Soga M, Kishimoto Y, kawaguchi J, Nakai Y, Kawamura Y, Inagaki S, Katoh K, Oohara T, Makino S, Oshima I. The FLS mouse: a new inbred strain with spontaneous fatty liver. Lab Anim Sci. 1999;49:269-275. [PubMed] |
| 11. | Kishina M, Koda M, Kato J, Tokunaga S, Matono T, Sugihara T, Ueki M, Murawaki Y. Therapeutic effects of the direct renin inhibitor, aliskiren, on non-alcoholic steatohepatitis in fatty liver Shionogi ob/ob male mice. Hepatol Res. 2014;44:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Soga M, Hashimoto S, Kishimoto Y, Hirasawa T, Makino S, Inagaki S. Insulin resistance, steatohepatitis, and hepatocellular carcinoma in a new congenic strain of Fatty Liver Shionogi (FLS) mice with the Lep(ob) gene. Exp Anim. 2010;59:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Sugihara T, Koda M, Kishina M, Kato J, Tokunaga S, Matono T, Ueki M, Murawaki Y. Fatty liver Shionogi-ob/ob mouse: A new candidate for a non-alcoholic steatohepatitis model. Hepatol Res. 2013;43:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Eriksson AK, van Harmelen V, Stenson BM, Aström G, Wåhlén K, Laurencikiene J, Rydén M. Endothelin-1 stimulates human adipocyte lipolysis through the ET A receptor. Int J Obes (Lond). 2009;33:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Lin CY, Lee TS, Chen CC, Chang CA, Lin YJ, Hsu YP, Ho LT. Endothelin-1 exacerbates lipid accumulation by increasing the protein degradation of the ATP-binding cassette transporter G1 in macrophages. J Cell Physiol. 2011;226:2198-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Yang YY, Tsai TH, Huang YT, Lee TY, Chan CC, Lee KC, Lin HC. Hepatic endothelin-1 and endocannabinoids-dependent effects of hyperleptinemia in nonalcoholic steatohepatitis-cirrhotic rats. Hepatology. 2012;55:1540-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Koda M, Bauer M, Krebs A, Hahn EG, Schuppan D, Murawaki Y. Endothelin-1 enhances fibrogenic gene expression, but does not promote DNA synthesis or apoptosis in hepatic stellate cells. Comp Hepatol. 2006;5:5. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Arthur MJ, Mann DA, Iredale JP. Tissue inhibitors of metalloproteinases, hepatic stellate cells and liver fibrosis. J Gastroenterol Hepatol. 1998;13 Suppl:S33-S38. [PubMed] |
| 19. | Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-A receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology. 2000;118:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Knowles JP, Shi-Wen X, Haque SU, Bhalla A, Dashwood MR, Yang S, Taylor I, Winslet MC, Abraham DJ, Loizidou M. Endothelin-1 stimulates colon cancer adjacent fibroblasts. Int J Cancer. 2012;130:1264-1272. [PubMed] |