INTRODUCTION
Having been established from the inner cell mass of the blastocyst, embryonic stem cells (ESCs) possess pluripotency and can theoretically differentiate into all kinds of embryonic tissue. During the last decade, a number of studies have reported the differentiation of ESCs into a range of embryonic tissues. These compelling results were done either by stimulating the cells with particular molecules or by simulating the environmental cues of the early embryo. Importantly, these differentiated cells can then be used in regenerative medicine and for drug discovery. Hence, it is important to elucidate the detailed involvement of signals and signaling pathways in these processes before these cells are used for therapeutic purposes. Compared to development in vivo, neurons that differentiate from ESCs in vitro seem to develop via a similar pattern and thus have become a promising field in terms of the medical applications in stem cell research.
Basically, a default model for development in vivo is hypothesized as that induction of neural differentiation reflects the earliest fate in determining neurons in the ectoderm. The neural inducers involved in the process have been found to be bone morphogenetic protein (BMP)-binding molecule called noggin in Xenopus[1] and FGF in chicken[2]. Utilizing a serum-free and patterning factor-free condition to cultivate mouse ESCs (mESCs), Tropepe et al[3] found that neural progenitors differentiated specifically from these stem cells. Neural differentiation occurs spontaneously and does not require the presence of any extrinsic neural inducer in a special culture system named serum-free culture of embryoid body-like aggregates with quick reaggregation (SFEBq)[4]. ESCs were found to selectively differentiate into neural progenitors efficiently (> 95% of total cells). The endogenous signals can also be minimized by adding the inhibitors of Wnt and/or Nodal such as dickkopf-1 and/or lefty-1, respectively. Furthermore, utilizing transforming growth factor β (TGFβ) antagonists to abolish the SMAD signaling is shown to enhance neural differentiation, especially in induced pluripotent stem cells and human ESCs (hESCs)[5]. All the results are in agreement with the ‘‘neural default model’’ of embryonic development.
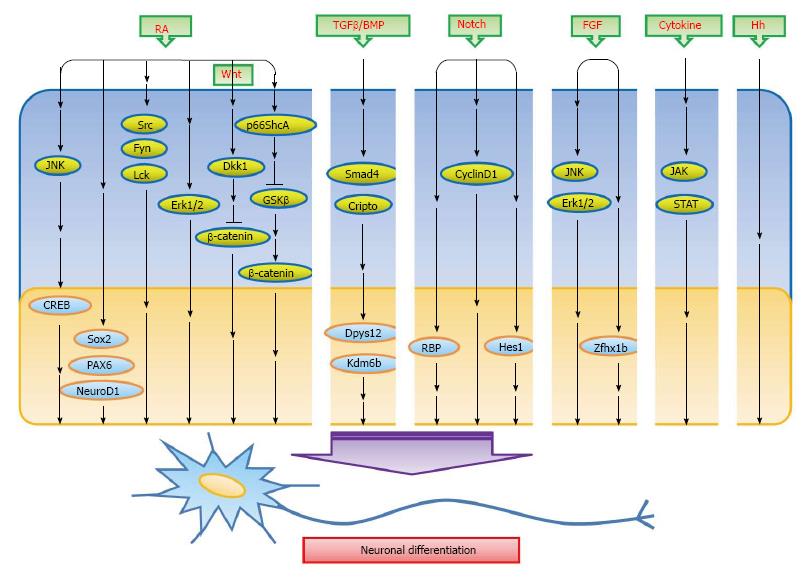
Neural cells were one of the first lineages to be developed from ESCs in vitro[6,7]. Many studies have effectuated subsequently to differentiate ESCs into the creation of different cellular subtypes such as neurons and glial cells. These ESCs can be surgically transferred back into vertebrate embryos and are then participated in brain development[8]. Work on ESCs over the last three decades has been concentrated on elucidating the characteristics of stem cells, particularly the differentiation approaches to obtain specific cell types such as neurons. Neural differentiation by ESCs is able to provide insights into the mechanisms involved in neural induction. In this review, we focus on obtaining an improving understanding of the overall signaling that underlies neural differentiation from ESCs in vitro. The inducers and pathways explored include retinoic acid (RA), Wnt/β-catenin, TGF/BMP, Notch, FGF, cytokine, Hedgehog, JNK/mitogen-activated protein kinase (MAPK) and others (Figure 1). This should help researchers with exploring the various areas of stem cell biology and also help to enhance the application of ESCs differentiation in regenerative medicine.
Figure 1 A simplified scheme outlining the signaling pathways described in the text.
The inducers that induce embryonic stem cells to differentiate into neurons reported thus far including RA, Wnt/β-catenin, TGF/BMP, Notch, FGF, cytokine, Hedgehog, JNK/MAPK and others. The inducers in the middle part of figure mediate signaling molecules that bring about the differentiation into neurons. → means stimulation. Some miscellaneous molecules/factors mentioned at the end of this text are not included in this figure. RA: Retinoic acid; TGF: Transforming growth factor; BMP: Bone morphogenetic protein; CREB: cAMP response element-binding protein; Dkk-1: Dickkopf-related protein 1; Erk: Extracellular signal-regulated kinase; FGF: Fibroblast growth factor; Hh: Hedgehog; JAK: Janus kinase; JNK: C-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase.
THE RETINOIC ACID INDUCTION PATHWAY
RA typically is used at a range of concentrations from 5 μmol/L to 5 mmol/L in order to facilitate the differentiation of ESCs into neural progenitor cells (NPCs). Shan et al[9] found that the active cyclic AMP response element-binding protein (CREB) protein was obviously increased after treatment with 5 μmol/L RA treatment during the differentiation/formation of embryoid body (EB). Inhibition of CREB activity was found to result in EBs switching the differentiation to other germ layers fate, while enhanced expression of CREB was found to augment NPCs differentiation. RA promoted the expression of active CREB by increasing the activity of c-Jun N-terminal kinase (JNK). These results show that JNK/CREB is likely to be a crucial factor in the RA-induction of NPCs differentiation[9]. In addition, Theus et al[10] found that neurons derived from ESCs after RA induction led to significant neurite growth. This event is associated with an spread expression pattern of Src kinase from the cell body to the neurite processes and an up-regulated expression of Src, Fyn and Lck[10]. These neurons were characterized by the expression of neurofilament, synaptophysin and NMDA as well as the presence of kainate currents; they were found to have become vulnerable to excitotoxicity and went on to form functional excitatory synapses. Since these events were abolished when the cells were grown in the addition of the Src family kinase inhibitor PP2, it seems likely that the pathway induced by RA is mediated via Src[10]. Furthermore, Tonge et al[11] demonstrated that neural differentiation of hESCs and embryonal carcinoma cells induced by RA needs both prolonged exposure of RA and cellular interaction that is done by the presence of a high cell density. These factors are required for the increase of the expression of various neural genes (NeuroD1, PAX6 and Sox2) and the development of a neuronal appearance. They also found that inhibition of GSK3β activity was able to block the RA-induced differentiation of neural lineage derived from ESCs. This finding suggests a role for properly modulated Wnt signaling in this process[11]. After RA induction for 1-5 d, Li et al[12] found that there is a dramatic increase in extracellular signal related kinase 1/2 (Erk 1/2) phosphorylation (p-Erk 1/2) and that this can be attenuated by treatment of U0126, a p-Erk 1/2 inhibitor. Furthermore, both the expression of associated cytoskeletal proteins and the number of NeuN-positive cells are dramatically reduced after the inhibition of p-Erk 1/2. As a result there was an increase in the differentiating ESCs of the nuclear translocation of STAT3, together with a decrease in the expression of NGF, BDNF and GDNF molecules. These results imply that phosphorylation/activation of Erk 1/2 is a key signaling that is essential for survival of ESCs and early neural differentiation[12]. Recently, Glaser et al[13] found that when mESCs were initiated to progress into neural differentiation with RA, the expression of Oct-4 and the P2X7 receptor, an ATP-gated cation channel, were reduced. Utilizing KN-62, a specific P2X7 receptor inhibitor, they found an increased number of SSEA-1 and type III β-tubulin expressing double-positive cells. This confirms the appearance of neuroectodermal differentiation and it would seem that the neural fate determination of mESCs is dependent on suppression of P2X7 receptor activity[13].
RA could also mediate crosstalk among other signaling pathways such as the Wnt/β-catenin, FGF, and Erk pathways in order to induce neural differentiation. This is based on the finding that 4-d of RA treatment substantially increases the synthesis of the Dickkopf-related protein 1 (Dkk-1), a Wnt antagonist, and induces the expression of the Wnt/Dkk-1 co-receptor LRP6[14]. When recombinant Dkk-1 was utilized, the EBs presented in a similar manner to treatment with RA, namely there was an induction of two neural markers, the distal-less homeobox gene (Dlx-2) and nestin gene. Dkk-1 overexpression was found to be able to block the Wnt pathway, as evidenced by a decrease of β-catenin protein in the nucleus. These findings show that the prevention of the canonical Wnt pathway is a prerequisite for neural differentiation of ESCs when this is induced by RA treatment[14]. Conversely, judging from the expression of neural marker Hoxc4, Otero et al[15] found that neural differentiation can be initiated by overexpressing β-catenin alone or combination with RA. Nevertheless, RA treatment was found to inhibit the β-catenin-induced production of tyrosine hydroxylase positive neurons, which suggests that the effects of RA are only partially dependent on β-catenin signaling. These results also suggest that β-catenin signaling enhances determination of neural lineage in ESCs. Moreover, β-catenin signaling could play a role of required co-factor in RA-induced pathway so as to permit the neural differentiation[15]. Papadimou et al[16] reported that p66ShcA is increased during neural induction of ESCs in vitro. Overexpression of p66ShcA in ESCs ablates GSK-3β kinase activation which in turn to stabilize β-catenin protein. In parallel, p66ShcA over-expression was found to result in both mESCs and hESCs undergoing neural induction as predicted and accelerated neural differentiation. Thus there seems to be a role for p66ShcA in the regulation of Wnt/β-catenin pathway as well as in ESCs neutralization. Based on the above, p66ShcA would seem to also participate in a part of the RA-induction pathway[16]. Furthermore, Engberg et al[17] monitor ESCs containing reporter genes that allowed the detection of markers associated with the early neural plate and the primitive streak and its progeny. When RA signaling is inhibited, they found that the change from neural to mesodermal fate develops. In addition, neural induction in ESCs needs RA to block Nodal signaling. Thus, the mechanism by which Wnt signaling pathway inhibits neural development could be interpreted as via facilitation of Nodal signaling pathway[17]. Stavridis et al[18] shows that retinoid repression of fibroblast growth factor (FGF) signaling is able to promote the onset of neural differentiation. Induction of FGF8 by RA and subsequent Erk activity under early differentiation conditions could function to ascertain the loss of self-renewal. Nevertheless, a progressing inhibition of FGF4 by RA would seem to be associated with an overall decrease in Erk activity at the later stage. The admission of a neural or a non-neural fate is therefore decided by an inhibition of FGF signaling. Hence, inhibition of FGF/Erk activity would enhance ESCs self-renewal, but a subsequent abolishment of FGF signaling seems to have the opposite effect and act as a driver for differentiation[18].
THE TGFβ/BMP PATHWAY
It has been speculated that a default mechanism for neural differentiation might be involved in regulating the property of neural stem cell identity directly from ESCs. As above-mentioned, Tropepe et al[3] characterized that the neural lineage of differentiation from a nascent stem cell is modulated negatively by TGFβ-related signaling. Moreover, differentiated mESCs in vitro with Smad4 or Cripto genes knockout have been found to produce increased numbers of neurons[19]. The profiles analysis of gene expression in vitro further demonstrates that cells bearing Smad4 gene deletion were inclined to possess expressing patterns of mid-hindbrain and anterior hindbrain. However, the Cripto knockout cells tended to express gene markers of rostral central nervous system (CNS) in addition to other previous genes. Thus it would seem that Smad4-/- ESCs exhibit differentiation of mesoderm while Cripto-/- ESCs develop into epidermal/neuroectodermal cell types[19]. To investigate the role of BMP-4 in the determination of either epidermal or neural fate, Gambaro et al[20] demonstrated that treatment of BMP-4 on murine ESCs results in the significant apoptosis of neural precursor cells which contain Sox-1 expression. Furthermore, counteraction of the SMAD pathway by overexpression of SMAD6, an inhibitor SMAD (I-SMAD), hinders the BMP4-induced apoptosis. Utilizing Noggin and SB431542, Chambers et al[5] shown that these two inhibitors of SMAD signaling are sufficient to allow the induction of neural differentiation derived from hESCs.
Genome-wide mapping was used to obtain plausible downstream candidates within the TGFβ/BMP pathway that are involved in ESCs differentiation. Fei et al[21] mapped the gene promoters on a genome-wide scale to search for the target sequences bound with SMAD1, SMAD4, and SMAD5. They found that these molecules were associated with many developmental regulators and these were abundant in terms of H3K4 and H3K27 trimethylation bivalent markers. These promoters were found to be repressed when cells were in the self-renewing state, whereas these promoters underwent rapid induction upon differentiation. In the same context, the results from SMAD loss-of-function experiments further supported the hypothesis that BMP mediating signaling via SMAD does not directly affect self-renewal, whereas is necessary for various processes relevant to differentiation. Within the various SMAD-associated genes, they were able to identify two regulators which have been known to participate in the early neural differentiation regulated by BMP. These genes are Dpysl2 (also known as Crmp2) and the H3K27 demethylase Kdm6b (also known as Jmjd3). Bertacchi et al[22] also adopted a global gene expression approach and were able to show that mESCs produce, secrete, and respond to BMPs during neural differentiation in vitro. Utilizing the analysis of several markers of dorsoventral and anterior/posterior identity, they found that the gene expression pattern of differentiated ESCs reflects the midbrain identity. They also revealed that the endogenous BMPs during neural differentiation principally function to inhibit the expression of genes with a telencephalic profile. This phenomenon was evidenced by treating ESCs with a number of BMP inhibitors or Noggin.
THE NOTCH PATHWAY
Lowell et al[23] explored the role of Notch receptors and ligands in mESCs. They found that genetic manipulation that the constitutively activated Notch does not change the phenotype of stem cells. Nonetheless, these cells differentiate exclusively and promptly into the neural lineage upon abolishment of self-renewal stimuli. Conversely, genetic or pharmacological interference with Notch signaling inhibits the determination of neural fate. The neural commitment enhancing by Notch needs parallel signaling through the FGF receptor. Since expression of Notch ligand in stromal cells also induces the neural differentiation of hESCs, it indicates this pathway is conserved within pluripotent stem cells[23]. Das et al[24] investigate the role of the Notch pathway by engineering a mESCs line such that there were short pulses of activated Notch. The alteration of Notch protein could be induced at the various stages of neural differentiation in vitro. The results show that activation of Notch signaling for 6 h specifically at day 3 during neural induction from ESCs was found to lead to dramatically increase cell proliferation. This outcome is associated with the cyclin D1 expression induced by Notch. In contrast, a decrease of cyclin D1 was observed during the development of the CNS in mouse embryos without Notch signaling. The ESCs containing a dominant negative form of cyclin D1 was found to abrogate the Notch-induced cell proliferation. These seem to indicate the presence of a special function for Notch in regard of temporal context. These findings also confirm that cyclin D1 is a crucial signaling molecule in Notch-induced differentiation/proliferation in ESCs[24].
Downstream of the Notch pathway, the protein RBP was also show to play a key role. Main et al[25] obtained RBP+/- mice using RBPJkloxP/loxP mice[26] bred with CMV-cre mice. After RBP-/- mESCs had been obtained and cultured at low density, they were found to behave in the same way as wild-type cells in terms of the origin of apical specification and neural progenitors. When ESCs undergo development through the rosette formation, RBP was found to be required for the modulation of neuronal differentiation and for the appropriate preservation of rosette structure. Utilizing inhibitors of Notch and/or loss-of-function analysis of Notch signaling resulted in the disintegration of neural rosettes and an acceleration of neuronal differentiation. Rosette integrity was also found to demand Rho kinase activity and actin polymerization in addition to requiring normal Notch signaling. However, it is worth noting that rosette maintenance is not required as a prior condition for regular neuronal differentiation. Various results demonstrate that Notch signaling also plays a role in the maintenance and organization of polarity during early nervous system development[25]. Furthermore, it was also showed that Hes1-high ESCs are inclined to a mesodermal fate, whereas Hes1-low ESCs are inclined to a neural fate[27]. Kobayashi et al[28] further showed that Hes1-low and Hes1-high ESCs are respectively correlated with cells that have undergone activation and inactivation of Notch signaling. Although Notch and Hes1 function in the same direction in most other cell types, the abovementioned results show that both signaling leads to opposite effect during ESCs differentiation. That is, Hes1 would seem to be not the downstream signaling molecule of Notch pathway during ESCs differentiation.
It was known that both activation of the Sonic Hedgehog (Shh) pathway and inhibition of the Notch pathway induce the neural differentiation during the neural tube development in vivo. To distinguish the effect of Shh and/or Notch signaling on ESCs-derived EBs, Crawford and Roelink used N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), an inhibitor of Notch, to investigate how critical the Shh signaling is in the EBs formation and neural differentiation in vitro. It was found that DAPT led to promote neuronal differentiation. On the other hand, more interneurons were identified in the absence of Shh. They also found that the effect of DAPT on EBs with an activated Shh signaling is associated with the premature disappearance of markers involved in the ventral neuronal precursors[29].
THE FGF PATHWAY
Chen et al[30] showed that FGF family includes FGF1, FGF2, and FGF4, but not FGF8b, are able to enhance the neurogenesis of mESCs during serum-free neural induction. They found that the enhanced neurogenesis by FGF is not mediated through a promotion of the proliferation of Sox1+ cells or via a rescue of apoptosis. It was found to involve the inactivation of JNK-1 and Erk-2, but did not involve p38 MAPK, which is known to inhibit neural formation by inhibiting ESCs differentiation. Furthermore, ESCs that lacked FGF4 or have been treated with a FGF receptor inhibitor were found to be resistant to neural and mesodermal induction[30]. Kunath et al[31] found that activated Erk 1/2 induced by FGF4 is a stimulus by which naive ESCs are able to be released from the self-renewal program. FGF4 is capable of initiating differentiation activity. The important role of Erk downstream signaling was further explored by an examination of Erk2 deficient ESCs, which fail to proceed to either mesodermal or neural differentiation and retain their pluripotency. On the other hand, FGF2 which functions for the maintenance of epiblast stem cells and hESCs was found to inhibit development of early neural cells by epiblast intermediates. Nevertheless, FGF2 alone is sufficient to enhance self-renewal of epiblast stem cells. Conversely, FGF8, the endogenous inducer for embryonic neural differentiation, promotes more homogenous neural induction that is accompanied by transient self-renewal of early neural cells. They also found that completely blocking of FGF signaling in epiblast cells enhances prompt neural induction as well as the succeeding neurogenesis. Therefore, FGF signaling pathway seems to play a variety of roles during the different stages of ESCs differentiation[31].
Dang et al[32] investigated the downstream molecules involved in FGF signaling. Sox1, enhancing the colony formation of definitive neural stem cells, is regularly used as a marker of neural precursors. Under typically culture conditions of ESCs in which the medium contains leukemia inhibitory factor (LIF) and serum, overexpression of Zfhx1b in these cells is essential to initiate the expression of Sox1. When mESCs were initiated to the neural differentiation, a prompt increase of Zfhx1b gene expression is observed and can be further potentiated by FGF signaling[32]. In the same content, utilizing siRNA to knockdown Zfhx1b in ESCs leads to decrease the developmental capability of these neural cells although the initial transition of ESCs to a neural cell fate is not affected. Taken together, these findings show that intercellular FGF signaling induces Zfhx1b and this is able to promote the development of definitive neural stem cells after an initial neural specification event has occurred.
THE CYTOKINE PATHWAY
Under culture conditions, LIF is used to prevent the differentiation of mESCs. However, He et al[33] found that utilizing inhibitors to abolish the STAT3 signaling which was activated by LIF could block the neuronal differentiation of ESCs. Furthermore, inhibition of the MEK signaling which was activated by LIF decreased the differentiation of ESCs into glial cells. During ESCs differentiation, LIF enhanced proliferation of cells and inhibited apoptosis of cells. In addition, LIF promoted the determination of neural progenitors although inhibited the differentiation of mesoderm and extraembryonic endoderm fates. Foshay et al[34] further examine the role of STAT3. The loss of STAT3, brought about by generating STAT3 dominant negative ESCs, led to the production of significantly fewer neural stem cells and this was associated with a decrease in the expression of the neural stem cell marker nestin. Further investigation revealed that the Sox2 promoter is directly regulated by STAT3. The decreased expression of Sox2 would induce the expression of nestin and result in a commitment to a neural stem cells fate. Using mouse embryonal carcinoma P19 cells, Pacherník et al[35] demonstrated that the effects of LIF on inducing neural differentiation were inhibited by blocking the JAK2/STAT3 signaling pathway. This is also partially true for the pro-neural effects of RA. Conversely, inhibition of the MEK1/Erk signaling pathway was found not to show any such effect. These data suggest that the cooperation between LIF and other factors, such as RA, may eventually converge into the STAT3 signaling pathway.
THE HEDGEHOG PATHWAY
Maye et al[36] demonstrated that mutants in the signaling molecules of the Hedgehog pathway within ESCs could lead to defect in the generation of neuroectoderm-forming EBs. These mutant ESCs are incapable to produce nestin positive neural stem cells or differentiate into mature neurons in respond to RA treatment. Moreover, ESCs lacking Ofd1, a gene that causes Oral-Facial-Digital 1 (OFD1) Syndrome when mutated, were found to show an increased tendency on neuronal differentiation. Furthermore, the OFD1 mutant ESCs-derived neurons are unable to differentiate into V3 interneurons, a cell type dependent on Hedgehog signaling[37]. These findings show a role for Hedgehog signaling in producing the neuronal and glial progenitors derived from ESCs.
THE JNK/MAPK PATHWAY
Amura et al[38] showed that there is an essential role for the JNK pathway during ESCs neurogenesis. ESCs lines containing homozygous destruction of the JNK1, JNK2, or JNK3 genes were introduced into differentiation protocol via the procedure at the EB formation. The outcome shows that neural differentiation was observed in wild-type EB cultures, JNK2-/- EB cultures, and JNK3-/- EB cultures except in JNK1-/- EB cultures. The identified inhibitors of ESCs neurogenesis, Wnt-4 and Wnt-6, were found to be increased their expression in JNK1-/- cultures as compared to wild-type, JNK2-/-, and JNK3-/- cultures. Furthermore, a genetic approach using JNK knockout ESCs has revealed a role for JNK1 in neural differentiation that involves repression of Wnt expression using a murine ESCs model[38]. Na et al[39] reexamined the role of Erk 1/2 in hESCs by using a chemically defined culture system. The results demonstrate that when the activity of Erk 1/2 is inhibited, the differentiation of neurons and mesendoderm is inhibited. However, these cells are still able to differentiate after BMP stimulation.
OTHER MOLECULES/FACTORS
Calcium, calcium receptor, and calcineurin
A number of studies have shown that calcium homeostasis is a crucial factor in determination of neural fate. This has been thoroughly discussed in a previous review[40]. It is worth noting that utilizing proteomic analysis or gene screening during neural induction in mESCs, four Ca2+-related proteins, namely neuronatin, translationally controlled tumor protein, pyruvate dehydrogenase E1/E2 subunits, and calreticulin, have been found to be altered in expression[41,42] . When neuronal cells are differentiated from ESCs lacking the Ca2+ release channel type 2 ryanodine receptors (RyR2−/−), Yu et al[43] found that the rate of neurogenesis was significantly blocked. Meanwhile, the expression of NeuroD, a neuronal transcription factor, and the activity of intracellular Ca2+ signaling were also inhibited in the RyR2-/- deficient mESCs. Moreover, neuronal differentiation in RyR2+/+ cells enhanced by activation of L-type Ca2+ channels or of GABA receptors was inhibited by RyR inhibitors. Therefore it would seem that cooperation between RyR2 channels and L-type Ca2+ is important for activity-dependent neurogenesis. Recently, Cho et al[44] found that neural induction is dependent on a Ca2+-activated phosphatase, calcineurin. They also have shown that calcium entry mediated by FGF stimulation activates calcineurin, which then directly and specifically dephosphorylates BMP-regulated Smad1/5 proteins.
Estrogen receptor
Utilizing an estrogen receptor (ER) agonist, Zhang et al[45] found that ERβ, but not ERα, stimulated calcium oscillations in neurons derived from ESCs. The increase of calcium oscillations and the phosphorylation of PKC, AKT and Erk1/2 induced by the ERβ agonist in ESCs derived neurons could be blocked by nifedipine, an inhibitor of L-type calcium channels. The result demonstrates that ERβ could modulate neuron activity via L-type voltage gated calcium channels.
Hox protein
In order to investigate the role of the Hox gene in neuronal differentiation, Bami et al[46] used a mESCs cellular model by combining efficient neural differentiation with inducible Hoxb1 expression. The profile of gene expression indicates that Hoxb1 could function as both activator and repressor in the short term, whereas as a repressor in the long term. Such a pattern of Hoxb1 activity was observed in the regulation of mESCs after RA induction.
Ceramide
It has been previously showed that bioactive lipids are important regulators of stem cell survival and differentiation[47]. It was found that the sphingolipid ceramide and its derivative, such as sphingosine-1-phosphate, are able to function synergistically during ESCs differentiation and the guided differentiation of mESCs toward neural and glial lineages[48].
Glycosaminoglycan
mESCs that lack heparan sulfate (HS) cannot process into neural specification whereas this phenomenon can be recovered by adding a highly sulfated glycosaminoglycan, one kind of soluble heparin[49]. Pickford et al[50] demonstrated that specific heparin polysaccharides or HS support the formation of Sox11 neural progenitor cells from wild-type ESCs. They also found that a number of receptor tyrosine kinases were affected by HS during the differentiation.
Ginsenoside Rg1
Ginsenoside Rg1, a saponin and major component in ginseng, has been shown to possess neuroprotective effects. Wu et al[51] explored the effect of Rg1 on the promotion of mESCs differentiation towards the neuronal lineage. They found Rg1 increased the phosphorylation of Akt and Erk 1/2 in a time dependent pattern through glucocorticoid receptor. Treatment with either LY294002, an inhibitor of PI3K, or U0126, an inhibitor of MEK, blocked the Rg1-induced neuronal differentiation.
Opioids
Kim et al[52] measured μ-opioid receptor and κ-opioid receptor expression in various cell types including ESCs and neural progenitors induced by RA. In the RA-induced ES cells, a biphasic profile of Erk activation after opioid stimulation was observed. Nevertheless, the proliferation of the neural progenitors was inhibited after opioid stimulation in which this phenomenon was Erk independent. The findings indicate that opioids could have opposite effects on ESCs self-renewal and ESCs differentiation.
Two pore channel 2
The nicotinic adenine acid dinucleotide phosphate (NAADP), located on membranes of lysosome, has a potent effect on mobilizing endogenous Ca2+. Two pore channel 2 (TPC2), voltage-gated ion channels, is shown to be the receptor of NAADP. Zhang et al[53] found that expression of TPC2 was decreased dramatically when the ESCs entry differentiation towards neural progenitor cells. During the late stages of neurogenesis, the expression of TPC2 reoccurred. Analysis of loss-of-function mutants of TCP2 found that TPC2 knockdown in mice accelerated mESCs differentiation into neural progenitors. This contrasted with the situation where there was TPC2 gain-of-function in a mouse model; this revealed that gain-of-function inhibited mESCs from entering the early neural differentiation. These findings suggest that TPC2 signaling plays a vital role in regulating the differentiation of mESCs into the neural lineage.
Nitric oxide
Employing various approaches, including ESC-derived neural precursor cells, Arnhold et al[54] studied the role of nitric oxide in initiating the differentiation of neurons. They found that specific blocking of the NOS-II isoform was able to bring about the inhibition of neurite outgrowth.
Chemically defined medium
When chemically defined medium (CDM) is used for growth, ESCs differentiation is highly neurogenic. Neural differentiation in CDM is shown to be dependent on endogenous FGF signaling. This process is able to be inhibited by BMP4 or LiCl in which they simulate Wnt pathway. The neural differentiation in CDM could be terminated by blocking Hedgehog activity endogenously. Therefore, a common developmental mechanism could be processing since the profile change of gene expression in stem cells cultivation in CDM and the ones in the early embryos are extremely similar[55].
Cell-Cell interactions
Parekkadan et al[56] observed that the presence of a previously specified Sox1-GFP+ cell in contact with undifferentiated ESCs was able to initiate a similar specification. This induction relied on the age of previously specified cells before co-culture. Further search for the cell adhesion molecules, it was found that connexin (Cx)-43 expression was associated with the age-dependent effect of cell contact in the experiments of cell pair. Both aberrant neuroectodermal specification and lineage commitment were seen in ESCs in which Cx-43 was knockout. Such an observation highlights the important role of gap junction signaling in the neuronal development.
Physical stimuli
Interestingly, physical stimuli are also able to affect the differentiation of ESCs and these phenomena have gained some attention recently. Piacentini et al[57] reported that the percentages of cells expressing type III β-tubulin, microtubule-associated protein 2, and calcium channel proteins (Cav1) were dramatically increased when differentiating neural stem cells are exposed to extremely low-frequency electromagnetic fields (ELFEFs, 1 mT, 50 Hz). An obviously increase in spontaneous firing were also found in these ELFEF-exposed neurons. Furthermore, they found that stimulation of ELFEF during the early differentiation could induce an increase of cells expressing CREB phosphorylation by which it is calcium channel dependent[57]. In another study, Maioli et al[58] created a Radio Electric Asymmetric Conveyer (REAC) that is able to deliver wireless fidelity radiofrequency (Wi-Fi RF) at 2.4 GHz. This radio wave is delivered by immersing the conveyer electrodes into the culture medium. Using such a device allows mESCs to be exposed to REAC and in such circumstances it was found that transcription of genes involved in differentiation, such as neuronal commitment (neurogenin1), were upregulated, while other genes, such as Sox2, Oct4, and Nanog, were downregulated. These findings mean that the physical environment is also able to regulate the fate of stem cells.
CONCLUSION
Some canonical pathways involved in cell size such as Hippo/Yap pathways and/or growth such as PI3K/Akt pathways seem to have little relationship with the initiation of neuronal differentiation from ESCs in vitro. The PI3K/Akt pathway is viewed as important to the maintenance of neuronal survival, but not to the differentiation process. In this context, Watanabe et al[59] show that the membrane bound Akt through myristoylation (myr-Akt) could maintain mESCs at the undifferentiated status without supplement of LIF in the medium. Once the myr-Akt was deleted, the dependence of LIF and ability of differentiation were recovered. They found that the PI3K/Akt signaling could regulate “stemness’ of many stem cell systems. Zhao et al[60] also found that insulin can rescue ESCs-derived neural progenitor cells from hypoxia-induced cell death. Such an effect is able to be inhibited by LY294002, an inhibitor of the phosphatidylinositol 3-kinase (PI3K). Nevertheless, Chuang et al[61] have recently reported that the mTOR pathway, a downstream pathway of PI3K, would seem to play a role in ESCs-derived neuronal differentiation. In order to reveal the role of raptor/mTOR in neurons differentiated from ESCs, we established raptor gene-trap mESCs and raptor knockdown mESCs using raptor RNAi infection followed by puromycin selection. Embryonic body growth in both cases was greatly reduced and the result was an unsuccessful differentiation of neurons. Furthermore, treatment with 1 μmol/L rapamycin over 48 to 72 h of treatment starting at the point when neuronal precursors began to differentiate from mESCs was found to bring about a gradual loss of neuritis together with a shrinkage of the soma and a decreased ratio of neurite length to cell number. Knockdown of raptor during neuronal differentiation from mESCs also resulted in a gradual loss of neurites and cell body shrinkage. The loss of neurite density that results from rapamycin treatment is able to be reversed by overexpression of S6K T389E. Therefore, raptor/mTORC1/S6K would seem to play a critical role in the differentiation and survival of neurons derived from mESCs[61]. Therefore, it seems likely that the mTOR pathway plays a pivotal role in neuronal differentiation of ES cells in vitro.
To comprehensive understand these pathways will definitely contribute greatly to stem cell biology and translational medicine. In conclusion, the pathways outlined here are simple and linear. However, it is still unclear how these pathways crosstalk with each other and/or what is the level of interplay between the pathways both temporally and spatially. The integration of these pathways into a comprehensive network will probably require more incisive investigative approaches. Generating specific types of neurons from ESCs in vitro has created high expectations in terms of possible medical treatments and/or potential cures for neuronal pathological diseases. Knowledge obtained by the research related to ESCs derived neurons in vitro should also provide a technical basis for regenerative medicine, applied medical research, and drug discovery.
P- Reviewer: Maioli NA, Sarkadi B S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ