Copyright
©The Author(s) 2020.
World J Stem Cells. Mar 26, 2020; 12(3): 188-202
Published online Mar 26, 2020. doi: 10.4252/wjsc.v12.i3.188
Published online Mar 26, 2020. doi: 10.4252/wjsc.v12.i3.188
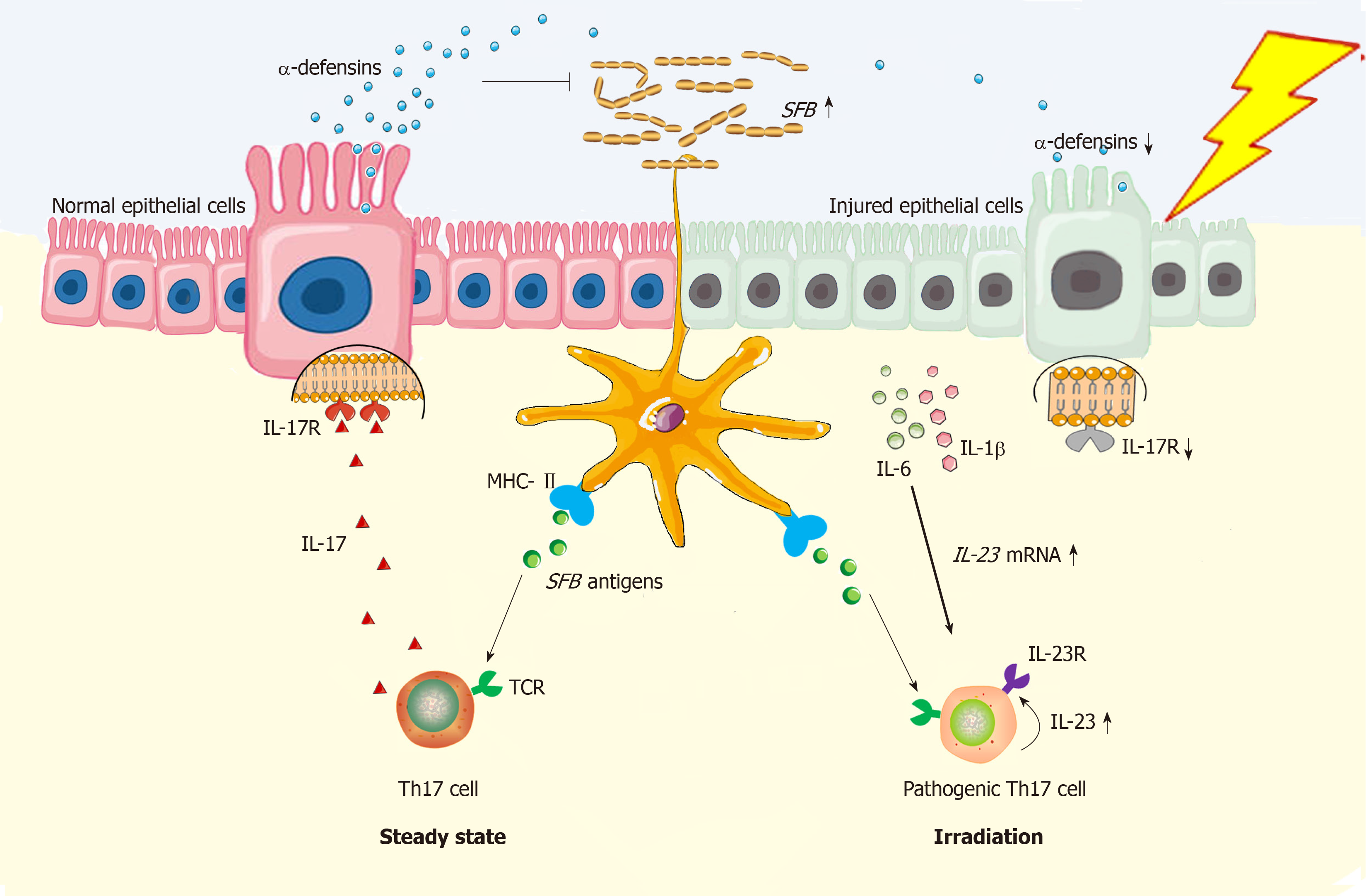
Figure 1 Schema of radiation exposure in pathogenic Th17 cell induction in gut.
In steady state, dendritic cells (DCs) are potent in Th17 induction in gut of mice because the T cell receptor recognizes the segmented filamentous bacteria (SFB) antigen presenting by DCs[28]; Meanwhile, MHC class II molecule on DCs can provide all essential signals for Th17 polarization[41]. Functionally, Th17 cells can stimulate synthesis of α-defensins by epithelial cells depending on interleukin (IL)-17/IL-17R interaction, thus protecting against SFB overgrowth in gut lumen[33]. However, under the irradiated condition, epithelial injuries will augment the local concentration of IL-1β and IL-6[31,35], which functionally upregulate expression of gene encoding IL-23[35,42]. By binding with IL-23R on Th17 cells, IL-23 is able to stimulate Th17 expansion[35]. Herein, both IL-23R/IL-22 loop and IL-23/IL-17 loop are able to increase Th17 cell-mediated immune response[26,43], thus enabling the inflammation in irradiated gut to persist. In this regard, Th17 cells are pathogenic. Besides, due to epithelial loss, low production of α-defensins will somewhat facilitate SFB overgrowth in gut lumen, thus facilitating Th17 induction as well. Collectively, Th17 cell induction will be robust in irradiated gut. DCs: Dendritic cells; SFB: Segmented filamentous bacteria; MHC-II: Major histocompatibility complex class II; TCR: T cell receptor; Th17: T helper cell 17; IL-17: Interleukin-17; IL-17R: Interleukin-17 receptor; IL-1β: Interleukin-1β; IL-6: Interleukin-6; IL-22: Interleukin-22; IL-23: Interleukin-23; IL-23R: Interleukin-23 receptor.
- Citation: Gao YL, Shao LH, Dong LH, Chang PY. Gut commensal bacteria, Paneth cells and their relations to radiation enteropathy. World J Stem Cells 2020; 12(3): 188-202
- URL: https://www.wjgnet.com/1948-0210/full/v12/i3/188.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i3.188