Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.428
Peer-review started: August 1, 2014
First decision: August 28, 2014
Revised: September 29, 2014
Accepted: October 23, 2014
Article in press: October 27, 2014
Published online: March 26, 2015
Tissue engineering essentially refers to technology for growing new human tissue and is distinct from regenerative medicine. Currently, pieces of skin are already being fabricated for clinical use and many other tissue types may be fabricated in the future. Tissue engineering was first defined in 1987 by the United States National Science Foundation which critically discussed the future targets of bioengineering research and its consequences. The principles of tissue engineering are to initiate cell cultures in vitro, grow them on scaffolds in situ and transplant the composite into a recipient in vivo. From the beginning, scaffolds have been necessary in tissue engineering applications. Regardless, the latest technology has redirected established approaches by omitting scaffolds. Currently, scientists from diverse research institutes are engineering skin without scaffolds. Due to their advantageous properties, stem cells have robustly transformed the tissue engineering field as part of an engineered bilayered skin substitute that will later be discussed in detail. Additionally, utilizing biomaterials or skin replacement products in skin tissue engineering as strategy to successfully direct cell proliferation and differentiation as well as to optimize the safety of handling during grafting is beneficial. This approach has also led to the cells’ application in developing the novel skin substitute that will be briefly explained in this review.
Core tip: Biomaterials and epithelial stem cells, and especially hair follicle stem cells are vital components for successful skin tissue engineering. Ignoring one of these components will decrease the opportunity for skin tissue engineering to foster complete healing through skin repair and will increase the failure of skin grafting in the clinical setting. The latest technology, new raw biomaterials and information on the significant contribution of stem cells are likely to be of great benefit to skin tissue engineering.
- Citation: Hilmi ABM, Halim AS. Vital roles of stem cells and biomaterials in skin tissue engineering. World J Stem Cells 2015; 7(2): 428-436
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/428.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.428
In classical bioengineered skin, the engineered epidermis is reconstructed from a skin autograft or allograft serving as a cellular dressing. The limitations addressed by this technique are pain and scar formation at the donor site, impaired wound healing and non-healing wounds, insufficient material to cover a large defect area and autoimmune rejection in the case of an allograft. The emergence of skin tissue engineering has led to robust innovation in skin substitutes and skin replacement products for burn and wound management. Diseased or injured skin may rule out a particular treatment because of wound depth. Superficial, partial and full-thickness wounds require diverse skin substitutes that simultaneously function as a primary dressing. Although various commercial skin substitutes are available, novel findings on fabrication techniques for biomaterials and on regulators of wound healing have highly encouraged scientists to develop new engineered skin substitutes that offer an effective remedy for wound care and wound management. The combination of stem cells or other cells with a specifically designed novel biomaterial has resulted in different impacts on engineered skin after wounding. An ideal biomaterial with multiple combinations of cultured cells and a collectively established broad knowledge of the healing process are the main criteria for the future development of skin substitutes. Eventually, skin substitutes that can be kept frozen, that are ready for use, inexpensive in cost, less labor intensive, and permanently adherent to the wound bed, that yield an impressive cosmetic outcome and that do not contain animal or human serum will definitely be in increased demand for skin tissue engineering purposes.
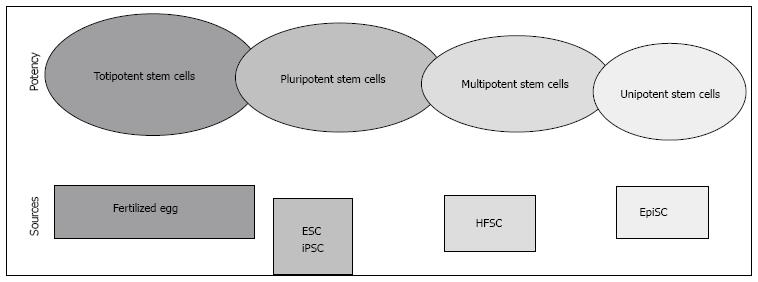
Stem cells have at least two advantageous properties. First, these cells are capable of dividing or renewing themselves for long periods of time with the identical morphology and phenotypic characteristics as their parent cells. This self-renewal mechanism is crucial for recovering degenerated and damaged cells, tissues or organs. Second, the differentiation of stem cells is a mechanism that can maintain the cellular and molecular integrity of adjacent or other cells. Stem cells differentiate into specialized cells to perform a specific function[1] after receiving internal or external signals, which are signs of signalling pathway changes in the cells’ microenvironment. Differentiation starts when the cells discontinue renewal. Differentiation abilities are used to rank stem cells’ potency (Figure 1). Stem cells that are able to differentiate into one cell type are known as unipotent stem cells, such as epidermal stem cells which regenerate differentiated epidermis[2]. Hair follicle stem cells (HFSC) are able to differentiate into multiple types of structures with specialized functions in the body including hair follicles[3], epidermis[4], sebaceous glands[5] and neurons[6]. These stem cells are known as multipotent stem cells. Pluripotent stem cells are stem cells that have the ability to differentiate into ectoderm, mesoderm and endoderm germ layers as demonstrated by embryonic stem cells and induced pluripotent stem cells[7]. Totipotent stem cells have the excellent ability to differentiate into whole tissues. This total potency can be observed in the fertilized eggs of humans or animals.
The skin is known as cutaneous or integumentary and its appendages constitute the integumentary system. The skin is the largest organ, contributing 15%-20% of body mass to form the external covering of the body. The skin has two main components: the epidermis and the dermis. The epidermis is derived from ectoderm, composed of a keratinized stratified squamous epithelium that grows continuously and simultaneously maintains its normal thickness by the process of desquamation[8]. The dermis is derived from mesoderm, composed of a connective tissue that provides mechanical support, strength and thickness to the skin. The hypodermis is not a part of the skin but lies beneath the dermis and is equivalent to the subcutaneous fascia. This layer contains adipose tissue arranged into lobules that are separated by connective tissue septa. The epithelial skin appendages are composed of the hair follicles and hairs, sweat glands, sebaceous glands, nails and mammary glands. The skin forms an effective barrier against pathogen invasion, chemicals and ultraviolet light[9]; participates in homeostasis[10] by regulating the body temperature and water loss; conveys sensory information; and function in pigmentation[11], physical appearance, wound repair and regeneration[12]. The skin is considered as a stem cell zoo, as it accommodates a variety of stem cell niches. A niche is the microenvironment in which stem cells remain quiescent. The dermal papilla is a stem cell niche for mesenchymal stem cells (MSC) which initiate hair follicle growth[13]. The superior bulge is a stem cell niche for HFSC[14] and melanocyte stem cells (MelSC)[15]. Multipotent HFSC not only function to maintain the hair growth cycle[16], but also give rise to sebaceous glands[17] and contribute to skin re-epithelialization[18], which form neo-epidermis by differentiating into keratinocytes within the wound. MelSC supply melanin for hair and skin pigmentation[19] and are concurrently involved in skin re-epithelialization[20]. The abundant fat in the subcutaneous layer contains adipose stem cells (ASC)[21] which are efficient in promoting skin repair[22]. Collectively, the evidence suggests that skin’s distinct resident stem cell pools have a crucial function in skin repair and regeneration. Therefore, it is very important for scientists to develop a good understanding of this idea before skin substitutes can be engineered in the future.
Skin tissue engineering is the first field within tissue engineering that successfully engineered tissue in the laboratory. As the largest organ, the skin has high potential risk of injuries and diseases. Skin damaged requires a skin replacement product from either a natural or an artificial source. Skin replacement products are in high demand for the treatment of burns and wounds, leading the industrial sector to highly invest in skin tissue engineering. Skin tissue engineering utilizes biomaterials, stem cells, connective tissues and an established broad knowledge of the mechanism of the acute and chronic healing processes. The main target of skin tissue engineering is to produce an excellent skin replacement product for application in wound repair, especially in the case of full-thickness skin loss, with the goal of less or no scar formation after wounding. However, skin replacement products are not able to replace damaged skin as completely as native skin can, leaving a scar. Consequently, the field of skin tissue engineering is challenging.
Collagen gel is the first biomaterial or skin replacement product that has been used in skin tissue engineering to replace the use of allografts and autografts. Essentially, to produce an excellent skin substitute, a biomaterial must be able to support cell growth and differentiation in a similar manner as in the cells’ original microenvironment. Most importantly, biomaterials with multiple graded pore sizes allow the acceleration of tissue reconstruction[23]. For example, microscopic pores improve cell attachment, proliferation and responses[24] and macroscopic pores that are at least 100 μm in diameter play a role in enhancing the ingrowth of cells and blood capillaries[25]. To allow three-dimensional (3-D) tissue reconstruction in vitro, a biomaterial should support uniform cell spreading into interconnected pores for the engineering specific tissue[26]. Biomaterials with a single pore size have certain limitations, only allowing one cell type to grow according to the pore size whereas, biomaterials with multiple graded pore sizes can reconstruct various types of tissues simultaneously[27]. A previous study has suggested that a pore size 20-120 μm in diameter is suitable for skin tissue engineering[28]. Biomaterials must have the ability to absorb the nutrients involved in wound healing and the exudate of the wound bed which are important factors in skin tissue engineering. The absorption ability is known as the water uptake ratio (WUR). Biomaterials with a high WUR are suitable for use in full-thickness wounds that contain excess exudates and inflammation that lead to impaired wound healing. Meanwhile, a lower WUR is suitable for partial thickness wounds[29]. An appropriate WUR enhances the biological activity of skin equivalents and contributes to hydrophilicity and the maintenance of 3-D structure. A moist wound bed is required to enhance dermal regeneration and wound closure which can be achieved if a scaffold has suitable water vapor permeability (WVP). The WVP depends on the scaffold thickness and the ratio of the scaffold area to the water surface area[30]. A suitable WVP is an important factor for wound dressing. If the WVP is too high, the wound bed will be dry and it may increase metabolic activity. In contrast, if the WVP is too low, the accumulation of exudates will trigger the onset of bacterial growth. Following the criteria mentioned above, a fabricated skin replacement product will retain the cell-cell and cell-biomaterial signaling, allowing the complete layer of skin to be engineered. Subsequently, the engineered skin substitute can be used for grafting purposes, this is how skin tissue engineering works. Most importantly, the engineered skin must be tolerated by the host, be retained permanently and later be able to degrade slowly over time. A permanent skin replacement product is a primary dressing that is ideal for application in great skin loss because it is used only once during treatment, until healing is completed. In contrast, a temporary skin replacement product must be changed many times during treatment which may increase the cost of wound management and wound care. Commercial skin replacement products are collagen, chitosan or fibrin-based and these products have been broadly used in clinical applications as shown in Table 1.
| Brand | Cell and biomaterial | Wound type |
| Apligraf®, United States | Human keratinocytes and fibroblasts cultured on collagen | Full thickness |
| Biobrane®, United Kingdom | Silicone membrane attached to a nylon mesh | Partial thickness |
| CellerateRX®, United States | Gel containing 65% collagen | Superficial |
| Cryoskin®, United Kingdom | Keratinocytes cultured on silicone | Superficial |
| Dermagraft®, United States | Cryopreserved human fibroblasts from foreskin cultured on a polyglactin mesh scaffold | Full thickness |
| EpiDex®, Switzerland | HFSC cultured on silicone | Full thickness |
| EpiFix®, United States | Human amniotic membrane | Full thickness |
| Integra®, United States | Semi-permeable silicone membrane composed of bovine tendon collagen | Full thickness |
| MyDerm™, Malaysia | Human fibroblasts and keratinocytes cultured on fibrin-silk | Full thickness |
| OASIS™, United States | Porcine small intestinal submucosa | Full thickness |
| OrCel™, United States | Human keratinocytes and fibroblasts cultured on bovine collagen | Full thickness |
| PriMatrix, United States | Collagen from fetal bovine dermis | Full thickness |
| Transcyte™, United States | Human newborn fibroblasts cultured on nylon mesh | Full thickness |
Apligraf is a permanent cellular based skin replacement product for the treatment of non-healing wounds including diabetic foot ulcer and venous leg ulcer. It was approved by the United States Food and Drug Administration’s guidelines (FDA) for use in non-infected, partial and full-thickness skin ulcers.
Biobrane is a temporary acellular based skin replacement product. It originated from porcine dermal collagen which is bonded to a nylon mesh and nylon membrane to form a biosynthetic skin dressing for use in superficial wound, partial-thickness wound and donor site wound dressing.
CellerateRX is a patented form of Type I bovine collagen for the treatment of diabetic or other impaired healing wounds. It is provided in a powder and a gel form which can be used as alone or a powder-gel combination.
Cryoskin is a cell spray-based skin replacement product and is prepared upon request by clinicians. It is made from allogeneic donor cells which are originally isolated from a newborn foreskin biopsy. Cryoskin spray is suitable for the treatment of chronic wounds and burns.
Dermagraft is a permanent cryopreserved cellular based skin replacement product. It composed of collagen which is isolated from neonatal foreskin biopsy and cultured on biodegradable mesh. Dermagraft was approved by the FDA for use in diabetic foot ulcers.
EpiDex is a permanent autologous epidermal skin replacement product that is isolated directly from epithelial stem cells of patients. The patient’s own hair was plucking using non-surgical procedure and then grafted onto a chronic wound.
EpiFix is a dehydrated amniotic membrane allograft. It is provided in sheets form, does not require FDA approval as human amniotic membrane is considered not significantly changed from its original structure. EpiFix is used in the treatment of acute and chronic wounds.
Integra is a bilayer engineered skin replacement product. It composed of porous matrix derived from bovine tendon collagen, glycosaminoglycan and a semi-permeable polysiloxane. Integra is used in the treatment of chronic and traumatic wounds.
MyDerm is an autologous bilayer engineered skin replacement product. It contained the mixture of fibrin-keratinocytes that seeded onto a piece of medical grade of silk. The mixture of fibrin-fibroblasts is then seeded onto fibrin-keratinocyte skin equivalent. MyDerm is used for the treatment of wounds and burns.
OASIS is a xenogeneic porous collagen matrix which derived from porcine small intestinal submucosa. It was approved by the FDA for the management of pressure ulcers, venous ulcers, diabetic ulcers and chronic vascular ulcers.
OrCel is an allogeneic bilayered cellular based matrix. It is biodegradable, made of composite bovine collagen which contained skin cells. OrCel was approved by the FDA for use in patients with dystrophic epidermolysis bullosa.
PriMatrix is a xenogeneic acellular based dermal matrix. It is derived from fetal bovine dermis. PriMatrix is provided in meshed, fenestrated and solid form. It was approved by the FDA for pressure and venous stasis ulcers.
Transcyte is an allogeneic bilayer skin replacement product for the treatment of burn. It made of human fibroblasts grown on nylon mesh and then combined with a synthetic epidermal layer. Transcyte has been approved by the FDA for temporary covering over burn wounds.
Collagen is the main protein component in connective tissue and its most abundant sources are found in mammals and marine animals. Collagen is easily obtained from porcine and bovine sources which provide bone, skin, tendon and many other parts of the body as raw materials. Unfortunately, prion disease that may transfer to humans after these materials’ use[31] has led scientists to have seek an alternative source. Currently, fish skin, seaweed, jellyfish and other marine sources are in high demand for isolating collagen and are the ideal sources for skin tissue engineering because prion disease transmission to humans is eliminated if marine-based sources are used compared with mammalian-based sources. Collagen-based biomaterials encode antimicrobial activity[32] and do not support colonization by bacteria in a full-thickness wound. The prevention of infection in wounds leads to minimized scar formation and promote wound healing[33]. Due to their biocompatibility and biodegradability, collagen biomaterials have been established for use in skin repair in the clinical setting since last decade with various forms such as a gel[34], sponge[35], film[36] and paste[37].
Chitosan is a biopolymer obtained from deacetylated chitin and the second most abundant biopolymer sources after collagen. Crude chitin can be found in shellfish. Chitosan is naturally insoluble unless in acidic solutions, and possesses a positive charge and low cytotoxicity which broadly imply excellence as a DNA carrier[38], protein nanocarrier[39], drug delivery system[40], siRNA nanovector[41] and growth factor carrier[42]. Based on its composition, the structure of chitosan is similar to that of glycosaminoglycans. Glycosaminoglycans are a component of the extracellular matrix and are an important substrate for tissue constructs[43]. Chitosan arrests bleeding in major hepatic injuries and is effective for use in patients who suffer major injury with clotting dysfunction[44]. Therefore, chitosan is preferable for tissue engineering and especially for engineered skin applications. The used of a novel technique to develop a chitosan sponge by electrospinning has produced a chitosan nanofiber that provides excellent substrate-cell adhesion, proliferation and differentiation[45]. During skin repair and regeneration, nanofibers contribute to increased vascularization, re-epithelialization and enhanced granulation tissue formation. The major skin cells, particularly epithelial and epidermal cells and also fibroblasts grow well on this type of biomaterial.
Fibrin-based biomaterials are well-established biological sealants for skin tissue engineering. However, the use of raw blood to isolate the fibrin has limited fibrin’s use as a novel skin substitute. Commercial fibrin in kit form can successfully heal a wound but is relatively expensive for the majority of patients. Fibrin gel is continuously in demand in plastics and reconstructive surgery as it minimizes subcutaneous seroma formation and decreases wound morbidity[46]. The combination of fibrin with bone marrow mesenchymal stem cells can improve the condition of scalded skin, providing strong self-repair capability and promising an acceptable cosmetic appearance with hair follicle formation. This application is more suitable for the treatment of patients with burns in emergency cases[47]. Long-lasting fibrin biomaterials ensure stable and functional angiogenesis by highly tunable and sustained delivery of growth factors[48].
Mesenchymal stem cells also known as mesenchymal stromal cells, have broadly contributed to cellular therapy and skin tissue engineering. MSC are spindle-shaped, or similar to morphology to but phenotypically different from fibroblasts. Bone marrow derived-MSC were first isolated in 1981[49] before MSC from other tissues were explored. Skin derived-MSC were first isolated from mice in 2001, and were initially named skin-derived precursors[50]. In the skin, MSC are predominantly found in the dermal papilla, functioning in secreting diverse growth factors after wounding to promote fibroblast proliferation and collagen formation and to elicit intrinsic stem cell differentiation[51], serving as a modulator to activate macrophages[52], and directly affecting hair follicle morphogenesis[53] and neo-dermis reconstruction[54]. MSC elicit leukocyte migration for skin homeostasis and produce hepatocyte growth factor and basic fibroblast growth factor to inhibit scar formation at the wound site[55]. The MSC-based skin substitute constructs have increased paracrine factor levels in promoting skin repair[56].
Apart from stem cells, other components of the dermis such as fibroblasts are important in skin tissue engineering[57]. Fibroblasts synthesize and deposit extracellular protein after wounding, producing growth and angiogenic factors that accelerate cell proliferation and angiogenesis[58]. Fibroblasts are also transformed into myofibroblasts that express contractile fibers. As a myofibroblast contracts, forces are applied to the collagen fibers, thus reducing the size of the wound[59]. Cultured fibroblasts in biomaterials have been reported to improve regeneration of the dermis[60] and epidermis[61] especially by re-epithelialization[57]. This specific role of fibroblasts, especially in improving scarring and the contraction of skin wounds has encouraged researchers to highly prioritize facilitating these cells’ use in skin replacement therapies[62]. Additionally, a previous study has demonstrated that fibroblasts from the dermis are the best source for skin tissue engineering applications compared with fibroblasts from other sources based on yields and reduced contraction of wounds[63].
Fat accommodates adipose tissue-derived stem cells usually known as adipose stem cells[21]. ASC are actually MSC therefore, ASC express the same surface markers and highly contribute to skin repair as well. ASC improve neo epidermis formation and skin aging[64] by accelerating angiogenesis[65] and fibroblast proliferation[66]. The use of ASC in skin repair increases the deposition of extra cellular matrix components such as collagen type-VI which has the potential to improve the protein deficiency[67]. A mixture of fibroblasts and ASC can improve the epidermal morphogenesis of tissue-engineered skin[68]. The ability of ASC to grow on porous biomaterial and to differentiate into fibrovascular, endothelial and epithelial tissues for skin repair[69] has proven these cells’ excellent capability as a cellular source for skin tissue engineering.
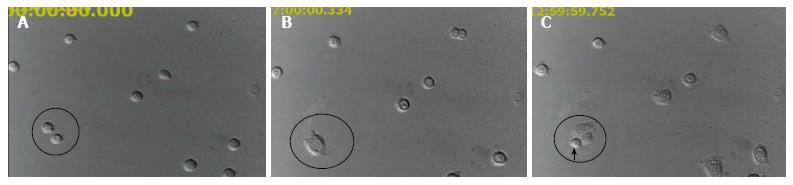
HFSC and their progenitors are directly involved in hair and skin regeneration during skin homeostasis[70]. Based on their anatomical classification and function, HFSC have mainly been called epithelial stem cells[71,72] and have been broadly known as epidermal stem cells[73,74] as well as bulge stem cells[75,76] in diverse studies. The critical role of HFSC in epidermal homeostasis[77] has led to their application in fabricating neo epidermis in skin tissue engineering. Due to their vital characteristic, HFSC are capable of proliferating rapidly and this phenomenon is shown in Figure 2.
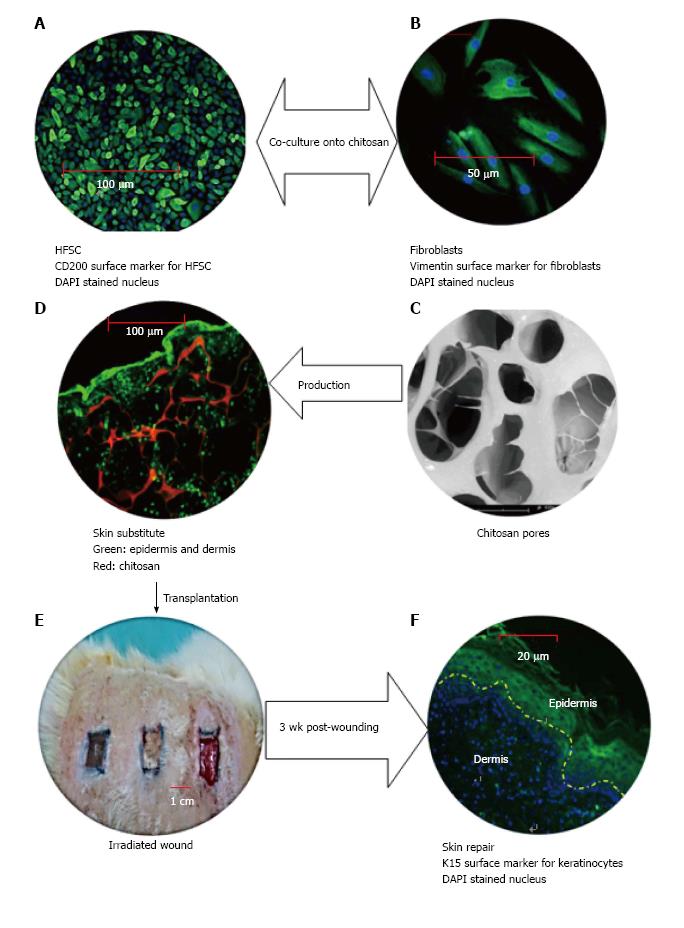
The use of HFSC in skin tissue engineering was successfully performed in chronic wounding in 2002, when Limat and Hunziker demonstrated that their HFSC-silicone constructs contributed to accelerated the healing of patients’ leg ulcers[78]. To date, the contribution of HFSC to ameliorating impaired healing due to radiation has stimulated the interest of tissue engineers in using epithelial stem cells for other tissue reconstruction. In Figure 3 shows that the chitosan based skin substitute which composed of HFSC-fibroblasts is an ideal source for skin tissue engineering. The chitosan composite was then used for transplanting into irradiated wound. Full-thickness wounds 1 cm by 1 cm in size were excised and treated using the novel skin substitute. As a result, a complete re-epithelialization occurred during skin repair after 3 wk post-wounding.
This review has summarized the fundamentals of stem cells and especially the terminology for stem cell potency which has occasionally created confusion in certain experiments. Stem cell potency provides the necessary guideline for skin engineering experiments. The importance of fibroblast, HFSC, MSC, ASC, chitosan, collagen and fibrin in skin tissue engineering highlights their critical role in the repair process. The use of stem cells and biomaterials is especially important in the case of impaired wound healing and in cases involving major excisional skin defects.
P- Reviewer: Hara M, Soker S S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Hilmi ABM, Halim AS, Noor NM, Lim CK, Idris Z, Pohchi A, Asma H, Wahab SFA, Tiede S, and Paus R: A simple culture method for epithelial stem cells derived from human hair follicle. Central European Journal of Biology. 2013;8:432-439. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Potten CS, Booth C. Keratinocyte stem cells: a commentary. J Invest Dermatol. 2002;119:888-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol. 2014;16:179-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Liang X, Bhattacharya S, Bajaj G, Guha G, Wang Z, Jang HS, Leid M, Indra AK, Ganguli-Indra G. Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis. PLoS One. 2012;7:e29999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol. 2012;363:138-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016-1020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Halim AS, Lim CK, Mohd Hilmi AB, Arman Zaharil MS. New Era of Regenerative Medicine: an Islamic Perspective. Cell and Tissue Culture: Research and Technology from Islamic Perspective. Gombak: IIUM Press 2014; 110-142. [Cited in This Article: ] |
| 8. | Ross MH, Kaye GI, Pawlina W. Histology: a text and atlas with cell and molecular biology. Philadelphia: Lippincott Williams & Wilkins 2003; . [Cited in This Article: ] |
| 9. | Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063-1072. [PubMed] [Cited in This Article: ] |
| 10. | Dean LG, Breslin A, Ross EZ. Is it hot in here? Thermoregulation and homeostasis through an exercise activity. Adv Physiol Educ. 2014;38:99-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Yu M, Finner A, Shapiro J, Lo B, Barekatain A, McElwee KJ. Hair follicles and their role in skin health. Exp Rev Dermatol. 2006;1:855-871. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Mohd Hilmi AB, Halim AS, Jaafar H, Asiah AB, Hassan A. Chitosan dermal substitute and chitosan skin substitute contribute to accelerated full-thickness wound healing in irradiated rats. Biomed Res Int. 2013;2013:795458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 14. | Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351-1354. [PubMed] [Cited in This Article: ] |
| 15. | Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Purba TS, Haslam IS, Poblet E, Jiménez F, Gandarillas A, Izeta A, Paus R. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays. 2014;36:513-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Niemann C, Horsley V. Development and homeostasis of the sebaceous gland. Semin Cell Dev Biol. 2012;23:928-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Takeda N, Jain R, Leboeuf MR, Padmanabhan A, Wang Q, Li L, Lu MM, Millar SE, Epstein JA. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013;140:1655-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19:924-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Sotiropoulou PA, Candi A, Mascré G, De Clercq S, Youssef KK, Lapouge G, Dahl E, Semeraro C, Denecker G, Marine JC. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Meruane MA, Rojas M, Marcelain K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2012;130:53-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184-198. [PubMed] [Cited in This Article: ] |
| 24. | Ma PX, Choi JW. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23-33. [PubMed] [Cited in This Article: ] |
| 25. | Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065-2073. [PubMed] [Cited in This Article: ] |
| 26. | Zeltinger J, Sherwood JK, Graham DA, Müeller R, Griffith LG. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7:557-572. [PubMed] [Cited in This Article: ] |
| 27. | Oh SH, Park IK, Kim JM, Lee JH. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials. 2007;28:1664-1671. [PubMed] [Cited in This Article: ] |
| 28. | Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA. 1989;86:933-937. [PubMed] [Cited in This Article: ] |
| 29. | Guptaa B. Textile-based smart wound dressings. Indian J of Fibre &. Textile Research. 2010;35:174-187. [Cited in This Article: ] |
| 30. | Hu Y, Topolkaraev V, Hiltner A, and Baer E. Measurement of water vapor transmission rate in highly permeable films. J Appl Polym Sci. 2001;81:1624-1633. [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Aguzzi A, Zhu C. Five questions on prion diseases. PLoS Pathog. 2012;8:e1002651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Abdillahi SM, Balvanović S, Baumgarten M, Mörgelin M. Collagen VI encodes antimicrobial activity: novel innate host defense properties of the extracellular matrix. J Innate Immun. 2012;4:371-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Zhou Y, Yang H, Liu X, Mao J, Gu S, Xu W. Potential of quaternization-functionalized chitosan fiber for wound dressing. Int J Biol Macromol. 2013;52:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Grunert P, Borde BH, Hudson KD, Macielak MR, Bonassar LJ, Härtl R. Annular repair using high-density collagen gel: a rat-tail in vivo model. Spine (Phila Pa 1976). 2014;39:198-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Shin YH, Seo YK, Yoon HH, Yoo BY, Song KY, Park JK. Comparison of hair dermal cells and skin fibroblasts in a collagen sponge for use in wound repair. Biotechnology and Bioprocess Engineering. 2011;16:793-800. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43-56. [PubMed] [Cited in This Article: ] |
| 37. | Shevchenko RV, Sibbons PD, Sharpe JR, James SE. Use of a novel porcine collagen paste as a dermal substitute in full-thickness wounds. Wound Repair Regen. 2008;16:198-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Lu H, Dai Y, Lv L, Zhao H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS One. 2014;9:e84703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Mattu C, Li R, and Ciardelli G: Chitosan nanoparticles as therapeutic protein nanocarriers: The effect of ph on particle formation and encapsulation efficiency. Polymer Composites. 2013;34:1538-1545. [DOI] [Cited in This Article: ] |
| 40. | Meng L, Huang W, Wang D, Huang X, Zhu X, Yan D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules. 2013;14:2601-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Liu X, Ma L, Mao Z, and Gao C. Chitosan-based biomaterials for tissue repair and regeneration. Chitosan for Biomaterials II: Springer 2011; 81-127. [Cited in This Article: ] |
| 42. | Tsuchiya N, Sato S, Kigami R, Kawano E, Takane M, Arai Y, Ito K, Ogiso B. Effect of a chitosan sponge impregnated with platelet-derived growth factor on bone augmentation beyond the skeletal envelope in rat calvaria. J Oral Sci. 2014;56:23-28. [PubMed] [Cited in This Article: ] |
| 43. | Hilmi AB, Halim AS, Hassan A, Lim CK, Noorsal K, Zainol I. In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. Springerplus. 2013;2:79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Millner R, Lockhart AS, Marr R. Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in the presence of moderate systemic heparinisation. Ann R Coll Surg Engl. 2010;92:559-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Dhurai B, Saraswathy N, Maheswaran R, Sethupathi P, Vanitha P, Vigneshwaran S, and Rameshbabu V: Electrospinning of curcumin loaded chitosan/poly (lactic acid) nanofilm and evaluation of its medicinal characteristics. Frontiers of Materials Science. 2013;7:350-361. [DOI] [Cited in This Article: ] |
| 46. | Köhler G, Koch O, Antoniou S, Lechner M, Mayer F, and Emmanuel K. Prevention of Subcutaneous Seroma Formation in Open Ventral Hernia Repair Using a New Low-Thrombin Fibrin Sealant. World J Surg. 2014;38:1-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Zhang W, Li Y, Fang G, Zhang K. Scalded skin of rat treated by using fibrin glue combined with allogeneic bone marrow mesenchymal stem cells. Ann Dermatol. 2014;26:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Sacchi V, Mittermayr R, Hartinger J, Martino MM, Lorentz KM, Wolbank S, Hofmann A, Largo RA, Marschall JS, Groppa E. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF164. Proc Natl Acad Sci USA. 2014;111:6952-6957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 49. | Fridenshteĭn AIa. [Stromal bone marrow cells and the hematopoietic microenvironment]. Arkh Patol. 1982;44:3-11. [PubMed] [Cited in This Article: ] |
| 50. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [PubMed] [Cited in This Article: ] |
| 51. | Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 52. | Ulivi V, Tasso R, Cancedda R, Descalzi F. Mesenchymal stem cell paracrine activity is modulated by platelet lysate: induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev. 2014;23:1858-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Ramos R, Guerrero-Juarez CF, Plikus MV. Hair follicle signaling networks: a dermal papilla-centric approach. J Invest Dermatol. 2013;133:2306-2308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 55. | Ennis WJ, Sui A, Bartholomew A. Stem Cells and Healing: Impact on Inflammation. Adv Wound Care (New Rochelle). 2013;2:369-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 572] [Cited by in F6Publishing: 509] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 57. | Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 722] [Cited by in F6Publishing: 797] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 58. | Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell. 2011;22:3791-3800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 59. | Holloway S, Harding K, Stechmiller JK, Schultz G. Acute and chronic wound healing. Wound care essential. 3rd ed. Ambler: Lippincott Williams & Wilkins 2012; 89. [Cited in This Article: ] |
| 60. | Souto LR, Rehder J, Vassallo J, Cintra ML, Kraemer MH, Puzzi MB. Model for human skin reconstructed in vitro composed of associated dermis and epidermis. Sao Paulo Med J. 2006;124:71-76. [PubMed] [Cited in This Article: ] |
| 61. | Wang HM, Chou YT, Wen ZH, Wang CZ, Chen CH, Ho ML. Novel biodegradable porous scaffold applied to skin regeneration. PLoS One. 2013;8:e56330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Levinson H. A Paradigm of Fibroblast Activation and Dermal Wound Contraction to Guide the Development of Therapies for Chronic Wounds and Pathologic Scars. Adv Wound Care (New Rochelle). 2013;2:149-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | van den Bogaerdt AJ, van Zuijlen PP, van Galen M, Lamme EN, Middelkoop E. The suitability of cells from different tissues for use in tissue-engineered skin substitutes. Arch Dermatol Res. 2002;294:135-142. [PubMed] [Cited in This Article: ] |
| 64. | Metral E, Santos MD, Amélie Thépot1 WR, Mojallal A, Auxenfans C, and Damour O. Adipose-derived Stem Cells Promote Skin Homeostasis and Prevent its Senescence in an In vitro Skin Model. J Stem Cell Res Ther. 2014;4:194. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Zhao J, Hu L, Liu J, Gong N, Chen L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res Int. 2013;2013:578479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Alexeev V, Arita M, Donahue A, Bonaldo P, Chu ML, Igoucheva O. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res Ther. 2014;5:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Lu W, Yu J, Zhang Y, Ji K, Zhou Y, Li Y, Deng Z, Jin Y. Mixture of fibroblasts and adipose tissue-derived stem cells can improve epidermal morphogenesis of tissue-engineered skin. Cells Tissues Organs. 2012;195:197-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 2009;27:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 70. | Hsu YC, Fuchs E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat Rev Mol Cell Biol. 2012;13:103-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 71. | Yang R, Zheng Y, Burrows M, Liu S, Wei Z, Nace A, Guo W, Kumar S, Cotsarelis G, Xu X. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat Commun. 2014;5:3071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 72. | Jaks V, Kasper M, Toftgård R. The hair follicle-a stem cell zoo. Exp Cell Res. 2010;316:1422-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 569] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 74. | Blanco S, Kurowski A, Nichols J, Watt FM, Benitah SA, Frye M. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 2011;7:e1002403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 75. | Demehri S, Kopan R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development. 2009;136:891-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Zhang Y, Yu J, Shi C, Huang Y, Wang Y, Yang T, Yang J. Lef1 contributes to the differentiation of bulge stem cells by nuclear translocation and cross-talk with the Notch signaling pathway. Int J Med Sci. 2013;10:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 866] [Cited by in F6Publishing: 858] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 78. | Limat A, Hunziker T. Use of epidermal equivalents generated from follicular outer root sheath cells in vitro and for autologous grafting of chronic wounds. Cells Tissues Organs. 2002;172:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |