Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.217
Revised: August 27, 2013
Accepted: September 18, 2013
Published online: October 26, 2013
AIM: To investigate reprogramming of human adipose tissue derived stem cells into insulin producing cells using non-integrated lentivirus harboring PDX1 gene.
METHODS: In this study, human adipose tissue derived stem cells (hADSCs) were obtained from abdominal adipose tissues by liposuction, selected by plastic adhesion, and characterized by flow cytometric analysis. Human ADSCs were differentiated into adipocytes and osteocytes using differentiating medium to confirm their multipotency. Non-integrated lentiviruses harboring PDX1 (Non-integrated LV-PDX1) were constructed using specific plasmids (pLV-HELP, pMD2G, LV-105-PDX1-1). Then, hADSCs were transduced with non-integrated LV-PDX1. After transduction, ADSCsPDX1+ were cultured in high glucose DMEM medium supplement by B27, nicotinamide and βFGF for 21 d. Expressions of PDX1 and insulin were detected at protein level by immunofluorescence analysis. Expressions of PDX1, neurogenin3 (Ngn3), glucagon, glucose transporter2 (Glut2) and somatostatin as specific marker genes were investigated at mRNA level by quantitative RT-PCR. Insulin secretion of hADSCsPDX1+ in the high-glucose medium was detected by electrochemiluminescence test. Human ADSCsPDX1+ were implanted into hyperglycemic rats.
RESULTS: Human ADSCs exhibited their fibroblast-like morphology and made colonies after 7-10 d of culture. Determination of hADSCs identified by FACS analysis showed that hADSCs were positive for mesenchymal cell markers and negative for hematopoietic cell markers that guaranteed the lack of hematopoietic contamination. In vitro differentiation of hADSCs into osteocytes and adipocytes were detected by Alizarin red and Oil red O staining and confirmed their multilineage differentiation ability. Transduced hADSCs+PDX1 became round and clusters in the differentiation medium. The appropriate expression of PDX1 and insulin proteins was confirmed using immunocytochemistry analysis. Significant expressions of PDX1, Ngn3, glucagon, Glut2 and somatostatin were detected by quantitative RT-PCR. hADSCsPDX1+ revealed the glucose sensing ability by expressing Glut2 when they were cultured in the medium containing high glucose concentration. The insulin secretion of hADSCsPDX1+ in the high glucose medium was 2.32 μU/mL. hADSCsPDX1+ implantation into hyperglycemic rats cured it two days after injection by reducing blood glucose levels from 485 mg/dL to the normal level.
CONCLUSION: Human ADSCs can differentiate into IPCs by non-integrated LV-PDX1 transduction and have the potential to be used as a resource in type 1 diabetes cell therapy.
Core tip: Common treatments for diabetes mellitus are based on insulin injections and pancreas transplantation, limited by hypoglycemia, shortage of donors, immunosuppression and organ rejection. Cell therapy using human adipose tissue derived stem cells (hADSCs) offers a novel strategy for diabetes treatment without tumor formation and ethical concerns. Different viral vectors have been used for pancreatic differentiation. However, integration of provirus into host chromatin has induced insertional mutagenesis and malignancy. This is the first study to investigate the application of non-integrated Lentiviral vectors harboring PDX1 for differentiation of hADSCs into insulin producing cells and its usage in treatment of diabetic rats.
-
Citation: Boroujeni ZN, Aleyasin A. Insulin producing cells established using non-integrated lentiviral vector harboring
PDX1 gene. World J Stem Cells 2013; 5(4): 217-228 - URL: https://www.wjgnet.com/1948-0210/full/v5/i4/217.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i4.217
Type 1 diabetes mellitus is a devastating condition that affects millions of people worldwide. It is characterized by autoimmune destruction of pancreatic β-cells, leading to insufficient insulin production and increasing blood glucose level. Treatment of diabetes mellitus has been commonly performed by frequent insulin injections or by pancreas replacement. Some problems, such as immunosuppression and difficult surgery, limit the transplantation of pancreas tissue. Now, application of stem cell therapy has been under intensive investigation as another candidate for pancreatic β-cell replacement.
Stem cells can be converted to obtain required metabolic functions using genetic and epigenetic manipulations. In this way, embryonic stem cells (ESCs) derived from blastocysts[1] and adult stem cells derived from different adult tissues[2] have been genetically manipulated to have abilities like β-cell function[3,4]. ESCs are pluripotent cells but are limited by immune rejection, tumorgenicity and ethical problems[5]. Adult stem cells are not limited as such and can be obtained from many adult tissues, such as skin[6], salivary gland[7], liver[8], spleen[9], bone marrow[10] and adipose tissue[11-13]. Among them, adipose tissue derived stem cells (ADSCs) are good candidates for possible clinical applications without ethical concerns. They can undergo mesodermal differentiation as well as ectodermal and endodermal differentiation both in vitro and in vivo[14,15]. ADSCs may become a useful target for β-cell replacement in diabetic patients because of their potential to adoption of characteristics[16].
Application of stem cell therapy for diabetes treatment has been under investigation by working on different kinds of genes, transfer vectors and differentiating mediums[17]. Many transcription factors, such as PDX1, neurogenin3 and Nkx2.2, have been used for pancreatic cells differentiation. Variable transforming vectors, such as adenovirus, retrovirus, adeno-associated virus and lentivirus, have been used for gene transfer to cells[18-21]. The homeodomain transcription factor PDX-1 is an important transcription factor of pancreatic islet development and function[22]. PDX-1 induces differentiation of ADSCs into insulin-producing cells[23,24] due to its ability to regulate downstream islet cell-specific gene expression and production of pancreatic hormones[25-28]. This gene can be packaged in recombinant viral vectors to express PDX1 protein in the nucleus[29].
Lentiviral vectors (LV) can deliver large cDNA to dividing and nondividing target cells[30]. These vectors transduce a variety of cell types, including embryonic and adult stem cells for both in vivo and ex vivo gene therapy applications[31]. However, integration of a viral genome into a host cell genome may induce insertional mutagenesis that has been highlighted by induction of malignancy in mouse models[32] and development of leukemia in five patients in two clinical gene therapy trials[33]. Improving safety and efficiency of LV has been achieved, for example, by modifications of packaging cassette on virus integrase gene or on other regions of virus genome. This virus is called non-integrated LV that cannot integrate into the host genome[34]. The aim of this study was to transform hADSCs with non-integrated LV harboring PDX1 to obtain functional pancreatic beta-like cells with less genome manipulation.
Human ADSCs were isolated from 80 to 100 mL aspirates from the abdominal fat tissue of normal donors. Informed consent was obtained from all participants and experiments performed according to the guidelines set with the Local Ethics Committee in the National Institute of Genetic Engineering and Biotechnology on Human Research. Lipoaspirate samples were collected into a sterile bottle with serum-free Dulbecco’s modified Eagle’s medium (DMEM). The samples were washed by phosphate buffered saline (PBS) and digested by collagenase solution (Sigma) at a final concentration of 0.075%. Digested tissues were centrifuged at 400 g for 10 min[15]. Pellets were resuspended in erythrocyte lysis buffer and centrifuged at 300 g for 10 min. Pellets were cultured at a density of 2 × 106 cells/mL in 25 cm2 plastic culture flask in low glucose DMEM containing 50% fetal bovine serum (FBS), 100 U/mL penicillin and 100 U/mL streptomycin for 24 h. Non adherent cells were washed away with PBS and hADSCs were cultured in DMEM containing 20% FBS and antibiotics. Upon 80% confluency, the cells were harvested using 0.25% Trypsin-0.02% EDTA for 1-2 min at 37 °C and were kept frozen in liquid nitrogen for later use.
Human ADSCs at the third passage were detached with trypsin-EDTA. The cells were centrifuged at 1500 rpm for 6 min and then were resuspended in PBS at the concentration of 1 × 106/mL. The fluorescent labeled antibodies (10 μL for each sample) were added and incubated for 30 min at room temperature[35,36]. The labeled cells were analyzed on a FACS Caliber (Becton-Dickinson, Franklin Lakes, NJ, United States) following labeled antibodies against human CD45-FITC/CD34-PE, CD31-FITC/CD73-PE, CD90-FITC/CD105-PE, CD11b-PE, CD44PE/ CD106-PEcy5, CD16-FITC/CD29-PE, CD14-PE/CD55-PE5 (Serotec, United States).
Human ADSCs were differentiated into adipocytes and osteocytes using differentiating medium to confirm their multipotency ability. For differentiation into adipocytes, third-passage hADSCs were plated at 30000 cells per cm2 in 6-well plates in adipocyte differentiation medium (Gibco) consisting of DMEM, 10% FBS, 0.5 mmol/L 3-isobutyl-1 methylxanthine, 1 mmol/L dexamethasone, 200 mmol/L indomethacin and 10 mmol/L insulin for two weeks. Medium was changed twice a week. Cultured cells were stained with Oil Red O to detect lipid accumulation in differentiated cells[4,14,37,38].
For osteogenic differentiation, third passage hADSCs were cultured in osteogenic medium containing DMEM, 10% FBS, 0.1 mol/L dexamethasone, 50 mol/L ascorbate-2-phosphate and 10 mmol/L glycerophosphate for two weeks. Cultured cells were stained with Alizarin Red to detect bone matrix in differentiated cells[3,14,37,38].
HEK293 cells were cultured in 75 cm2 plastic culture flask in high glucose DMEM and were prepared to a level of confluency. The transfer construct LV-105 harboring PDX1 (GeneCopia) was co-transfected with the enveloped plasmid pMD2G (InvivoGen) and the packaging plasmid pLV-HELP (InvivoGen) into HEK 293T cell culture. The culture medium was changed 14 h after transfection. One day after medium change, active lentiviral vectors were released in culture medium. Culture medium was removed and viral vectors become concentrated with MILLIPORE falcons and kept in -80 °C for further usage[36].
Total RNA was extracted using RNA X PLUS (CinnaGen Co) and first-strand cDNA was prepared using cDNA RT Kit (Fermentas). RT-PCR was prepared in a 20 μL reaction volume by appropriate primers for PDX1 gene (Table 1). After amplification, 5 μL of products were loaded on a 2% agarose gel. DNA bands were stained and visualized by UV transilluminator.
| Gene | Gene ID | Size (bp) | Strand | Sequence (5'–3') | Annealing temperature |
| Somatostatin | NM_001048.3 | 114 | F | GCACCCCGAGAACGCAAAGC | 56 |
| R | TGTGGGGGCGAGGGATCAGAG | ||||
| Ngn3 | NM_020999.3 | 241 | F | GTAGAAAGGATGACGCCTCAACC | 54 |
| R | TCAGTGCCAACTCGCTCTTAGG | ||||
| Glucagon | NM_002054.4 | 187 | F | CAGTGCGCCTTGGTGCAGAAGT | 58 |
| R | GGAACGTTGCCAGCTGCCTTG | ||||
| Glut2 | NM_000340 | 300 | F | AGCTTTGCAGTTGGTGGAAT | 52 |
| R | AATAAGAATGCCCGTGACGA | ||||
| PDX1 | NM_000209.3 | 201 | F | GTCCGGTGCCAGAGTTCAGT | 63 |
| R | CCCAGTCTCGGTTCCATTCG | ||||
| β-actin | NM_001101.3 | 161 | F | GAGACCTTCAACACCCCAGCC | 56 |
| R | AGACGCAGGATGGCATGGG |
The MTT assay was performed on transduced hADSCs to determine the cytopathic effect of non-integrated LV-PDX1. The transduced and untransduced hADSCs culture mediums were replaced with a volume of 100 μL high glucose DMEM medium containing 10% tetrazolium dye 3-(4,5-dimethylthiazole-2-yll-2,5-diphenyltetrazolium bromide (MTT) (Sigma) solution in the 96-well plates and incubated for 3 hours at 37 °C. The medium was changed with 100 μL DMSO solution and incubated for 10 minutes at room temperature to develop the purple color. The optical density (OD) of DMSO solution was measured at 580 nm to determine the relative cell viability.
Third passage human ADSCs (2 × 105 cells/mL ) were cultured in a 25 cm2 plastic culture flask in low glucose DMEM and 10% FBS for 24 h. Concentrated non-integrated LV-PDX1 (100 μL) was added to cultures and incubated for 12 hours. The viral transduction was repeated twice to obtain better PDX1 expression in hADSCs and exposed to 2.5 μg/mL puromycin for 2 d to obtain stable transduction[36]. The selection was continued with 2 μg/mL puromycin for 3 wk until individual colonies expressing PDX1 appeared.
Human ADSCsPDX1+ were cultured in the medium containing high glucose (25 mmol/L) DMEM, 2% FBS, 10 mmol/L nicotinamide and 2% B27 for 7 d. Culture medium was later supplemented with FGFβ (1 μL/mL) for 14 d[35]. A parallel negative control of untransduced hADSCs with the same condition in the culture medium was prepared to compare the morphological changes.
Expression of beta cell marker genes consisted of PDX1, Ngn3, Glut2, glucagon and somatostatin and were determined in transduced and untransduced hADSCs in three weeks of cell culture in comparison to β-actin gene which was used as housekeeping B acting gene using quantitative RT-PCR[4,36]. Total mRNA was extracted from all triplicate groups cell culture using RNA X PLUS (CinnaGen Co). The first and second cDNA was prepared using cDNA RT Kit (Fermentas). RT-PCR was performed using AccuPower® 2X Greenstar qPCR Master Mix Kit (Bioneer) in Rotor-GeneTM 6000 (Corbett) thermal cycler. Primer sequences and their annealing temperature and products length are shown in Table 1. The identity of PCR products was confirmed by electrophoresis and sequencing. Their relative gene expression data were analyzed using 2−∆∆Ct method[39].
Transduced and untransduced hADSCs (105) were seeded on glass cover slides in triplicate cultures using 6-well culture plates for 3 wk. Cells were washed with PBS containing 0.05% TWEEN 20 and fixed with cold absolute methanol for 5 min and washed with PBS 1% Triton X-100 for permeabilization of the cells. Cells were treated with 1% BSA (bovine serum albumin) for 20 min at room temperature and washed with PBS 1% Tritone X-100. Cells were reacted separately with primary rabbit anti PDX1 antibody (1:1000, Abcam) and primary guinea pig polyclonal anti insulin (1:200, Abcam) overnight in 37 °C. After washing with PBS 1% Tritone X-100, they were incubated with FITC-conjugated goat anti-rabbit IgG (1:250, Abcam) and FITC-conjugated goat anti-guinea pig IgG for 45 min in a dark room. Cells were reacted with 4,6-diamidino-2-phenylindole in a dark room for 10 minutes and washed with PBS[21]. The signals were observed under a fluorescence microscope (Zeiss, Axioplan, Germany) and the images were taken with connected digital camera.
To assay the insulin secretion of transduced cells, triplicate culture groups from transduced and untransduced hADSCs were prepared and washed by PBS buffer before low glucose DMEM (5.5 mmol/L glucose) containing 2% FBS was added in the media and incubated for 24 h. Transduced and untransduced hADSCs were washed again with PBS and incubated in high glucose DMEM (25 mmol/L glucose) containing 2% FBS for 24 h[35]. Culture mediums were collected and measured insulin contents using electrochemiluminescence test[36] performed in Bahar Laboratory, Tehran, Iran.
Hyperglycemia was induced in 20 Sprague-Dawley adult male rats through intraperitoneal injection of 120 mg/kg of alloxan monohydrate (Sigma) that resolved in sodium citrate solution[40]. All experimental procedures were approved and supervised by the National Institute of Genetic Engineering and Biotechnology Animal Care and Use Committee which conforms to the principles laid down by the National Academy of Science for the care and use of laboratory animals.
Rats were housed individually in special clear sided cages at 22 °C and 12:12 light: dark cycle. After two weeks adaptation period, rats were fasted for 12 h before an intraperitoneal injection of 120 mg/kg of alloxan. Blood glucose level was determined using a blood glucose meter before and after alloxan injection. Rats were diabetic if their blood glucose ranges reached levels between 400 and 600 mg/dL and were maintained stably for one week. Diabetic rats were divided into three groups, each contained 7 diabetic rats. One group of diabetic rats received an intraperitoneal transplant of 4 × 106 semi differentiated hADSCsPDX1+ (5 d after transduction) that was harvested with trypsin and resuspended in 500 μL of PBS. Another group of diabetic rats were injected IP with 4 × 106 untransduced hADSCs. The third group of diabetic rats was not injected as control diabetic rats. Blood glucose levels were monitored twice a week after cell injection using the glucose oxidase method by ACCU-CHEK active strips (Roche Diagnostics, Germany).
For gene expression and insulin secretion comparison among transduced and untransduced hADSCs, statistical analysis was performed by one-way analysis of variance using SPSS version 12.0 (SPSS, Chicago, United States). In all statistical analyses, P < 0.05 was statistically judged significant and all P values were two-sided.
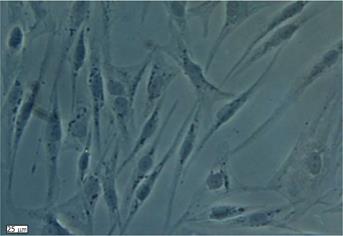
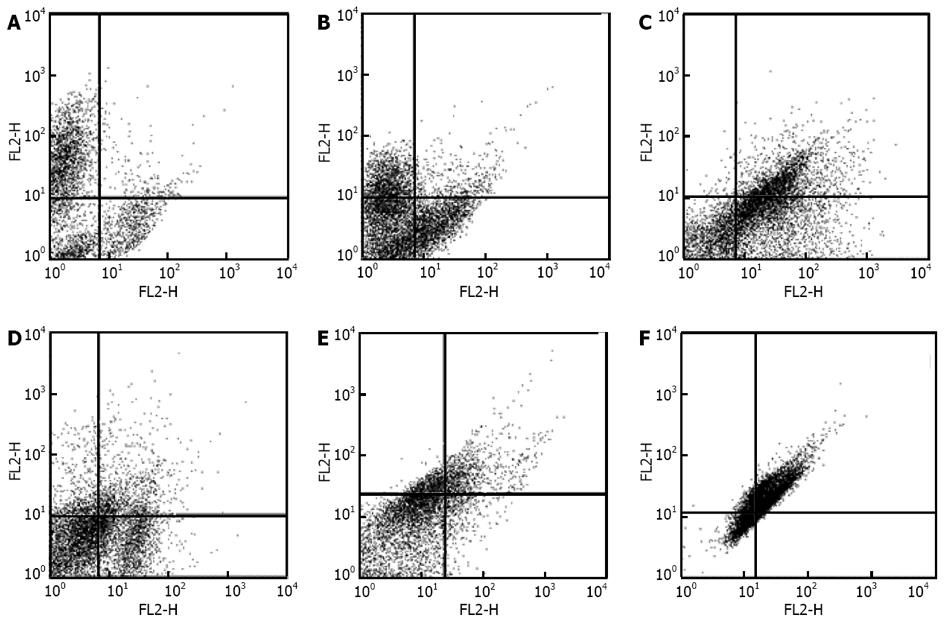
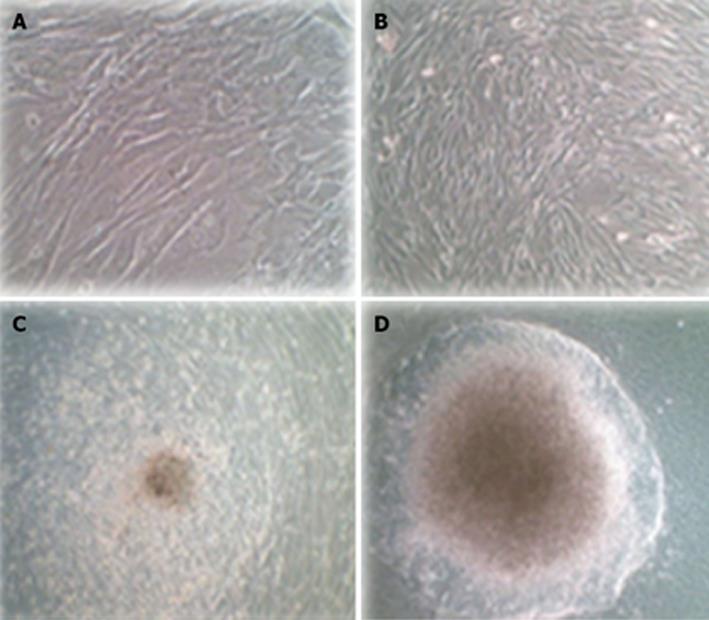
Human ADSCs were isolated from abdominal adipose tissue through collagenase digestion successfully and expanded in DMEM culture medium at a density of 2 × 104 cells/mL after plastic adherence selection. Hematopoietic cells were removed during subsequent changes of medium and passaging. Human ADSCs exhibited their fibroblast-like morphology and made colonies after 7-10 d of culture (Figure 1). The hADSCs were harvested and labeled with antibodies against CD73, CD90, CD29, CD34, CD31, CD45, CD105, CD16, CD29, CD14, CD55, CD106 and CD44. Determination of hADSCs identity by FACS analysis showed that hADSCs were negative for CD31, CD34 and CD45, which guaranteed the lack of hematopoietic contamination. These cells expressed high levels of CD44, CD73, CD90, CD105, CD29, CD106 and CD55 (Figure 2).
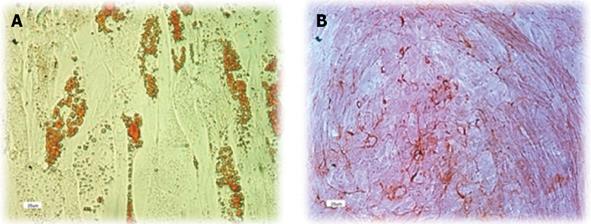
Human ADSCs were differentiated into mesodermal lineage, such as adipocytes and osteocytes, by a differentiating medium for confirming their multilineage differentiation ability. In vitro differentiation of hADSCs into osteocytes and adipocytes was detected by Alizarin red and Oil red O staining. Human ADSCs contained adipose (Figure 3A) and calcium granules (Figure 3B) after 3 wk of treating with adipogenic and osteogenic mediums.
The actual non-integrated LV-PDX1 vector was amplified and packaged in HEK 293 cells. The sign of viral production was an early color change in the medium from red to yellow, compared to non transfected HEK 293. As viral production proceeded, some of the cells rounded up and detached from the plate. The culture medium contained active non-integrated LV-PDX1 vector and was collected two days after transfection. The activity and identity of isolated non-integrated LV-PDX1 were confirmed by infecting the new culture of HEK293 cells followed by RNA extraction and cDNA synthesis. PDX1 mRNA expressions were detected by RT-PCR in HEK293.
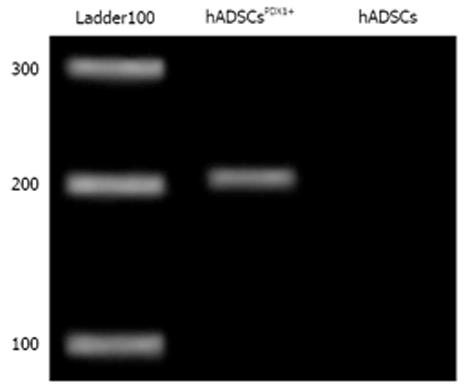
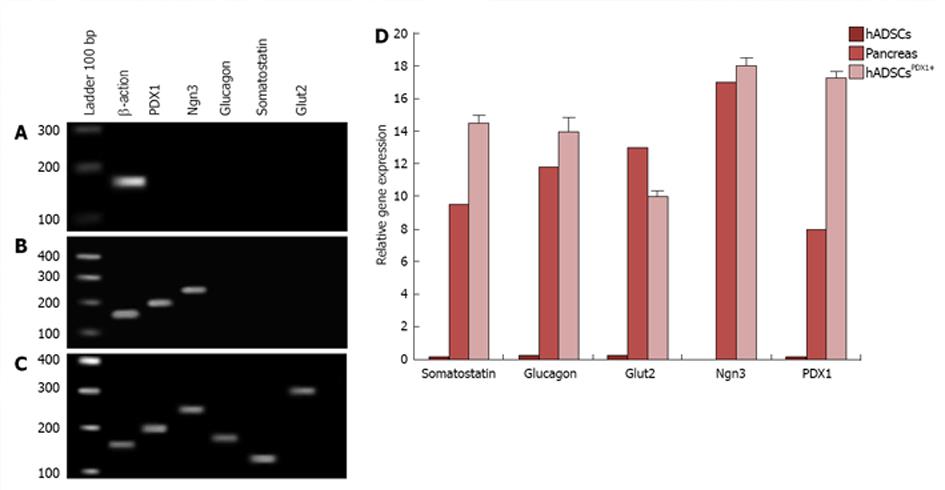
Transduced hADSCs culture was screened against puromycin antibiotic for 2 wk for hADSCsPDX1+ selection from untransduced hADSCs. Expression of PDX1 was confirmed by amplification of 201 bp fragments from hADSCsPDX1+, compared to no amplification from control hADSCs (Figure 4).
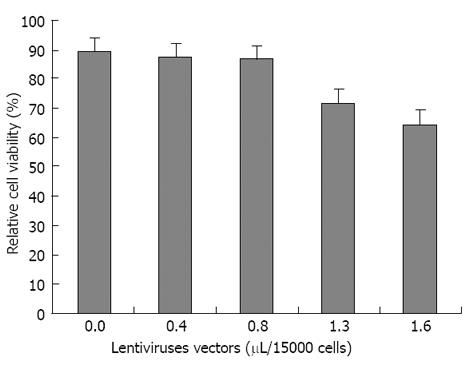
The optimum volumes for viral suspension of non-integrated LV-PDX1 that has been used in hADSCs transductions were obtained in MTT test as 0.8 μL/15000 hADSCs. Increasing the volume of non-integrated LV-PDX1 more than 0.8 μL/15000 hADSCs resulted in significant decrease in viability and optical density (OD) of cells (Figure 5).
Untransduced hADSCs were typically adherent as a spindle shape (Figure 6A). Transduced hADSCs+PDX1 became oval-shaped cells (Figure 6B) and clusters appeared using culture medium containing high glucose (25 mmmol/L) DMEM, nicotinamide and B27 (Figure 6C). These cells continued to differentiate and eventually resulted in larger clusters under high glucose (25 mmmol/L) DMEM, nicotinamide and B27 supplemented with FGFβ (Figure 6D).
Differentiation of hADSCsPDX1+ towards insulin producing cells was performed using specific culture media consisting of high glucose DMEM, 2% B27, 2% FBS, 10 mmol/L nicotinamide and βFGF. To investigate endocrine pancreatic cell characteristics, expression of pancreatic marker genes such as PDX1, Ngn3, glucagon, somatostatin and Glut2 were studied by quantitative RT-PCR from hADSCsPDX1+, hADSCs and pancreatic cells cDNAs isolated in the 1st and 3rd week after first viral transduction (Figure 7). Expressions of PDX1 and Ngn3 were high after the first week and decreased in the 3rd week in hADSCsPDX1+ culture compared to the untransduced one. On the other hand, expression of somatostatin, glucagon and Glut2 increased in the 3rd week compared to the 1st week (equal to 0.0), which indicates that differentiated hADSCsPDX1+ had obtained the glucose-sensing ability after 3 wk (Figure 7). Reduction in Ngn3 expression in the 3rd week in hADSCsPDX1+ is an indication for the phenotypical differentiation toward beta cell characteristics. However, no differentiations happened in untransduced hADSCs since no expressions were detected for PDX1, Ngn3, Glut2 and glucagon and somatostatin genes were observed in untransduced hADSCs after the 1st and 3rd week of culture.
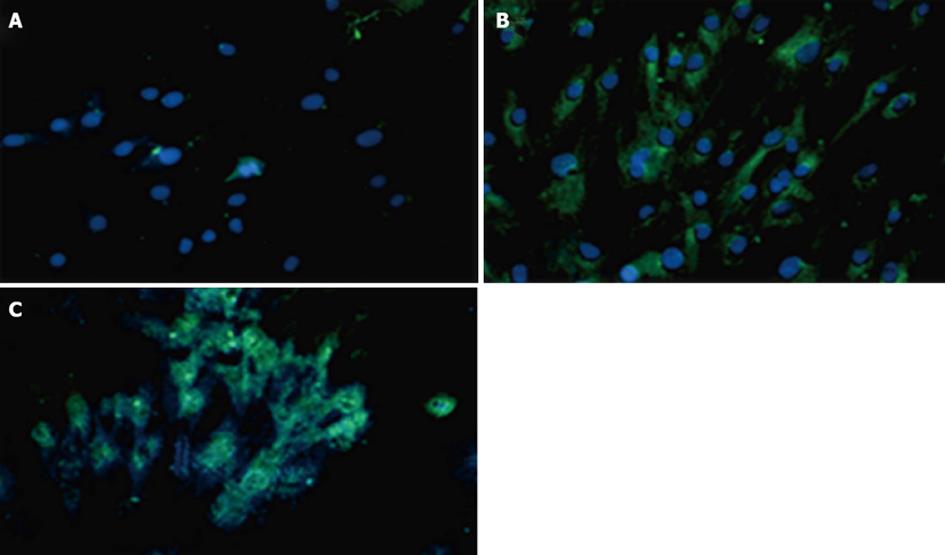
Expressions of PDX1 and insulin proteins were shown in transduced hADSCs+PDX1 using anti-PDX1 and anti-insulin antibodies, which were represented as green dots in the immunofluorescence assay (Figure 8). This result illustrated that hADSCsPDX1+ are capable of activating the endogenous insulin promoter in high glucose medium containing 25 mmol/L glucose.
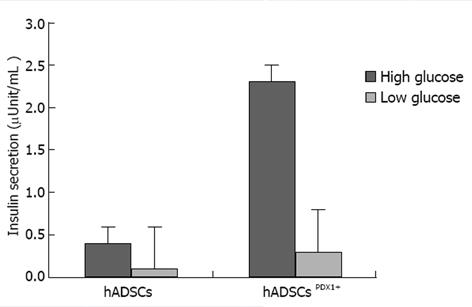
Induction of insulin excretion from hADSCsPDX1+ 2.32 fold was shown using electrochemiluminescence assay, a sensitive method for insulin secretion assay, in high glucose medium compared with untransduced hADSCs (Figure 9).
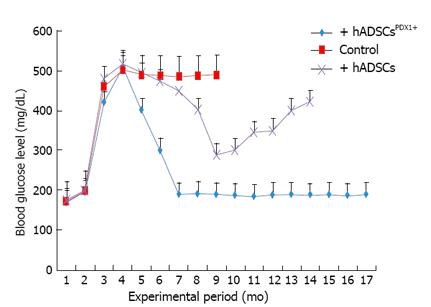
The potential of differentiated hADSCsPDX1+ to diabetes treatment was confirmed by hADSCsPDX1+ transplantation into 20 alloxan-induced diabetic rats. Two rats died during the anesthetic procedure and the remaining hyperglycemic rats were divided into three groups, each contained 6 diabetic rats. Fasting blood glucose level of all twenty rats before alloxan injection was 160-200 mg/dL. After 12-14 d of receiving 120 mg/kg alloxan, the blood glucose levels in diabetic rats increased to 400-500 mg/dL with approximately 20% weight loss. After two weeks of stable hyperglycemia, two groups of diabetic rats were injected with 3-4 × 106 semi differentiated hADSCsPDX1+ (5 d after transduction) and untransduced hADSCs respectively by IP injection and the remaining third group was kept as a control for stability of diabetes in rats. The blood glucose level of diabetic rats transplanted with hADSCsPDX1+ was gradually decreased and normalized within 3-4 d after transplantation. After transplantation, the blood glucose level of untransduced hADSCs transplanted rats decreased 10 d after transplantation transiently and increased again. Tumor formation was not observed in any transplanted rats. Control untransplanted diabetic animals remained hyperglycemic and died within 6 months (Figure 10). Our results suggested that the differentiated hADSCsPDX1+ may have responded to hyperglycemia in diabetic rats, which represent their possible glucose sensing ability.
Mesenchymal stem cells such as hADSCs are attractive therapeutic candidates for insulin dependent diabetes treatment in the clinical setting due to their immunomodulatory properties. hADSCs can modulate immune responses in recipients by releasing co-stimulatory and regulatory cytokines to control autoreactive T cells, B cells proliferation and differentiation, natural killer cells proliferation and dendritic cells maturation, antibody production and chemotactic abilities[41]. hADSCs express intermediate levels of major histocompatibility complex (MHC) class I molecules and no level of MHC class II molecules on their cell surface that allow their transplantation across MHC barriers[41]. They stimulate the production of regulatory T cells that inhibit lymphocyte proliferation in allogeneic transplants[42].
Human ADSCs were isolated from abdominal fat tissues and characterized by flow cytometric analysis. The phenotype of hADSCs was negative for hematopoietic markers, such as CD34 and CD45, and was positive for MSC markers, such as CD44, CD73, CD90, CD105, CD29, CD106 and CD55. Insulin production and secretion of differentiated hADSCsPDX1+ were detected using immunofluorescence analysis (Figure 8) and electrochemiluminescence (ECL). Insulin secretion measured by ECL was equal to 2.32 μU/mL in hADSCsPDX1+ culture medium but was 0.0 μU/mL in control hADSCs culture medium (Figure 9).
Differentiation of mesenchymal stem cells into beta like cells without gene transformation has been reported[35,43]. However, some animal products such as neuronal conditioned medium have been used for induction of differentiation that may have some complications in clinical usage. Animal products may transmit pathogens, induce antibodies and contaminate with prion particles, nanobacteria, mycoplasma and endotoxins. Proteins exist in animal products that may contaminate stem cells and induce a possible risk of transmitting unknown infectious agents and the risk of initiating xenogeneic immune responses. These antigenic responses may affect the viability, safety and efficacy of transplanted MSCs. In addition to these technical problems, it is inhumane to kill many animals for the collection of animal products[44].
Lentiviral vectors can deliver transgenes to a wide variety of dividing and nondividing cells and maintain stable long-term transgene expression[45]. However, integration of a provirus into host chromatin has induced some adverse issues, such as insertional mutagenesis and promoter interference and positional effects have been mentioned by induction of malignancy in some therapeutic trials in human and mouse models[31,32]. Development of non-integrated lentiviral vectors to deliver transgenes has provided adequate safety and efficiency in clinical applications due to modifications in the packaging cassette that have limited the potential risk for insertional mutagenesis and replication competent of lentiviruses[34]. This is the first study to investigate the application of non-integrated LV harboring PDX1 in differentiation of hADSCs into islet-like insulin producing cells and in treatment of insulin dependent diabetes in diabetic rat models. Previous attempts used integrated LV harboring PDX1 for differentiation of bone marrow mesenchymal stem cells into islet-like insulin producing cells[36]. In order to promote insulin secretion, some chemical materials, such as B27, nicotinamide and βFGF, were added into the culture medium of hADSCsPDX1+. Insulin production of hADSCsPDX1+ was glucose responsive, which represented efficiency of non-integrated LV vector in strong exogenous gene expression in transplanted cells over other viral vectors[28]. The nuclear targeting of non-integrated LV vectors enables transduction of both dividing and non-dividing cells, a suitable attribute for a gene therapy vector[29].
The ectopic PDX1 activated a number of genes, such as Glut2 and Ngn3, which are crucial in cellular insulin production and secretion in respect to sensing environmental glucose level. Nng3 is not found in mature pancreatic islets[46] and low expression level of Ngn3 observed in differentiated hADSCsPDX1+ after 21 d compared to 7 d, which indicated that these cells may partly resemble mature islet precursor cells that retain its proliferation ability. Detection of alpha and delta cell phenotypes related gene expression, such as glucagon and somatostatin, could be a reflection for multiple islet genes expression in differentiated hADSCs.
Experimental data for the therapeutic effects of hADSCsPDX1+ in the diabetic animal models are necessary tools for safety analyzing before human clinical applications. Alloxan-induced diabetic rats have been used for in vivo analysis of allogeneic transplantation of hADSCsPDX1+. Differentiated hADSCsPDX1+ could normalize diabetic rat blood glucose levels after three days of cell transplantation without any immune responses. No tumor formation was observed even after 12 mo of follow up of treated rats.
In conclusion, our results demonstrate that hADSCs is a suitable source of cells capable of differentiating into insulin producing and secreting cells, both phenotypically and functionally by non-integrated LV-PDX1 and appropriate microenvironment chemical materials. This hADSCsPDX1+ may be used as a source for autologous or allogenic transplantation for β-cell replacement in the treatment of insulin dependent diabetes.
Diabetes mellitus is characterized by autoimmune destruction of pancreatic β-cells, leading to insufficient insulin production. Now, application of stem cell therapy has been under intensive investigation as a new candidate for pancreatic β-cells replacement. Adipose tissue derived stem cell is an attractive therapeutic candidate for insulin dependent diabetes treatment in the clinical setting due to its immunomodulatory properties. Application of stem cell therapy for diabetes treatment has been under investigation by working on different kinds of genes, transfer vectors and differentiation medium. Lentiviral vectors (LV) can transduce a variety of cell types both in vivo and ex vivo. Many transcription factors, such as PDX1, Ngn3 and Nkx2.2, have been used for pancreatic cells differentiation. The homeodomain transcription factor PDX-1 is an important transcription factor of pancreatic islet development and function.
In the area of treatment of diabetes mellitus using stem cells, the research hotspot is how to differentiate the stem cells by chemicals and gene transduction with different vectors and genes. Many transcription factors, such as PDX1, Ngn3 and Nkx2.2, have been used for pancreatic cells differentiation. Previous attempts used adenoviruses or integrated LV harboring PDX1 for differentiation of mesenchymal stem cells into islet-like insulin producing cells.
In previous diabetes treatment methods, it was found that some animal products such as neuronal conditioned medium used for induction of pancreatic differentiation may have some complications in clinical usage. Animal products may transmit pathogens, induce antibodies and transmit contaminations with prion particles, nanobacteria, mycoplasma and endotoxins. Also, integration of a provirus into host chromatin has induced some adverse issues, such as insertional mutagenesis and malignancy, in some therapeutic trials in human and mouse models. Adenoviral vectors induce an immunological response and transient expression of transgenes. In previous attempts, insulin expression has been reported from partially differentiating embryonic stem cells and mesenchymal stem cells derived from different tissues. Embryonic stem cells are pluripotent cells but are limited by the immune rejection, tumorgenicity and ethical problems. The novelty of this study was to show the application of non-integrated LV harboring PDX1 that is not able to integrate into the host genome and to solve the induced insertional mutagenesis problem that can induce possible malignancies in cell differentiation.
The study shows that non-integrated LV-PDX1 can be used as a powerful vector in differentiation of hADSCs towards beta like cells. These cells have less potential for malignancy and can be used with less concern in autologous or allogenic β-cell replacement in the treatment of insulin dependent diabetes.
Diabetes mellitus (type 1): Type 1 diabetes mellitus is a devastating condition that affects millions of people worldwide. Diabetes mellitus is characterized by autoimmune destruction of pancreatic β-cells, leading to insufficient insulin production and increasing blood glucose level; PDX1: The homeodomain transcription factor PDX-1 is an important transcription factor of pancreatic islet development and function. PDX-1 has ability in regulating of downstream islet cell-specific gene expression and production of pancreatic hormones.
Overall, a very nice study. It is well written.
P- Reviewer Traub M S- Editor Wen LL L- Editor Roemmele A E- Editor Wang CH
| 1. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1063] [Cited by in F6Publishing: 961] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 2. | Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Müller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 608] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 3. | Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, Liu Q, Liu D, Chen L, Pei X. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol. 2007;211:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, Favrot M, Benhamou PY. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 646] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 7. | Okumura K, Nakamura K, Hisatomi Y, Nagano K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto T. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. [PubMed] [Cited in This Article: ] |
| 9. | Kodama S, Davis M, Faustman DL. Regenerative medicine: a radical reappraisal of the spleen. Trends Mol Med. 2005;11:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hassan HT, El-Sheemy M. Adult bone-marrow stem cells and their potential in medicine. J R Soc Med. 2004;97:465-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F. Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J. 2009;23:3494-3505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 14. | Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 676] [Cited by in F6Publishing: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 15. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4817] [Cited by in F6Publishing: 4869] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 16. | Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2132] [Cited by in F6Publishing: 1891] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 18. | Cao H, Chu Y, Zhu H, Sun J, Pu Y, Gao Z, Yang C, Peng S, Dou Z, Hua J. Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif. 2011;44:19-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci USA. 2009;106:9715-9720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1544] [Cited by in F6Publishing: 1473] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 21. | Zhu FF, Zhang PB, Zhang DH, Sui X, Yin M, Xiang TT, Shi Y, Ding MX, Deng H. Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia. 2011;54:2325-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Stoffers DA, Thomas MK, Habener JF. Homeodomain protein IDX-1: a master regulator of pancreas development and insulin gene expression. Trends Endocrinol Metab. 1997;8:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Kojima H, Nakamura T, Fujita Y, Kishi A, Fujimiya M, Yamada S, Kudo M, Nishio Y, Maegawa H, Haneda M. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes. 2002;51:1398-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030-1037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 25. | Marshak S, Totary H, Cerasi E, Melloul D. Purification of the beta-cell glucose-sensitive factor that transactivates the insulin gene differentially in normal and transformed islet cells. Proc Natl Acad Sci USA. 1996;93:15057-15062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Miller CP, McGehee RE, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994;13:1145-1156. [PubMed] [Cited in This Article: ] |
| 27. | Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Watada H, Kajimoto Y, Umayahara Y, Matsuoka T, Kaneto H, Fujitani Y, Kamada T, Kawamori R, Yamasaki Y. The human glucokinase gene beta-cell-type promoter: an essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells. Diabetes. 1996;45:1478-1488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 952] [Cited by in F6Publishing: 864] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 30. | Kafri T, van Praag H, Gage FH, Verma IM. Lentiviral vectors: regulated gene expression. Mol Ther. 2000;1:516-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 186] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Hamaguchi I, Woods NB, Panagopoulos I, Andersson E, Mikkola H, Fahlman C, Zufferey R, Carlsson L, Trono D, Karlsson S. Lentivirus vector gene expression during ES cell-derived hematopoietic development in vitro. J Virol. 2000;74:10778-10784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Li Z, Düllmann J, Schiedlmeier B, Schmidt M, von Kalle C, Meyer J, Forster M, Stocking C, Wahlers A, Frank O. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 471] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 33. | Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132-3142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1295] [Cited by in F6Publishing: 1292] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 34. | Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 35. | Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 242] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Talebi S, Aleyasin A, Soleimani M, Massumi M. Derivation of islet-like cells from mesenchymal stem cells using PDX1-transducing lentiviruses. Biotechnol Appl Biochem. 2012;59:205-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, Arner P, Rydén M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Karaoz E, Genç ZS, Demircan PÇ, Aksoy A, Duruksu G. Protection of rat pancreatic islet function and viability by coculture with rat bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2010;1:e36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16283] [Cited by in F6Publishing: 17497] [Article Influence: 1093.6] [Reference Citation Analysis (0)] |
| 40. | Dhasarathan P, Theriappan P. Evaluation of anti-diabetic activity of Strychonous potatorum in alloxan induced diabetic rats. J Med Med Sci. 2011;2:670-674. [Cited in This Article: ] |
| 41. | Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A, Verrecchia F, Larghero J. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837-3844. [PubMed] [Cited in This Article: ] |
| 43. | Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Naaijkens BA, Niessen HW, Prins HJ, Krijnen PA, Kokhuis TJ, de Jong N, van Hinsbergh VW, Kamp O, Helder MN, Musters RJ. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348:119-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | An DS, Wersto RP, Agricola BA, Metzger ME, Lu S, Amado RG, Chen IS, Donahue RE. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34(+) cells. J Virol. 2000;74:1286-1295. [PubMed] [Cited in This Article: ] |
| 46. | Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607-1611. [PubMed] [Cited in This Article: ] |