Published online Jul 27, 2014. doi: 10.4254/wjh.v6.i7.513
Revised: May 15, 2014
Accepted: May 28, 2014
Published online: July 27, 2014
AIM: To assess a protocol for treating patients with multiple synchronous colonic cancer liver metastases, which are unresectable in one stage.
METHODS: Patients enrolled in the “liver first” protocol presented with colon-only (not rectal) cancer and multiple synchronous hepatic metastases (type II or III). All patients showed good performance status (ECOG PS 0-1) and were treated with curative intent. Complete oncologic staging including positron emission tomography-computed tomography was performed in order to rule out extrahepatic disease. If bowel obstruction was imminent, an intraluminal colonic stent was placed endoscopically. Subsequently, all patients received standardised neo-adjuvant chemotherapy, that is, FOLFOX or XELOX regimens combined with an antiangiogenic agent (bevacizumab or cetuximab). Provided that a response to chemotherapy was observed, patients underwent either one or two hepatectomies with or without portal vein embolization followed by the indicated colectomy. Further chemotherapy was administered after each procedure. Re-staging was performed after each chemotherapeutic treatment. Disease progression at any stage resulted in discontinuation of the protocol and conversion to palliative disease management.
RESULTS: Prospectively recorded data from 11 consecutive patients (8 men) were analysed for this study. Their mean age at the time of their first assessment was 65.7 (SD ± 15.3) years. Six (54.6%) patients presented with type III metastatic disease. The minimum and maximum follow-up periods were 7.3 and 39.6 mo, respectively. The mean overall survival of all patients was 16.5 (95%CI: 10.0-23.2) mo. A colonic stent had to be placed in 5 (45.5%) patients due to the onset of an intraluminal obstruction. Four (36.4%) patients succeeded in completing all planned surgical operations. Their mean overall survival was 27.2 (95%CI: 15.1-39.3) mo and the mean disease-free survival was 7.7 (95%CI: 3.0-12.5) mo. Patients, who were obliged to shift to palliative treatment due to disease progression, had a mean overall survival of 10.5 (95%CI: 8.6-12.4) mo. None of these patients underwent palliative colectomy. No postoperative mortality was recorded.
CONCLUSION: The implementation of a structured “liver first” approach protocol for the treatment of patients with extensive, liver-limited colon cancer metastatic disease may be beneficial.
Core tip: Complete tumour burden resection remains the only possible curative therapy for liver-limited colon cancer metastatic disease. However, there are different approaches regarding treatment of the primary tumour and its hepatic metastases, if the latter are synchronous and unresectable with one surgical procedure. For this subgroup of patients, a “liver first” approach protocol is introduced in order to assess standardised treatment as well as to prevent overtreatment in cases of undetected extra-hepatic metastatic dissemination or disease progression.
- Citation: Kardassis D, Ntinas A, Miliaras D, Kofokotsios A, Papazisis K, Vrochides D. Patients with multiple synchronous colonic cancer hepatic metastases benefit from enrolment in a “liver first” approach protocol. World J Hepatol 2014; 6(7): 513-519
- URL: https://www.wjgnet.com/1948-5182/full/v6/i7/513.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i7.513
Approximately every second patient who suffers from colorectal cancer (CRC) will at some point be diagnosed with either synchronous or metachronous metastatic disease[1,2]. Liver is the most frequently affected organ. Resection of the complete tumour load has long been accepted as the only therapeutic option that results in improved long-term survival or even cure[3]. During the past decade a significant prolongation of overall survival and an increase in survival rates has been reported. This development is based on the improvement of systemic chemotherapy and introduction of antiangiogenic agents, but also on the utilisation of advanced surgical strategies and equipment[4-6].
Whereas in metachronous resectable disease, the timing of necessary operative procedures seems obvious, various approaches are currently being implemented if resectable (or potentially resectable) hepatic metastases, with no evidence of extrahepatic disease, are detected at the time of the primary tumour diagnosis[7]. The “classic” approach consists of targeting the primary tumour first, followed by chemotherapy and resection of the hepatic metastases[8]. This strategy remains essential, if diagnosis of the disease coincides with an existing acute lower gastrointestinal bleeding or significant bowel obstruction. The “simultaneous” approach includes resection of the primary tumour as well as any hepatic metastases in one stage. This option is often preferred, especially in experienced centres, when a minor hepatectomy is sufficient in clearing the existing tumour load[9]. Finally, the “reverse” strategy has been introduced in recent years[10,11]. In this approach, liver specific procedures such as portal vein embolization and hepatectomies come first, followed by colectomy. All operative procedures take place either after chemotherapy alone or after combination with radiotherapy, when the diagnosis is rectal cancer. The rationale behind this strategy is that patients with multiple hepatic metastases are more likely to become incurable by not timely confronting the extensive liver metastatic disease.
Important criteria for choosing the appropriate therapeutic plan are patient’s performance status, primary tumour location, disease extent, available diagnostic and therapeutic tools and methods, as well as the centre’s medical and surgical team experience. Due to the complexity of the disease, the patient population is heterogenic. In addition, conclusions regarding best possible management are based on retrospective series of patients suffering from CRC and liver metastases[12]. Therefore, treatment of those patients is routinely based on patient and centre specific (“individually tailored”) approaches rather than generally accepted guidelines.
For this study, a subgroup of CRC patients was defined, that is, patients who had been diagnosed with stage IV colonic (not rectal) cancer and presented with multiple, bilobar, synchronous, liver-only metastases, that were either potentially resectable after more than one procedure (type II) or initially unresectable, but possibly resectable after tumour downsizing (type III)[13,14]. These patients were enrolled in a prospective “liver first” approach protocol which included staging, certain oncologic therapy and surgical therapeutic steps. The aim of the study was to assess the implementation of this algorithm, especially in terms of applicability and safety.
This study was conducted in a tertiary care private hospital according to the guidelines of the Declaration of Helsinki of the World Medical Association[15]. The hospital’s ethics committee approved the study protocol. Written informed consent was obtained from all patients. Their enrolment was discussed during and approved by the hospital’s weekly tumour board. All patients were treated with curative intent.
Nomenclature regarding the extent of hepatic resections is that endorsed by the International Hepato-Pancreato-Biliary Association[16]. Decisions on resectability were taken by the hepato-pancreato-biliary surgeons of our centre based on the recommendations made on the Consensus Conferences on the Multidisciplinary Treatment of Colorectal Cancer Metastases[17,18]. Postoperative complications are reported according to the Dindo-Clavien classification[19].
Inclusion criteria for patients enrolled in the “liver first” protocol included the diagnosis of colon-only (not rectal) cancer and synchronous, multiple, bilobar, liver metastases (type II or III), age ≥ 18 years, no previous disease-specific therapeutic management and Eastern Cooperative Oncology Group (ECOG) performance status grade 0 or 1. Patients who were diagnosed with extrahepatic disease were excluded.
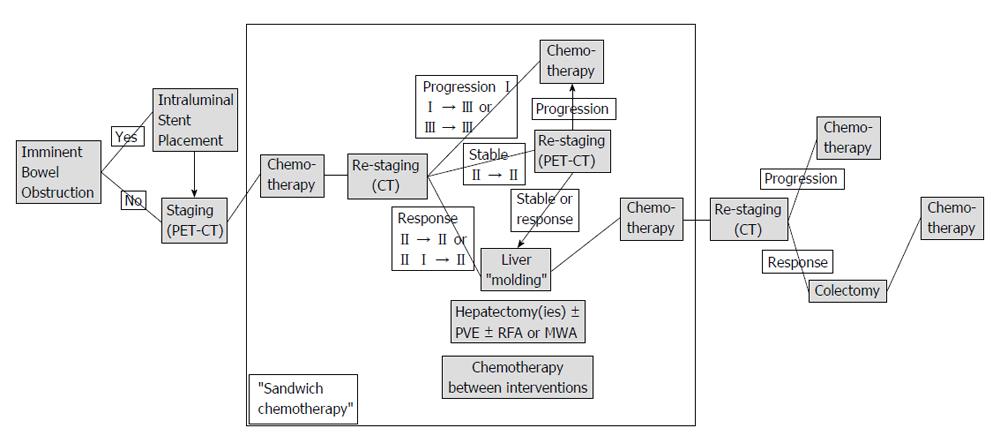
The protocol was performed within the scope of an intent-to-treat study. Initially, a complete oncologic staging, that is clinical examination, blood tests, liver function tests, tumour marker determination, coloscopy, primary tumour histology, abdominal and thoracic cross-sectional imaging, positron emission tomography-computed tomography (PET-CT), was performed. In the case of an imminent bowel obstruction, an intraluminal colonic stent was placed by endoscopy (Figure 1). All patients then received standardised neo-adjuvant chemotherapy including an antiangiogenic agent. In the case of post-chemotherapy disease response, patients underwent either portal vein embolization, in order to achieve an increase in the future liver remnant, or/and one or two hepatectomies. If indicated, radiofrequency ablation or microwave ablation was performed intraoperatively. In between, (sandwich) chemotherapy was administered. This particular protocol phase was called “liver molding”. If the disease remained stable, a PET-CT scan was performed in order to assess the neoplasm’s response to chemotherapy. Following the “liver molding” phase, chemotherapy and re-staging was repeated. Only in the case of absence of extrahepatic disease at this stage, patients underwent the indicated colectomy. Adjuvant chemotherapy regimens were administered. On the other hand, disease progression at any stage of the protocol resulted in its discontinuation and conversion to palliative disease management.
First-line chemotherapy comprised of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX4), or capecitabine and oxaliplatin (XELOX) combined with a vascular endothelial growth factor inhibitor (bevacizumab). In second-line chemotherapy, oxaliplatin was replaced by irinotecan and/or bevacizumab was replaced by an epidermal growth factor receptor inhibitor (panitumumab), the latter was administered if patients had non-mutated disease (KRAS wild-type).
Continuous and categorical variables were recorded and analysed with descriptive statistics. Survival analysis was performed by the use of Kaplan-Meier curves. Statistical analysis was performed by means of the IBM SPSS Statistics Package, version 19.9 (SPSS Inc., Chicago, IL, United States).
For this study, prospectively collected data were analysed. Between July, 2010 and October, 2011 eleven consecutive patients (eight men) who met the inclusion criteria were enrolled in the “liver first” protocol. Demographic and clinical characteristics at the time of their first assessment are displayed in Table 1. Patients’ mean age was 65.7 (SD ± 15.3) years. Seven patients (63.6%) presented with the primary tumour located in the sigmoid colon. Five patients (45.5%) presented with type II metastatic disease. Six patients (54.6%) presented with type III metastatic disease. The number of hepatic metastases ranged between seven and more than thirty, while their size ranged between 2 cm and 16 cm. A colonic stent was placed in five patients (45.5%) before the start of neo-adjuvant therapy due to an imminent intraluminal obstruction. Four patients (36.4%), all presenting with type II metastatic disease at the time of first assessment, completed all scheduled surgical procedures and correspondingly the entire protocol. They underwent two or three operations (mean: 2.75), including the indicated colectomy as the last operative step. Pathology confirmed negative margins (R0) of all resected specimens. One out of five “type II” patients (20.0%) suffered disease progression before reaching the time point of the planned hepatectomy. In only one out of six “type III” patients (16.7%) the neoplasm was able to be converted to “type II” following neo-adjuvant chemotherapy. No palliative colectomy was necessary for the seven patients who had to be allocated to palliative therapy due to disease progression (Table 2).
| Patients | Gender | Age (yr) | Primary colonic tumour location | Metastatic type (liver-limited) | Colonic obstruction > stent placement |
| 1 | Male | 67 | Sigmoid | II | - |
| 2 | Male | 75 | Sigmoid | II | - |
| 3 | Female | 37 | Sigmoid | III | - |
| 4 | Male | 79 | Sigmoid | III | √ |
| 5 | Male | 79 | Descending | III | √ |
| 6 | Male | 40 | Sigmoid | II | √ |
| 7 | Female | 75 | Sigmoid | II | - |
| 8 | Male | 59 | Descending | III | - |
| 9 | Female | 78 | Descending | III | √ |
| 10 | Male | 59 | Sigmoid | III | √ |
| 11 | Male | 75 | Ascending | II | - |
| Patients | Metastatic type (liver-limited) | Hepatectomy 1 | Hepatectomy 2 | Colectomy | Disease-free | Overall |
| Survival period (mo) | ||||||
| 1 | II | RE and wedge and RFA-MWA | - | √ | 8.23 | 39.57 |
| 2 | II | Right | Left lateral and RFA-MWA | √ | 2.20 | 14.17 |
| 3 | III | - | - | - | - | 13.97 |
| 4 | III | - | - | - | - | 7.33 |
| 5 | III | - | - | - | - | 13.37 |
| 6 | II | Laparoscopic left lateral | Right | √ | 15.27 | 39.17 |
| 7 | II | Left lateral | Right | √ | 5.27 | 15.57 |
| 8 | III | - | - | - | - | 9.43 |
| 9 | III | Laparoscopic left lateral | - | - | - | 7.80 |
| 10 | III | - | - | - | - | 11.5 |
| 11 | II | - | - | - | - | 10.1 |
The minimum and maximum follow-up periods were 7.3 mo and 39.6 mo, respectively. The mean overall survival of all patients was 16.5 (95%CI: 10.0-23.2) mo. Patients who were able to complete the “liver first” protocol had a mean disease-free survival of 7.7 (95%CI: 3.0-12.5) mo and a mean overall survival of 27.2 (95%CI: 15.1-39.3) mo. On the contrary, patients, who were obliged to shift to palliative treatment due to disease progression during the period of their enrolment did not became free of disease at any time point and had a mean overall survival of 10.5 (95%CI: 8.6-12.4) mo (Table 2).
With regard to severe complications associated with chemotherapy, one patient suffered from upper gastrointestinal bleeding after receiving the FOLFOX and bevacizumab regimen. Two severe postoperative complications (Grade III) were documented. One patient suffered an anastomotic site bleeding following sigmoidectomy, which was confirmed and treated by endoscopy and blood transfusions, and one patient suffered a bile leakage following hepatectomy, requiring percutaneous drainage. Furthermore, no postoperative (90-d) mortality was recorded.
Patients presenting with metastatic CRC represent a large, but significantly heterogeneous population as distinctions can be made based on primary tumour location, extension of metastatic spread and diagnosis time point of metastases (synchronous vs metachronous). Currently, complete neoplasm resection is regarded as the only curative therapeutic option for those patients[20]. Despite broadening resectability criteria in recent years, only a selected group (20%-30%) will be candidates for curative resection[21]. Historically, the first step of implementing therapeutic treatment was to resect the primary colorectal tumour and subsequently target hepatic metastases (“classic” approach). Due to improvements in both chemotherapy and surgical techniques, simultaneous resection of primary and liver-limited secondary disease (“combined” approach) or the prioritised resection of liver metastases (“reverse” approach) are being performed in experienced centres[22,23].
For this study, we selected a patient cohort as homogenous as possible. To be more specific, we included patients with synchronous liver-only metastatic disease that was diagnosed at the same time as the primary tumour and was either resectable in more than one stage or potentially resectable after successful downsizing. We excluded patients with rectal cancer because of the “interference” of radiotherapy treatment phases with the specific protocol steps. We also excluded patients who had to be treated with the “classic” approach, for example patients with ileus secondary to complete bowel obstruction. In addition, patients who could be treated with the “combined” approach, for example due to the presence of a solitary liver metastasis, were also excluded. Finally, we excluded patients with potentially resectable extrahepatic neoplasm dissemination.
In theory, the proposed “liver first” protocol may take advantage of the fact that neo-adjuvant chemotherapy in CRC patients provides an assessment of tumour biology[24]. Its effectiveness influences future therapeutic strategies because it may downsize the existing tumour load, so that initially unresectable metastases may become resectable[25]. Adding biological agents reportedly increases oncologic response and resectability rate[26]. On the other hand, this approach helps to avoid unnecessary operative procedures, and thus potential complications and delay in chemotherapy administration in patients whose neoplasm’s biology is not favourable.
Upfront colectomy in the treatment of CRC with synchronous hepatic metastases in the context of the curative or even palliative setting became controversial the last few years. Even though some authors conclude that upfront colectomy is beneficial in terms of overall survival, this standpoint has been challenged because the rate of primary-related complications seems low, even when using modern antiangiogenic therapy[27-30]. In our small cohort of patients, we did not encounter any primary-related complications. Whenever a bowel obstruction was imminent, a stent placement prevented acute surgery and enabled the protocol enrolment for each patient. In fact, one of five patients who received a colonic stent completed all planned operations and thus, the stent was resected with the colectomy specimen.
In spite of meticulous and repeated staging, three out of four patients (75.0%), who completed the “liver first” protocol and became disease-free, were finally diagnosed with recurrence (mean disease-free survival of 7.7 mo). This trend coincides with several large retrospective series[31,32]. A recent study suggests that pathologic characteristics of the primary colorectal tumour are more prognostic than relevant metastatic features[33].
A significant limitation of this study is the absence of a control group with matched diagnosis for comparing the “reverse” with the “classic” approach. Another important limitation is that the number of patients enrolled in the applied protocol is small.
The main goal of this work was to examine the feasibility and safety of realising a prospective “liver first” approach protocol-to our knowledge, it is the first one - for patients with liver-limited metastatic colon cancer. It focuses on a specific subgroup, namely patients with synchronous, multiple, bilobar hepatic metastases that are resectable after several interventions or disease downsizing. Treatment for these patients is usually “individually tailored” since the criterion of metastatic load resectability and the availability of therapeutic options may differ significantly among medical teams. Even though the number of patients is low, a noticeable trend can be observed, that is, patients who showed disease progression during the various steps of this algorithm had a worse outcome than those patients who succeeded in completing the protocol and became disease free, even for a short period of time. Furthermore, patients with disease progression avoided at least one operation (colectomy) without developing primary-related complications that needed surgical intervention.
In conclusion, the implementation of a structured “liver first” approach protocol for the treatment of patients with extensive, liver-limited colon cancer metastatic disease is feasible, safe, and may be beneficial. The application of such a protocol requires strict multidisciplinary decision-making process and therapeutic management.
Liver-limited colon cancer metastatic disease is a common entity in oncological and surgical practice. Complete tumour burden resection combined with systemic chemotherapy currently constitutes the only possible curative therapy.
No consensus has yet been reached concerning both the timing and the sequence of primary tumour and synchronous, multiple hepatic metastases resection in case this cannot be achieved in one stage (“simultaneous” approach). Depending on the patient’s clinical situation and the existing medical expertise, the primary tumour is either targeted upfront (“classic” approach) or subsequent to one or more liver resections (“reverse” or “liver first” approach).
For this subgroup of patients, a structured “liver first” approach protocol has been introduced and implemented in order to assess standardised treatment as well as to prevent overtreatment in cases of undetected extra-hepatic metastatic dissemination or disease progression.
This study suggests that, regarding the treatment of patients with multiple synchronous colonic cancer liver metastases, which are unresectable in one stage, the application of a “liver first” approach protocol, which is based on a strict multidisciplinary decision-making process and therapeutic management is feasible, safe and potentially beneficial.
A synchronous colorectal cancer metastasis is usually defined as metastatic neoplasmatic tissue that is detected either concurrently with diagnosis of the primary tumour or three to twelve months after the diagnosis. With respect to the described treatment protocol, a synchronous colorectal cancer metastasis was defined as metastatic neoplasmatic tissue which was diagnosed at the same time as the primary tumour. In contrast, metachronous metastases were identified at a later stage.
The present manuscript deals with a novel and very interesting approach protocol to treat patients with colon cancer and hepatic metastasis.
P- Reviewer: Galvao FHF, Ramos S S- Editor: Song XX L- Editor: Webster JR E- Editor: Liu SQ
| 1. | Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 373] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Bova R, Kamphues C, Neuhaus P, Puhl G. [Impact of time of occurrence of liver metastases (synchronous vs. metachronous) on early postoperative outcome and long-term survival of colorectal cancer patients]. Zentralbl Chir. 2014;139:220-225. [PubMed] [Cited in This Article: ] |
| 3. | Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 622] [Cited by in F6Publishing: 645] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Wang CC, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol. 2012;18:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Padman S, Padbury R, Beeke C, Karapetis CS, Bishnoi S, Townsend AR, Maddern G, Price TJ. Liver only metastatic disease in patients with metastatic colorectal cancer: impact of surgery and chemotherapy. Acta Oncol. 2013;52:1699-1706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Donati M, Stavrou GA, Oldhafer KJ. Current position of ALPPS in the surgical landscape of CRLM treatment proposals. World J Gastroenterol. 2013;19:6548-6554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Tzeng CW, Aloia TA. Colorectal liver metastases. J Gastrointest Surg. 2013;17:195-201; quiz p.201-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Gennari L, Doci R, Bignami P, Bozzetti F. Surgical treatment of hepatic metastases from colorectal cancer. Ann Surg. 1986;203:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, de Saussure W, Choti MA, Gindrat I, Aldrighetti L, Barrosso E. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg. 2013;216:707-716; discussion 716-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93:872-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 221] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Mentha G, Roth AD, Terraz S, Giostra E, Gervaz P, Andres A, Morel P, Rubbia-Brandt L, Majno PE. ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg. 2008;25:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, Gerstel E, Roth A, Majno PE, Mentha G. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg. 2012;256:772-778; discussion 778-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-657; discussion 657-658. [PubMed] [Cited in This Article: ] |
| 14. | Choti MA. Defining resectable metastatic CRC: indications, outcomes, and controversies. Managing CRC: the resectable and potentially resectable patient-A multidisciplinary approach. New Jersey: CMPMedica-United Business Media 2008; 9-15. [Cited in This Article: ] |
| 15. | Adopted by the 18th World Medical Association General Assembly (Helsinki, Finland, June 1964) and amended by the 64th World Medical Association General Assembly. 2013;. [Cited in This Article: ] |
| 16. | The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2: 333-339. HPB (Oxford). 2002;4:99-100. [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 237] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford). 2013;15:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 19. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18532] [Cited by in F6Publishing: 21909] [Article Influence: 1095.5] [Reference Citation Analysis (0)] |
| 20. | Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J Gastrointest Surg. 2009;13:486-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Poultsides GA, Paty PB. Reassessing the need for primary tumor surgery in unresectable metastatic colorectal cancer: overview and perspective. Ther Adv Med Oncol. 2011;3:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Brouquet A, Mortenson MM, Vauthey JN, Rodriguez-Bigas MA, Overman MJ, Chang GJ, Kopetz S, Garrett C, Curley SA, Abdalla EK. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg. 2010;210:934-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Jamal MH, Hassanain M, Chaudhury P, Tran TT, Wong S, Yousef Y, Jozaghi Y, Salman A, Jabbour S, Simoneau E. Staged hepatectomy for bilobar colorectal hepatic metastases. HPB (Oxford). 2012;14:782-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Mayo SC, Pawlik TM. Colorectal hepatic metastasis - current therapeutic approach. EGHR. 2011;7:54-60. [Cited in This Article: ] |
| 25. | Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353. [PubMed] [Cited in This Article: ] |
| 26. | Nordlinger B, Adam R, Arnold D, Zalcberg JR, Gruenberger T. The role of biological agents in the resection of colorectal liver metastases. Clin Oncol (R Coll Radiol). 2012;24:432-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ferrand F, Malka D, Bourredjem A, Allonier C, Bouché O, Louafi S, Boige V, Mousseau M, Raoul JL, Bedenne L. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer. 2013;49:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Scheer MG, Sloots CE, van der Wilt GJ, Ruers TJ. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann Oncol. 2008;19:1829-1835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD, Paty PB. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379-3384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 30. | Cirocchi R, Trastulli S, Abraha I, Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G, Platell C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD008997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Topal B, Kaufman L, Aerts R, Penninckx F. Patterns of failure following curative resection of colorectal liver metastases. Eur J Surg Oncol. 2003;29:248-253. [PubMed] [Cited in This Article: ] |
| 32. | Tan MC, Butte JM, Gonen M, Kemeny N, Fong Y, Allen PJ, Kingham TP, Dematteo RP, Jarnagin WR, D’Angelica MI. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxford). 2013;15:803-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Cardona K, Mastrodomenico P, D’Amico F, Shia J, Gönen M, Weiser MR, Paty PB, Kingham TP, Allen PJ, De Matteo RP. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol. 2013;20:148-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |