Copyright
©The Author(s) 2023.
World J Stem Cells. May 26, 2023; 15(5): 354-368
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.354
Published online May 26, 2023. doi: 10.4252/wjsc.v15.i5.354
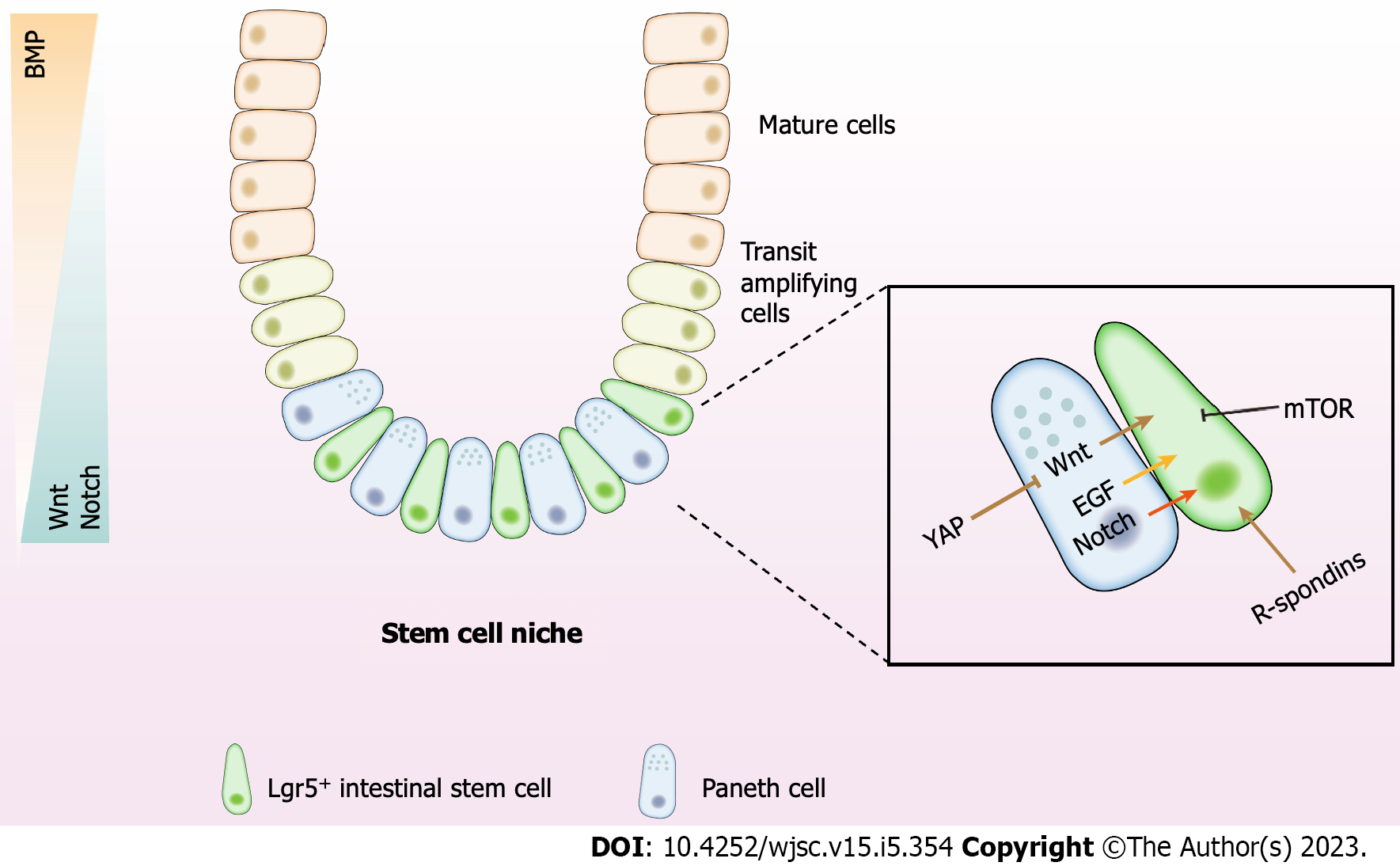
Figure 1 The intestinal stem cell niche and regulatory signals.
The intestinal epithelium consists of crypts and villi. The crypts generate a constant stream of new cells that differentiate and migrate upward into the villi. These Lgr5+ intestinal stem cells reside at the bottom of the crypts and are wedged between Paneth cells, which protect and nurture stem cells. Above the stem cell zone is the lineage-committed progenitor cells, also known as the transit amplifying zone, divided to fuel the rapid epithelial cell turnover. Mature epithelial cells originate from the crypt and move up toward the villus tip. The Wnt and Notch signaling exhibit high activity in the stem cell niche. Activation of the signals decreases along with the increased distance from the crypt bottom. While the BMP activity stands in the opposite direction. WNT, R-spondin, epidermal growth factor, and mammalian target of rapamycin are secreted by Paneth cells or mesenchymal cells. YAP works through the Wnt signaling to maintain the crypt–villus integrity. Besides, Paneth cells provide essential Notch signals to stem cells by expressing Notch ligands. The signaling network in the niche establishes the baseline for self-renewal, fate determination, proliferation, and differentiation of intestinal stem cells. BMP: Bone morphogenetic protein; EGF: epidermal growth factor; mTOR: Mammalian target of rapamycin.
- Citation: Wang Z, Qu YJ, Cui M. Modulation of stem cell fate in intestinal homeostasis, injury and repair. World J Stem Cells 2023; 15(5): 354-368
- URL: https://www.wjgnet.com/1948-0210/full/v15/i5/354.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i5.354