Copyright
©The Author(s) 2020.
World J Stem Cells. Aug 26, 2020; 12(8): 706-720
Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.706
Published online Aug 26, 2020. doi: 10.4252/wjsc.v12.i8.706
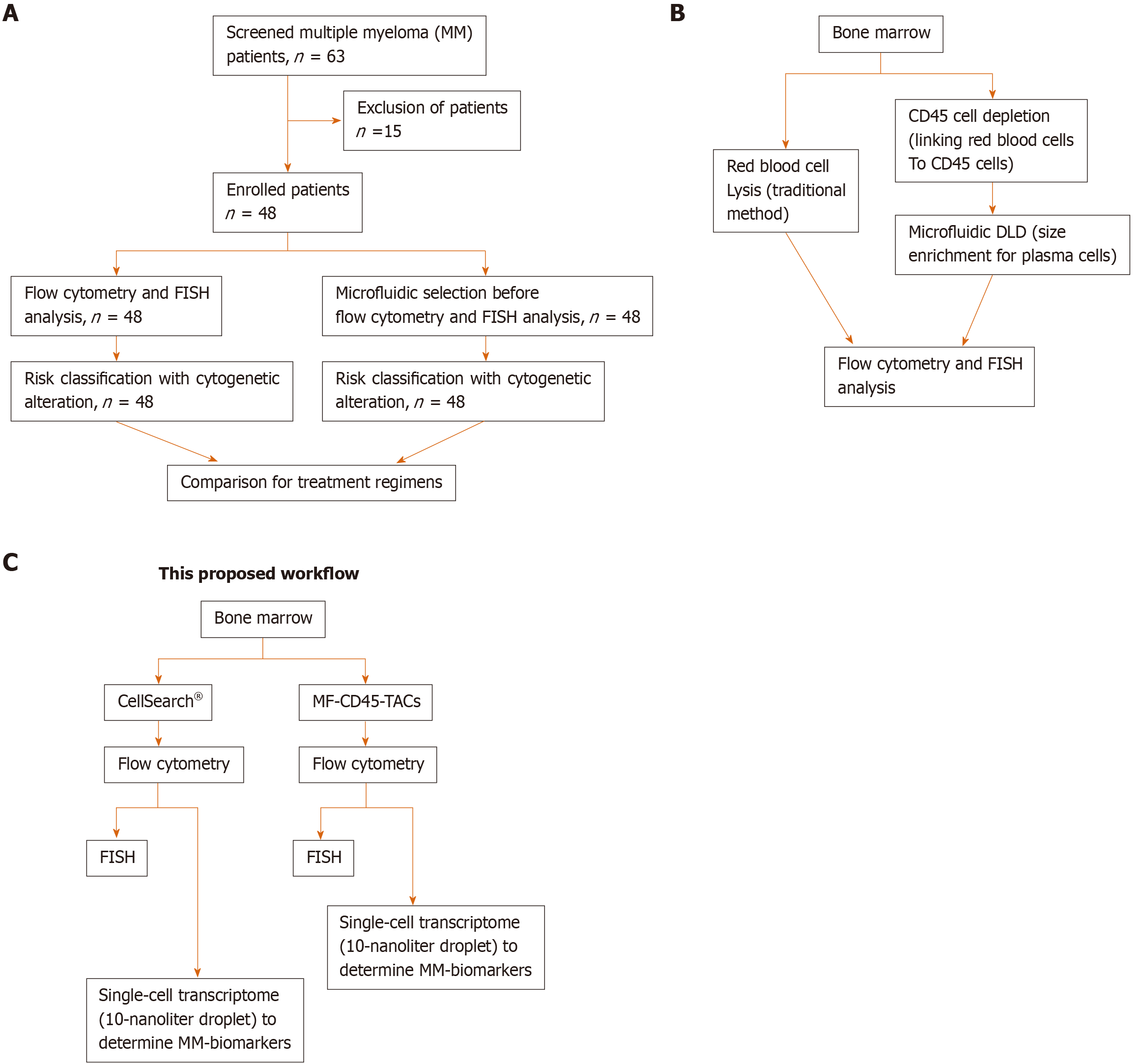
Figure 3 Schematic designs of the proposed workflow.
A: Consolidated Standards of Reporting Trials diagram. A total of 63 patients were screened for eligibility. Only 48 patients were newly diagnosed with multiple myeloma before receiving any treatment. These patients were enrolled, and their bone marrow obtained at diagnosis was divided into two aliquots: One aliquot underwent traditional flow cytometry and FISH analysis, and the other aliquot was subjected to microfluidic selection for enrichment of CD45-PCs, then subjected to flow cytometry and FISH analysis. Results from both methods were compared; B: Comparison of traditional method to microfluidic method (MF-CD45-TACs). MF-CD45-TACs significantly enrich plasma cells for flow cytometry and FISH assays and improve the accuracy of these assays; C: This proposed workflow (Note that we can use both bone marrow and circulating multiple myeloma cells[76]).
- Citation: Lee LX, Li SC. Hunting down the dominating subclone of cancer stem cells as a potential new therapeutic target in multiple myeloma: An artificial intelligence perspective. World J Stem Cells 2020; 12(8): 706-720
- URL: https://www.wjgnet.com/1948-0210/full/v12/i8/706.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i8.706